INTRODUCTION
Dating rock paintings is a key tool for archaeological studies in order to better understand the relative chronology between different human occupations of the sites, and how these prehistoric groups used to communicate. However, rock paintings are difficult to date because of the complexity of their composition with the use of a large range of materials from organic or inorganic origins, and to some extent, a mix of both. Watchman (Reference Watchman1993) gives an extensive list of datable organic compounds that includes charcoals, plant fibres and binders such as vegetable glue, animal oil, blood or honey as well as eggs, which were used in the production of rock paintings. Many studies describe how to date these types of organic materials because sufficient carbon can be extracted for a correct AMS date (Van der Merwe et al. Reference Van der Merwe, Sealy and Yates1987; Loy et al. Reference Loy, Jones, Nelson, Meehan, Vogel, Southon and Cosgrove1990; Russ et al. Reference Russ, Hyman, Shafer and Rowe1990; Valladas et al. Reference Valladas, Tisnerat, Cachier, Arnold, Bernaldo de Quiros, Cabrera Valdés, Clottes, Courtin, Fortea-Perez, Gonzalez-Sainz and Moure-Romanillo2001; Valladas Reference Valladas2003; Cole and Watchman Reference Cole and Watchman2005; Morwood et al. Reference Morwood, Walsh and Watchman2010; Bonneau et al. Reference Bonneau, Brock, Pearce and Pollard2011; Beck et al. Reference Beck, Genty, Lahlil, Lebon, Tereygeol, Vignaud, Reiche, Lambert, Valladas, Kaltnecker, Plassard, Menu and Paillet2013). However, the problem of dating the paintings arises when the carbon content of the paint layers becomes too small or even non-existent. Most rock paintings have no organic carbon available because the pigments were made from minerals such as iron oxides or aluminium silicates. In other cases, some paintings have carbon but in amounts that are too low even for AMS dating, which uses a gas ion source to analyze micrograms of carbon. This is often the case with open-air rock paintings, which are exposed to various environmental conditions that can largely degrade the pigment. A further complication is that obtaining pigment samples from rock painting motifs is often restricted, due to the damaging nature of the procedures. These cultural sites are fragile and can be considered one of the fundamental heritages of humanity. Thus, in order to date such important archaeological sites, research must design new, less destructive approaches.
Indirect stratigraphic dating of rock art can be considered one solution. To extract and date sample material overlaying and/or underlying the prehistoric painted artworks can give a better estimate of when the paintings were made. This approach can be applied on calcite crusts, silica skin with trapped organic matter, or calcium oxalate deposits (Watchman Reference Watchman1993). In this study, we concentrated on radiocarbon (14C) dating the calcium oxalate deposits covering rock paintings at some of the painted sites in Omandumba East and West, Erongo Mountains, Namibia, to provide a chronological limit, a terminus ante quem, for the age of the Namibian rock art. Combining this approach with the relative stylistic sequences might help to build a stronger archaeological context and to better understand the cultural exchanges in the different occupational phases of the sites. Various ways to date oxalate material have been applied in different regions all over the world including different carbon extraction techniques (Steelman et al. Reference Steelman, Rickman, Rowe, Boutton, Russ, Guidon and Jakes2002; Watchman et al. Reference Watchman, O’Connor and Jones2005; Ruiz et al. Reference Ruiz, Hernanz, Armitage, Rowe, Viñas and Rubio2012; Jones et al. Reference Jones, Levchenko, King, Troitzsch, Wesley, Williams and Nayingull2017). For this study, we tested the chemical extraction of oxalates from the mineral crust sampled on the studied archaeological sites. The aim was to establish, validate and define a robust and reliable protocol to separate the oxalate material from the organic residues of the crusts. The composition of bulk samples, and of the different phases after a pretreatment, which includes the oxalate extracts and residues, was monitored by Fourier transform infrared spectroscopy (FTIR). We first describe the formation of the oxalate skins on the rocks and the distinctiveness of the Namibian open-air sites. Then we present our protocols and discuss the different analyses and dating results. Finally, the reliability of this method and the new opportunities for further studies are discussed.
Archaeological Context and Oxalate Formation
The region of Central Namibia is well-known for its dense concentration of prehistoric rock paintings. The tradition is attributed to the hunter-gatherer communities who inhabited the region and who are the ancestors of the present-day San populations (Scherz Reference Scherz1970; Rudner Reference Rudner1982, Reference Rudner1983; Pager Reference Pager1989; Kinahan Reference Kinahan1990; Lenssen-Erz Reference Lenssen-Erz, Bubenzer, Bolten and Darius2007; Richter and Vogelsang Reference Richter, Vogelsang, Limprecht and Biesele2008, etc.)
In Namibia, as everywhere in the world, establishing chronology is one of the vital components in rock art studies. However, it is generally notoriously difficult due to the absence of organic material. As a result, paintings are often dated in association with other artefact assemblages from various archaeological contexts. Such relative dates do not necessarily produce the actual age of the rock paintings and it is possible that the paintings could be older or younger than this relative age determination. A classic example is that of the Apollo 11 cave site slabs known as “art mobilier” or mobile art from southern Namibia, recovered from the Middle Stone Age assemblages, and dated to 25,000 BP (Wendt Reference Wendt1972). It is possible, however, that the artwork could be even older as demonstrated by Vogelsang et al. Reference Vogelsang, Richter, Jacobs, Eichhorn, Linseele and Roberts2010) who date the art between 31.1 Ka and 32.9 Ka using an optical stimulated luminescence (OSL) dating technique.
The lack of direct dating leads to significant uncertainties in the chronology and attribution of these prehistoric artworks. Since 2007, a Franco-Namibian archaeological team has excavated the ornate site of Leopard Cave in the Erongo Montains (Erongo, Namibia), in order to document the use of pigments linked to the production of rock art, and to refine the chronological framework of hunter-gatherer occupation in this region (Pleurdeau et al. Reference Pleurdeau, Imalwa, Détroit, Lesur, Veldman, Bahain and Marais2012; Mauran et al. Reference Mauran, Lebon, Détroit, Caron, Nankela, Pleurdeau and Bahain2019). Pigments from rock paintings in this region are mainly composed of red iron oxides. However, during the study of the rock art pigments with in situ pXRF analyses (Elio XGLab, Brücker), results showed that black pigments appear to have an organic origin such as charcoal. Moreover, red or black pigments were widely impacted and degraded by weathering actions and as a result, the main part of the rock paintings is covered by white/gray accretions. In situ pXRF analysis suggested that these mineral crusts contain gypsum (CaSO4·2H2O) and also calcium oxalate minerals such as weddellite (CaC2O4·2H2O) and whewellite (CaC2O4·H2O) which contain carbon. Dating this material could therefore offer a minimum age limit, or the terminus ante quem, for the age of the paintings.
Generally, the crystallization of calcium oxalate hydrates can occur on various kinds of surfaces and this complex mechanism has been studied for a long time to better understand the origin of the dated carbon (Watchman Reference Watchman1993; Hedges et al. Reference Hedges, Ramsey, Van Klinken, Pettitt, Nielsen-Marsh, Etchegoyen, Niello, Boschín and Llamazares1998). However, the main criticism levelled against oxalate dating is the uncertainty about the source of carbon during the oxalate formation process. For instance, is the carbon derived only from atmospheric carbon dioxide used by oxalate-forming micro-organisms or does it also originate from the rock substrate (Watchman Reference Watchman1993; Bednarik Reference Bednarik2002; Aubert Reference Aubert2012)? Research projects conducted under different climatic conditions in different areas worldwide showed that carbon is brought by micro-organisms which absorb or assimilate carbon from the atmosphere and not from the surfaces on which they grow, particularly when the substrate is pure rock with no carbon content (Chen and Blume Reference Chen and Blume2000; Beazley et al. Reference Beazley, Rickman, Ingram, Boutton and Russ2002; Hess et al. Reference Hess, Coker, Loutsch and Russ2008; Rusakov et al. Reference Rusakov, Vlasov, Zelenskaya, Frank-Kamenetskaya and Vlasov2016). It is therefore accepted that the origin of the oxalate ions [C2O4]2– is biogenic with the participation of lichens or fungi (Aspergillus and Penicillium). Evidence shows that lichen activity excretes oxalic acids, which react with calcium coming from the rock substrate such as granite. With the activity of bacteria (Bacillus), this complicated multifactor process begins and Ca2+ and [C2O4]2– ions begin to crystallize first with the formation of weddellite (CaC2O4·2H2O) and then whewellite (CaC2O4·H2O) after dehydration. In the Erongo Mountains where our study was conducted, the geological rocks are granitic with an absence of calcite or other carbon-bearing minerals that could possibly react during oxalate crystallization. It seems highly probable that the only source of the carbon in oxalates of this area is the biogenic activity of micro-organisms with an atmospheric signature of different deposits. In Ruiz et al. Reference Ruiz, Hernanz, Armitage, Rowe, Viñas and Rubio2012), it is also highlighted that, for open air sites, calcium oxalate is a useful material because the crust, which is insoluble in water and inert under UV radiation, is very stable and resistant to weathering. Thus, the accretions are preserved over long periods of time. Studying the different lamination layers can help to refine the chronology between production of the rock art and crystallization of the oxalate skin.
Finally, a special characteristic of open-air sites, such as cave shelters in Namibia, favors the rapid production of calcium oxalate on cave walls (Figure 1). The rocks are colonised by many medium-sized terrestrial mammals, the rock hyrax (Procavia capensis), also called rock badger, and the rock rabbit, commonly referred to as the “rock dassie”. They typically live in groups of 10–80 animals in habitats with rock crevices to escape from predators. These colonies urinate and defecate on granites. Urine secretion crystallizes as a white precipitate that can form high run-off on the rock surface over time. Prinsloo (Reference Prinsloo2007) demonstrated that urine precipitate is composed of vaterite (CaCO3) and monohydrocalcite (CaCO3·H2O). These molecular components constitute a favorable matrix for the development of microbial activity leading to the formation of oxalate crusts.

Figure 1 (a) Photograph of rock hyrax (Procavia capensis); (b) Crevice hosting rock hyrax colony and displaying urine run-off and oxalate concretions. (c) Animal figure (likely kudu) partially covered by oxalate deposits. (d) a rock painting panel (Ghost Cave (GC)—Omandumba East, Erongo Mountains—Namibia).
A final criticism often levelled at oxalate dating is the possibility that the crust may trap other carbon components that can modify the carbon age of the crust formation. For example, windblown material such as charcoal, pollen or sediment can be deposited later in the pores of the whewellite and skew the results. For this reason, dating bulk samples is not ideal and a chemical extraction as proposed in Jones et al. Reference Jones, Levchenko, King, Troitzsch, Wesley, Williams and Nayingull2017) is critical. With the adequate pretreatment, it is possible to isolate a pure oxalate extract from the rest of the crust and to remove “the residue” composed of all the exogenous organic materials trapped during or after crystallization of the calcium oxalate.
MATERIALS AND METHODS
Samples Selected by FTIR Analysis
Different types of natural calcium oxalate accretions were taken from four different rock-painting sites in the Erongo Mountains (Namibia): Black Gnu Wall (BGW), Ghost Cave (GC), Elephant Wall (EW), and Leopard Cave (LC). Samples were extracted and collected on painted panels, adjacent to the figures but not directly on them. In all cases, the sampling was done very carefully with surgical blades and protective gloves to avoid any direct contact or other contamination. The quantities were limited to 400 mg to avoid degrading the painted rock surface and to preserve the overall visual appearance of the painted surfaces. We collected thin and thick crust as well as accretions directly from hyrax urine run-off and far from the defecation zones.
Macroscopic samples were analyzed in Attenuated Total Reflection mode (FTIR-ATR) using the Quest-ATR Accessory (Specac) mounted on a Vertex 70 spectrometer (Bruker). Spectra were collected with a spectral resolution of 4 cm–1 in the range of 4000 and 400 cm–1 by accumulation of 32 scans. Microscopic samples were analyzed by FTIR microscopy. A few grains of powder were then pressed into a diamond cell and analyzed in transmittance mode using a Hyperion FTIR microscope (Bruker) coupled with the Vertex FTIR spectrometer. Spectra were recorded between 4000 and 650 cm-1 with a spectral resolution of 4 cm–1 and the accumulation of 64 scans. The sample composition was determined by comparing spectra obtained with the spectral database of mineralogical references from the collection of the Muséum National d’Histoire Naturelle, Kimmel Center (Weiner Reference Weiner2010) and RRUFF (Lafuente et al. Reference Lafuente, Downs, Yang, Stone, Armbruster and Danisi2015).
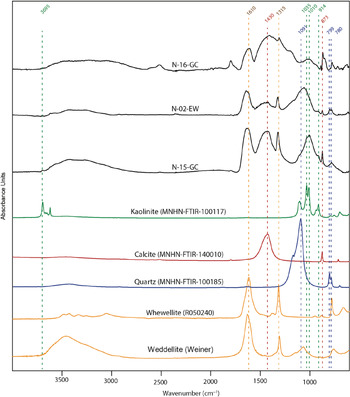
FTIR analyses carried out on bulk samples confirmed the presence of various amounts of oxalates (most likely whewellite) in all crusts sampled (Figure 2). However, oxalates were not the only compounds found and not even the main component of these alteration deposits. Gypsum, clays (kaolinite), quartz, and possibly feldspath were identified (Table 1). The two samples collected on accretions displaying urine run-off (GC-N-15 and GC-N-16) contained calcite, confirming the observation of Prinsloo (Reference Prinsloo2007) on precipitated hyrax urine. To test the oxalate extraction protocol, we selected samples with high or low quantities of oxalate (GC-N-15, GC-N-16, LC-N-11a, LC-N-11b, LC-N-13) to establish whether these contaminants were well removed during the chemical treatment.

Figure 2 FTIR spectrum of a selection of samples from Ghost Cave (N-15-GC and N-16-GC) and Elephant Wall (N-02-EW) in comparison to mineral reference spectra from MNHN, Kimmel Center and RRUFF (weddellite, whewellite, quartz, calcite and kaolinite).
Table 1 Mineral composition of sampled crusts determined by FTIR spectroscopy. Oxalate corresponds most probably to whewellite as shown in Figure 2.

As a result, samples corresponding to various preservation conditions were selected (Table 1). At Ghost Cave, sample GC-N-16 was collected on a very thick white deposit (more than 0.5 cm in some places) displaying hyrax urine run-off on the upper part. Sample GC-N-15 was collected from a thinner white crust surrounded by brown/yellow to black urine run-off (Figure 1d). At Leopard Cave, samples LC-N-11-a and b are oxalate crusts collected on very thin, whitish accretions without any indication of recent activities. Sample LC-N-13 corresponds to yellow crust, sometimes quite dark. The appearance of this crust is different to those observed at Ghost Cave since it is not in a urine run-off defecation zone and appear dry visually.
Sample Preparation
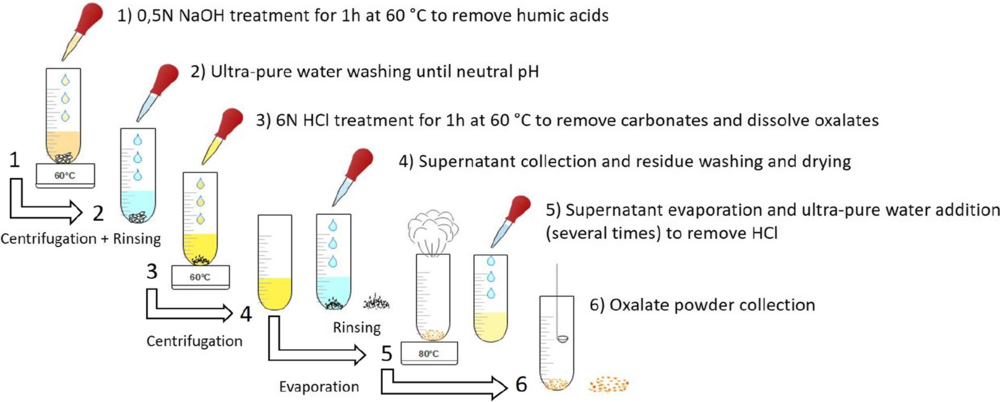
The protocol used in this study (Figure 3) was recently developed by Jones et al. Reference Jones, Levchenko, King, Troitzsch, Wesley, Williams and Nayingull2017). Around 400 mg of powdered calcium oxalate crust was introduced in a 0.5N NaOH solution at 60°C for one hour to remove humic and fulvic acids. After centrifugation, the supernatant was removed and the remaining solid material was collected. Extensive rinsing with ultra-pure water was applied until reaching neutral pH. The sample was then placed in a 6N HCl solution at 60°C for one hour to remove potential carbonates and to dissolve whewellite and weddellite. The solution was once again centrifuged and the supernatant containing the oxalic acid was isolated. The precipitate or “residue” which contains various solids such as silica, sediments, pollen, or windblown material was also collected, rinsed in ultra-pure water until neutral pH and dried to be further analyzed, dated and compared to the results of the oxalic acids. The acid supernatant was then dried at 80°C and part of the HCl was vaporised and removed. To obtain a better removal of HCl, we introduced ultra-pure water several times, followed by multiple evaporation steps. It is very important to remove as much HCl as possible because during combustion in the oven (850°C), the concentrated acid fumes produced can cause the quartz tube to explode, leading to the loss of the sample. After the last evaporation, we collected a dry powder containing the oxalate phase. Three aliquots were taken for the study: one for the Elemental Analyzer (THERMO Scientific Flash 2000) to determine the total organic carbon content (%TOC), a second one for FTIR analysis to verify that carbon comes from the oxalate phase only, and the third one for the 14C dating. The same analyses were performed on the “residue” to compare the carbon content, the age and also to determine the efficiency of the protocol for removing contaminants from the bulk oxalate crust. For the 14C dating, the dried oxalate powder and the residues were sealed in separate quartz tubes with an Ag wire and an excess of CuO and combusted at 850°C as described in Dumoulin et al. Reference Dumoulin, Comby-Zerbino, Delqué-Količ, Moreau, Caffy, Hain, Perron, Thellier, Setti, Berthier and Beck2017). It is important to measure the %TOC of a sample prior to combustion in order to obtain AMS targets with C contents between 0.5 mg to 1 mg of carbon. The CO2 was converted to graphite and analyzed on the LMC14 AMS facility “ARTEMIS’’ (Moreau et al. Reference Moreau, Caffy, Comby, Delqué-Količ, Dumoulin, Hain, Quiles, Setti, Souprayen and Thellier2013).

Figure 3 Different steps in the oxalate extraction protocol used at LMC14.
RESULTS AND DISCUSSION
FTIR and Elemental Analysis
After the chemical treatment and separation of the oxalate powder and the residue, the two fractions were analyzed by FTIR. The spectra (Figure 4a) show that the oxalate powder was almost pure, in some cases with the presence of gypsum but without any other carbon contaminant, whereas the spectra of the residues (Figure 4b) show various types of materials such as silica, gypsum or clay (Kaolinite). The residue of sample LC-N-13 (labeled C14-55264) was not totally dissolved and still contained a small amount of oxalate. A longer treatment in strong acid (HCl 6N) may have been necessary. For the other samples the extraction of oxalate was efficient, and the spectra show that the residues are free of whewellite. It is also interesting to note that calcite was totally removed with the pretreatment (C14-A55236). These FTIR analyses validate the efficiency of the chemical protocol to separate pure oxalate powder from the other components of the sample. The residue contains mineral compounds such as clays (kaolinite) often associated to organic compounds. These have to be removed to obtain a reliable radiocarbon dating of the calcium oxalate crust. The organic compounds of the residue may distort the dating results if combustion is directly applied to the bulk accretion sample.

Figure 4 Examples of FTIR spectra obtained for (a) oxalate extracts C14-55236 and C14-55237) and (b) residues after oxalate extraction (C14-55262 and C14-55264) compared to mineral references of weddellite, whewellite, gypsum, and Opal from RRUFF, Kimmel Center (Weiner Reference Weiner2010), and MNHN spectral databases.
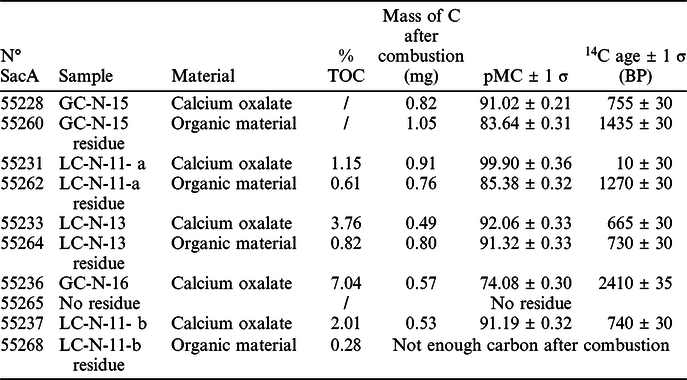
The results of the elemental analysis are reported in Table 2. The %TOC in oxalates is variable. The lowest value of %TOC (1.15%) is observed for sample LC-N-11- a and the highest value (7.04%) for GC-N-166. The %TOC of the residues is low, between 0.28% for LC-N-11-b Residue and 0.82% for LC-N-13 Residue. These values are in accordance with the FTIR results and are explained by the fact that the residues are mainly composed of minerals with low amounts of carbon-containing materials.
Table 2 Total organic carbon results (%TOC) and radiocarbon dating results (not calibrated) of the pure oxalate phase and the residues of natural calcium oxalate accretions from rock-painting sites in Erongo Montains (Namibia).

Radiocarbon Dating
The AMS radiocarbon dating of both fractions was obtained for all the samples except for two residue samples (Table 2). We observed that the ages of the residues were older than those of the pure oxalate fractions. The residue carbon dates were between 730 ± 30 BP for SacA55264 and 1435 ± 30 BP for SacA55260, directly depending on the type of residue and the amount of material trapped during or after formation of the accretion. When the extraction was complete, as for the pair SacA55231 and SacA55262, we observed no cross-contamination since we obtained an old residue of 1270 ± 30 BP for a modern oxalate fraction. On the other hand, when the residue still contained oxalate the two results are close, e.g. for the pair SacA55233 and SacA55264. For that reason, it appears essential to perform the carbon separation with strong 6N HCl acid to dissolve weddellite and whewellite and to ensure total removal of the non-reactive residues. A simple bulk oxalate crust radiocarbon dating would have given a wrong age, much older than the age of the accretion crystallization. This occurs especially when the ages are very far from each other, as for SacA55228 and SacA55260 or for SacA55231 and SacA55262. Without this step of oxalate dissolution, it would be nearly impossible to separate oxalate from the clay or other contaminants. The combination of dating results with the FTIR analyses shows the efficiency of this protocol to remove calcite as well as other organic compounds that do not react with the alkali and acid treatment.
The presence of urine stains nearby or on oxalate deposits cannot be systematically related to a modern age. Sample SacA55231 which was very thin and far from the urine run-off provides a modern age of 10 ± 30 BP for the oxalate and a much older age for the residue (SacA55262). The very thick oxalate accretion from Ghost Cave covered by “fresh” urine run-off provided the oldest ages with 2410 ± 35 BP (SacA55233). This indicates that fresh urine contaminations are discarded during sample treatment. The ages obtained suggest that places suitable for the settlement of rock hyrax colonies can be inhabited for long periods of time, which explains the significant thickness of the crust observed on this site. It confirms that the oxalate crusts are very stable, insoluble and resistant (Ruiz et al. Reference Ruiz, Hernanz, Armitage, Rowe, Viñas and Rubio2012) and that modern urine cannot dissolve older oxalate crusts.
Of the five radiocarbon ages obtained on the oxalate fractions, three of them ranged from 650–750 BP: one at Ghost Cave (755 ± 30 BP; SacA55228) and two at Leopard Cave (665 ± 30 BP; SacA55233 and 740 ± 30 BP; SacA55237). These similar ages question the origin of the processes leading to the formation of these oxalate accretions. At Leopard cave, these oxalate deposits are placed either in crevices, or at the limit of water run-off zones on the wall. Leopard Cave is geomorphologically protected from direct rainfall and the run-off is only active during periods of heavy or erratic rains which are rather rare but not unusual in this region of Namibia. The age obtained corresponds to the beginning of the Little Ice Age which may have been a period marked by intense precipitations in this area of Southern Africa (Heine Reference Heine2004). These precipitations could have promoted the formation of the crust, or the increase in rock-hyrax populations and thus the amount of secretions accumulated. In any case, the formation of these oxalate crusts is probably a multi-factorial process, and a better understanding of their formation is essential for the reliable use of this proxy for constraining ages. In addition, it is important to note that the protocol used in this study provides reliable dating whatever the source of contaminants. For the 3 samples mentioned above, similar oxalate ages were obtained for different preservation conditions evidenced by different residue ages and contaminant amounts. These results highlight that a chronology based on oxalate crust formation can be achieved.
CONCLUSION
This study was a first attempt to date oxalate for indirect stratigraphic dating of rock art in Namibia. We have shown that the pretreatment selected for the samples coming from open-air caves was appropriate to isolate pure oxalate from the crust. The chemical treatment was efficient to remove inorganic contaminants such as calcite but also organic compounds brought by wind and trapped during crystallization of the calcium oxalate crust. Oxalate dissolution by the strong acid step (HCl 6N) is required to remove all the compounds such as minerals, sediments, charcoal or pollen that do not react with the classical acid-alkali-acid treatment. This chemical separation allowed us to date only the carbon signature of the oxalate fraction. It is essential to target only the oxalate fractions from the crust because significant differences were found between the ages of the residues (often older) and the pure oxalate powders. This protocol is quite easy to implement and does not need special laboratory glassware.
This study confirms the importance of the sampling strategy on site. Highly variable ages were obtained on the same site and highlight the importance of collecting the layers of oxalate as close as possible to the rock painting. Pre-screening by FTIR is not only useful to be performed on the bulk accretion to check the presence of oxalates but also on the oxalate fraction obtained after the chemical treatment to control the purity of the powder. Dating the residues gives information about the contaminants removed from the samples; if access to AMS is limited, this step is not essential for the study of rock paintings. On the other hand, elemental analysis is recommended to estimate the amount of oxalates necessary to produce enough CO2.
Oxalate dating seems to be a good alternative to determine a terminus ante quem of non-datable rock painting either because the carbon contents are too low or non-existent or because direct sampling of the painting is not ethically allowed. The particularity of the presence of hyrax population is not a problem because the old crusts seem to be very stable and insoluble. The next step in this study will be to date selected calcium oxalate deposits covering rock paintings to obtain an estimation of the minimum age limit of this rock art.
ACKNOWLEDGMENTS
The authors are very grateful to Ms. and Mr. Rüst and their family for their kind permission to access and analyze the rock art sites located on the Omandumba farm. The authors wish to express their sincere gratitude to the National Heritage Council of Namibia for allowing these analyses according to the permit 11/2015 renewed and extended with the renewal permit 04/2017 given to D. Pleurdeau. The authors also thank the French Ministry of Foreign Affairs and the French embassy in Namibia for their support of the MANAM project. The authors also thank Sorbonne Universités for financial support through the Chaire Polyre funding the PhD project of G.M, and the APaNam project funded by the Observatoire des Patrimoines de Sorbonne Universités (OPUS). The authors also wish to thank the “Plateau de Spectrométrie Infrarouge” of the “Plateforme Analytique du MNHN” for access to the instruments. The LMC14 is funded by five French organizations: CEA, CNRS, IRD, IRSN, and MC. This is LSCE contribution number 6970.