INTRODUCTION
American trypanosomiasis or Chagas' disease is a debilitating illness caused by Trypanosoma cruzi, and is highly prevalent in Latin America where it affects about 10–16 million people, causing the death of around 12 500 patients annually. Economic hardship and political problems have spurred migration from Chagas-endemic countries to developed countries (Schmunis, Reference Schmunis2007; WHO, 2006). The infection is characterized by an acute phase that results in 2–8% mortality, in which the parasite circulates in the bloodstream as trypomastigotes and proliferates within the cytoplasm of a variety of cells as amastigotes. While in the chronic phase, most patients remain asymptomatic, and 30–40% of cases develop cardiac symptoms or digestive lesions (Prata, Reference Prata2001; Dantas et al. Reference Dantas, Salomão, Barbosa and Castro2006). Introduced in the 1960s and 1970s, nifurtimox and benznidazole are the currently accepted nitroderivatives for treatment of this disease. These compounds are active in the acute stage of Chagas' disease, but their efficacy during the chronic phase is still controversial. Moreover, the therapeutic dose is very close to the toxic dose and severe side-effects have been reported during their clinical use, including polyneuritis, lymphadenopathy, dermatitis, anorexia, allergic dermopathy, and depression of bone marrow. There is an urgent need to develop new compounds or novel strategies to make Chagas' disease chemotherapy more effective and less toxic (Coura and Castro, Reference Coura and Castro2002; Urbina and Docampo, Reference Urbina and Docampo2003; Urbina, Reference Urbina2009).
Marine algae have been used in traditional remedies in Asian countries including China, Japan, and Korea (Wang et al. Reference Wang, Zhang, Duan and Li2009). Species of Laurencia (order Ceremiales, family Rhodomeleceae) have proved to be a rich source of halogenated secondary metabolites, predominantly sesquiterpenes, diterpenes, and C15 non-terpenoids (Iliopoulou et al. Reference Iliopoulou, Roussis, Pannecouque, De Clercq and Vagias2002: Kladi et al. Reference Kladi, Vagias, Stavri, Rahman, Gibbons and Roussis2008). The probable role of these halogenated metabolites is to defend the algae against marine herbivores (Vairapan et al. Reference Vairappan, Suzuki, Abe and Masuda2001; Iliopoulou et al. Reference Iliopoulou, Roussis, Pannecouque, De Clercq and Vagias2002; Salgado et al. Reference Salgado, Viana, Andrade, Leal, Gama, Attias, Pereira and Amado Filho2008; Sudatti et al. Reference Sudatti, Rodrigues, Coutinho, Gama, Salgado, Amado Filho and Pereira2008). Published reports describe important biological activities of seaweeds, including antibacterial, antifungal, anti-leishmanial, anti-trichomonal, antihelmintic, antimalarial, antioxidative, antiviral, antipyretic, analgesic, anti-inflammatory, and anticoagulant (Schaeffer and Krylov, Reference Schaeffer and Krylov2000; Vairappan et al. Reference Vairappan, Suzuki, Abe and Masuda2001; Vairappan, Reference Vairappan2003; Matsuhiro et al. Reference Matsuhiro, Conte, Damonte, Kolender, Matulewicz, Mejías, Pujol and Zúñiga2005; Kang et al. Reference Kang, Khan, Park, Cho, Lee, Fujii and Hong2008; Kladi et al. Reference Kladi, Vagias, Stavri, Rahman, Gibbons and Roussis2008; Moo-Puc et al. Reference Moo-Puc, Robledo and Freile-Pelegrin2008; Freile-Pelegrin et al. Reference Freile-Pelegrin, Robledo, Chan-Bacab and Ortega-Morales2008; Mayer et al. Reference Mayer, Rodríguez, Berlinck and Hamann2009; Wang et al. Reference Wang, Zhang, Duan and Li2009).
In the present study, we assessed the in vitro anti-trypanosomal activity of the sesquiterpene elatol, the major constituent of the Brazilian red seaweed Laurencia dendroidea (Hudson) J. V. Lamouroux, against Trypanosoma cruzi. We used electron-microscopy techniques to evaluate the effect of elatol on the morphology and ultrastructure of the parasite.
MATERIALS AND METHODS
Algal material and obtention of elatol
Specimens of Laurencia dendroidea, a seaweed that occurs in the intertidal zone along almost the entire Brazilian coast, were collected at Cabo Frio Island, Rio de Janeiro state (22°59′S, 42°59′W). This seaweed was previously described as L. obtusa, but through molecular techniques, it was recently identified as L. dendroidea (Cassano, Reference Cassano2009). The specimens of L. dendroidea used in this study were identified by Dr Mute Toyota Fujii, and voucher specimens were deposited in the herbarium of the Instituto de Botânica, São Paulo state, Brazil (SP number: 399789).
The specimens of L. dendroidea were collected by hand during low tide and transported to the laboratory between sheets of moist paper in coolers. In the laboratory, this algal material was air-dried in the dark at room temperature, in order to avoid photolysis and thermal degradation. Dried seaweeds were submitted to exhaustive extraction in hexane or dichloromethane, and a crude extract was obtained. The solvent was eliminated in a rotary evaporator, and the elatol was isolated on pre-coated TLC plates, identified by TLC (thin-layer chromatography: Merck Al TLC 20×20 cm silica gel 60F254) and 1H NMR (nuclear magnetic resonance). The spectra were measured on a Varian Unity Plus spectrometer, operating at 299·9 MHz for 1H and 75·0 MHz for 13C, as proposed by Da Gama et al. (Reference Da Gama, Pereira, Soares, Teixeira and Yoneshigue-Valentin2003), and compared with the literature (Sims et al. Reference Sims, Lin and Wing1974; Konig and Wright, 1997).
Parasites and cells
Epimastigote forms of T. cruzi, Y strain were maintained at 28°C by weekly transfers in liver infusion tryptose medium – LIT (Camargo, Reference Camargo1964), supplemented with 10% inactivated foetal bovine serum (FBS) (Gibco Invitrogen Corporation, NY, USA). Trypomastigote forms were obtained from the supernatant of a monolayer of infected LLCMK2 cells in Dulbecco's modified Eagle's medium (DMEM, Gibco Invitrogen Corporation, New York, USA) in 5% CO2 at 37°C. LLCMK2 (epithelial cells of monkey kidney – Macaca mulatta) were maintained in DMEM supplemented with 2 mm L-glutamine, 10% FBS, and 50 mg/l gentamicin, buffered with sodium bicarbonate.
Anti-proliferative activity of elatol on the epimastigote form
Epimastigote forms of T. cruzi (106 cells/ml) were cultured in LIT medium supplemented with 10% FBS, in the absence or presence of different concentrations (3·0 to 300·0 μm) of elatol (from stock solution in 1% dimethyl sulfoxide). Parasites were incubated at 28°C for 96 h in 24-well microplates. After that, cell growth was determined by counting the parasites with a Neubauer haemocytometer (Improved Double Neubauer) and the results were expressed as the percentage of inhibition in relation to the control cultured in medium alone. The IC50 (concentration that inhibited 50% parasite growth) and IC90 (concentration that inhibited 90% parasite growth) were determined by logarithm regression analysis of the data obtained. Benznidazole (N-benzyl-2-nitro-1-imidazolacetamide) – Rochagan® (Roche Pharmaceuticals, Rio de Janeiro, Brazil) was used as the reference drug. Each experiment was conducted in duplicate and repeated at least 3 times.
Effect of elatol on the viability of the trypomastigote form
Trypomastigote forms (107 cells/ml) were resuspended in DMEM medium supplemented with 20% FBS, containing 10% mouse blood, in the absence or presence of different concentrations (0·3 to 60·0 μm) of elatol. Parasites were incubated at 37°C in a 5% CO2 air mixture for 24 h in 96-well microplates. The IC50 (concentration which lysed 50% of the parasites) was calculated in accordance with the Pizzi-Brener method (Brener, Reference Brener1962). Crystal violet was used as the reference drug. Each experiment was conducted in duplicate and repeated at least 3 times.
Activity of elatol on the intracellular amastigote forms
To assess the in vitro activity against intracellular T. cruzi amastigotes, LLCMK2 cells were seeded at a concentration of 2·5×105 cells/ml in 24-well microplates containing glass cover-slips and DMEM medium, and then maintained at 37°C for 24 h to allow cell adhesion to the cover slips. Trypomastigotes were added to the wells at a concentration of 10 parasites per host cell and incubated for 24 h. Then, non-internalized trypomastigotes were washed and the infected LLCMK2 cells were treated with different concentrations (1·5 to 12·0 μm) of elatol for 96 h at 37°C with 5% CO2 atmosphere, following fixation in methanol and Giemsa staining. The number of amastigotes was determined by counting at least 200 cells in duplicate cultures, and the results were expressed as the survival index. The survival index was obtained by multiplying the percentage of infected cells by the number of amastigotes per infected LLCMK2 cell. Each experiment was conducted in duplicate and repeated at least 3 times.
Red blood cell lysis assay
The potential haemolytic effect of elatol was evaluated in this assay. A 4% suspension of fresh defibrinated human blood was prepared in sterile 5% glucose solution. Several concentrations (1·2 to 60·0 μm) of the elatol were added to individual test tubes and gently mixed, and the tubes were incubated at 37°C. After 1 h of incubation, the visual reading was made, and after 2 h the samples were centrifuged at 250 g for 5 min. The absorbance of the supernatant was determined at 540 nm for estimation of haemolysis. The results were expressed as the percentage of haemolysis. Amphotericin B (Cristalia, São Paulo, Brazil) was used as the reference drug, Triton X-100 (Vetec, Rio de Janeiro, Brazil) was used as the positive control, and the cell suspension alone was used as the negative control. Each experiment was conducted in duplicate and repeated at least 3 times.
Cytotoxicity assay
A suspension of LLCMK2 cells was seeded at a concentration of 2·5×105 cells/ml in a 96-well microplate containing DMEM medium supplemented with 10% FBS, and then maintained at 37°C in 5% CO2 air mixture for 24 h until confluence was achieved. Thereafter, the cells were treated with different concentrations (0·3 to 300·0 μm) of elatol for 96 h under the same conditions as above. Control wells without elatol were included, and Benznidazole was used as the reference drug. Subsequently, the sulforhodamine B colourimetric assay was carried out. Absorbance was read in a 96-well plate reader (BIO-TEK Power Wave XS) at 530 nm. The 50% Cytotoxicity Concentration (CC50) was extrapolated by linear regression analysis. The cytotoxicity of elatol on LLCMK2 cells was also compared with the activity against trypomastigote and intracellular amastigote forms of T. cruzi, by using the selective index (SI) (ratio: CC50 LLCMK2 cells/IC50 protozoa). All experiments were performed in duplicate. The means were determined from at least 3 experiments.
Electron microscopy
Epimastigote, trypomastigote, and intracellular amastigote forms of T. cruzi were treated with elatol and then processed for electron microscopy. Parasite cells were harvested and washed twice with PBS, and fixed with 2·5% glutaraldehyde in 0·1 m sodium cacodylate buffer at 4°C. For transmission electron microscopy (TEM), cells were post-fixed in a solution containing 1% OsO4, 0·8% potassium ferrocyanide, and 10 mm CaCl2 in 0·1 M cacodylate buffer, dehydrated in an increasing acetone gradient, and embedded in Epon® resin. Next, ultrathin sections were stained with uranyl acetate and lead citrate, and images were obtained on a Zeiss 900 TEM. For scanning electron microscopy (SEM), epimastigote and trypomastigote forms of T. cruzi were fixed as before. Next, small drops of the sample were placed on a specimen support with poly-L-lysine. The samples were dehydrated in graded ethanol, critical-point dried in CO2, coated with gold, and observed on a Shimadzu SS-550 SEM.
Statistical analyses
Statistical analysis was performed with the program GraphPad Prism 4 (GraphPad Software, San Diego, California, USA). Student's t-test was applied, and a P value less than 0·05 was regarded as significant. All experiments were performed in duplicate. The means and standard deviations were determined from at least 3 experiments.
RESULTS
Chemical composition of L. dendroidea
The physical and spectroscopic properties of the compound elatol are shown in Table 1, and were identical with previously reported data (König and Wright, Reference König and Wright1997). Elatol (Fig. 1) is a colourless oil, and has been described in several species of the genus: Laurencia elata (Sims et al. Reference Sims, Lin and Wing1974), Laurencia obtusa (Hay et al. Reference Hay, Fenical and Gustafson1987), and Laurencia rigida (König and Wright, Reference König and Wright1997). The oil plays several ecological (Hay et al. Reference Hay, Fenical and Gustafson1987, Reference Hay, Duffy and Fenical1988; de Nys et al. Reference de Nys, Leya, Maximilien, Afsar, Nair and Steinberg1996; König and Wright, Reference König and Wright1997; Steinberg et al. Reference Steinberg, De Ny and Kjelleberg1998; Da Gama et al. Reference Da Gama, Pereira, Soares, Teixeira and Yoneshigue-Valentin2003) and pharmacological roles (Vairappana et al. Reference Vairappan, Suzuki, Abe and Masuda2001; Bansemira et al. Reference Bansemira, Justa, Michalikb, Lindequista and Lalk2004).
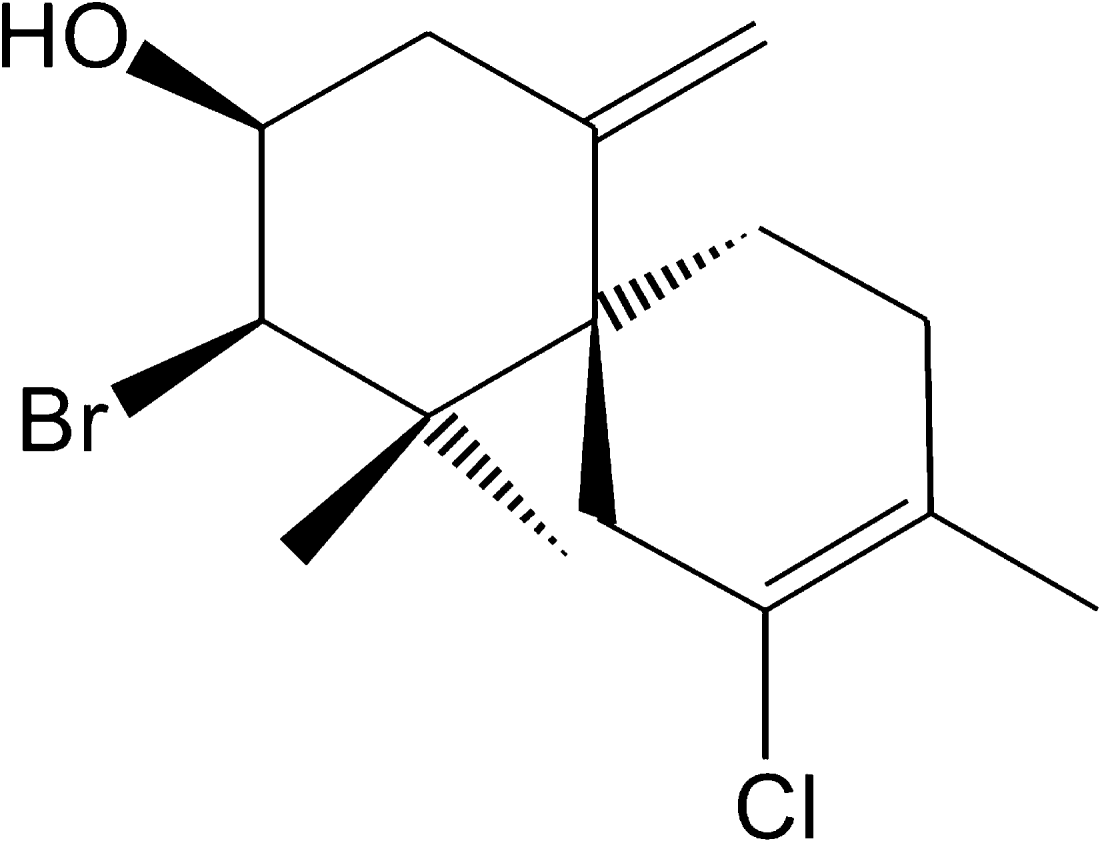
Fig. 1. Chemical structure of elatol, the major secondary metabolite isolated from Laurencia dendroidea.
Table 1. 1H NMR (CDCl3, 299·9 MHz) and 13C NMR (CDCl3, 75·0 MHz) data for elatol

a nH, Number of hydrogens; m, multiplicity; J (Hz), coupling constant; n.o., not observed.
Anti-trypanosomal activity of elatol
Elatol was initially tested in vitro against epimastigote forms of T. cruzi Y strain. Figure 2 A shows that elatol had a dose-dependent activity after 96 h of treatment, exhibiting an IC50 of 45·4±1·9 μm and IC90 of 138·8±3·0 μm. At 300·0 μm of the compound (the highest concentration tested), the parasites were completely arrested. The IC50 of the reference drug benznidazole was 7·8±1·4 μm. Figure 3A shows the activity of elatol on trypomastigote forms of the parasite. After 24 h of treatment, the effective concentration (IC50) was 1·38±0·15 μm. The IC50 of the reference drug crystal violet was 12·8±2·6 μm. Elatol showed better activity against trypomastigotes than did the reference drug. We also investigated the activity of elatol against intracellular amastigotes during 96 h of incubation (Fig. 4A). The elatol showed a strong effect against intracellular amastigotes, with an IC50 and IC90 of 1·01±0·65 μm and 3·0±1·8 μm, respectively. The survival indices were calculated as 427·5 for 1·5 μm, 186·5 for 3·0 μm, 98·0 for 6·0 μm, and 35·5 for 12·0 μm, while the control was determined as 1137·7. The EC50 value for benznidazole, the reference drug, was 24·3±1·4 μm. Therefore, elatol showed better activity against intracellular amastigotes than did the reference drug. All results were significant at P⩽0·05 as compared to the control group, by Student's t-test. Direct observation of treated parasites by light microscopy showed a dose-dependent effect of elatol on intracellular amastigotes until the infection was completely eliminated (Fig. 4 B, C, D).

Fig. 2. Effects of elatol against the epimastigote form of Trypanosoma cruzi Y strain. (A) Activity of the compound on growth of Trypanosoma cruzi. The protozoa were cultured for 96 h in the presence of concentrations of 3·0, 15·0, 30·0, 150·0, and 300·0 μm, and an untreated control. Each experiment was conducted in duplicate and repeated at least 3 times. The results were analysed as percentages of growth inhibition in relation to untreated parasites. Bars represent standard errors. All results were significant at P⩽0·05 as compared to the control group, by Student's t-test; morphological alterations were observed by s.e.m. (B) Control; (C) Parasites treated with IC50 and (D) Parasites treated with IC90 for 96 h. Scale bars=1 μm.
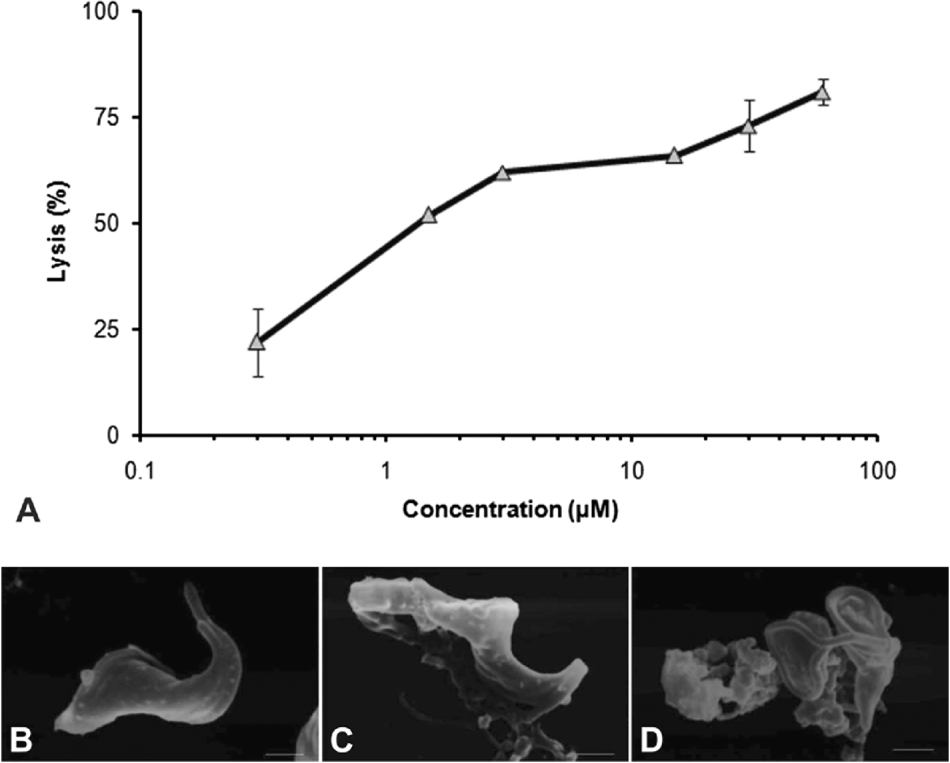
Fig. 3. Activity of elatol on trypomastigotes, the infective form of Trypanosoma cruzi. (A) The parasites (107 cells/ml) were re-suspended in the absence or presence of different concentrations of the compound (0·3, 1·5, 3·0, 15·0, 30·0, and 60·0 μm). Each experiment was conducted in duplicate and repeated at least 3 times. All results were significant at P⩽0·05 as compared to the control group, by Student's t-test; morphological alterations were observed by s.e.m. (B) Control, (C) parasites treated with IC50 and (D) parasites treated with IC90 for 96 h. Scale bars=1 μm.

Fig. 4. Effect of elatol on the Trypanosoma cruzi – LLCMK2 cell interaction. (A) LLCMK2 cells were infected with the trypomastigote form and then treated with elatol. The survival indices were determined by the equation: number of infected cells×mean of amastigotes per cell. The data represent the mean values from 3 independent experiments. All results were significant at P⩽0·05 as compared to the control group, by Student's t-test. (B) Light microscopy of T. cruzi-infected LLCMK2 cell line after 5 days, untreated. The cells were Giemsa-stained and examined under a light microscope at 40x magnification. Cells with intracellular amastigotes (arrow-heads). (C) Cells treated with 1·0 μm of elatol; (D) cells treated with 3·0 μm of elatol. Scale bars=20 μm.
Cytotoxicity and haemolytic assay
In Table 2, the cytotoxicity of elatol from L. dendroidea on LLCMK2 cells was compared with the activity against trypomastigote and intracellular amastigote forms of T. cruzi. Elatol caused no cytotoxic effect against the cell line after 96 h of treatment with concentrations up to 27·0±0·51 μm. The compound was more selective (about 20·0 times) for trypomastigotes than for LLCMK2 cells. The results for intracellular amastigote forms showed that elatol is 26·7 times more selective against the parasites than the mammalian cells. In the haemolytic assay, we evaluated the toxicity of elatol to human red blood cells (Fig. 5). The treatment with elatol did not affect red blood cell integrity at concentrations that inhibit the growth of forms of T. cruzi. At 60·0±0·44 μm (the highest concentration tested), elatol caused only 21% haemolysis. In contrast, cells treated with Amphotericin B (AMPB) showed 75% haemolysis with this concentration. We also observed that the red blood cell control with or without 1·0% DMSO did not show haemolysis, whereas the Triton X-100 positive control showed 100% haemolysis.
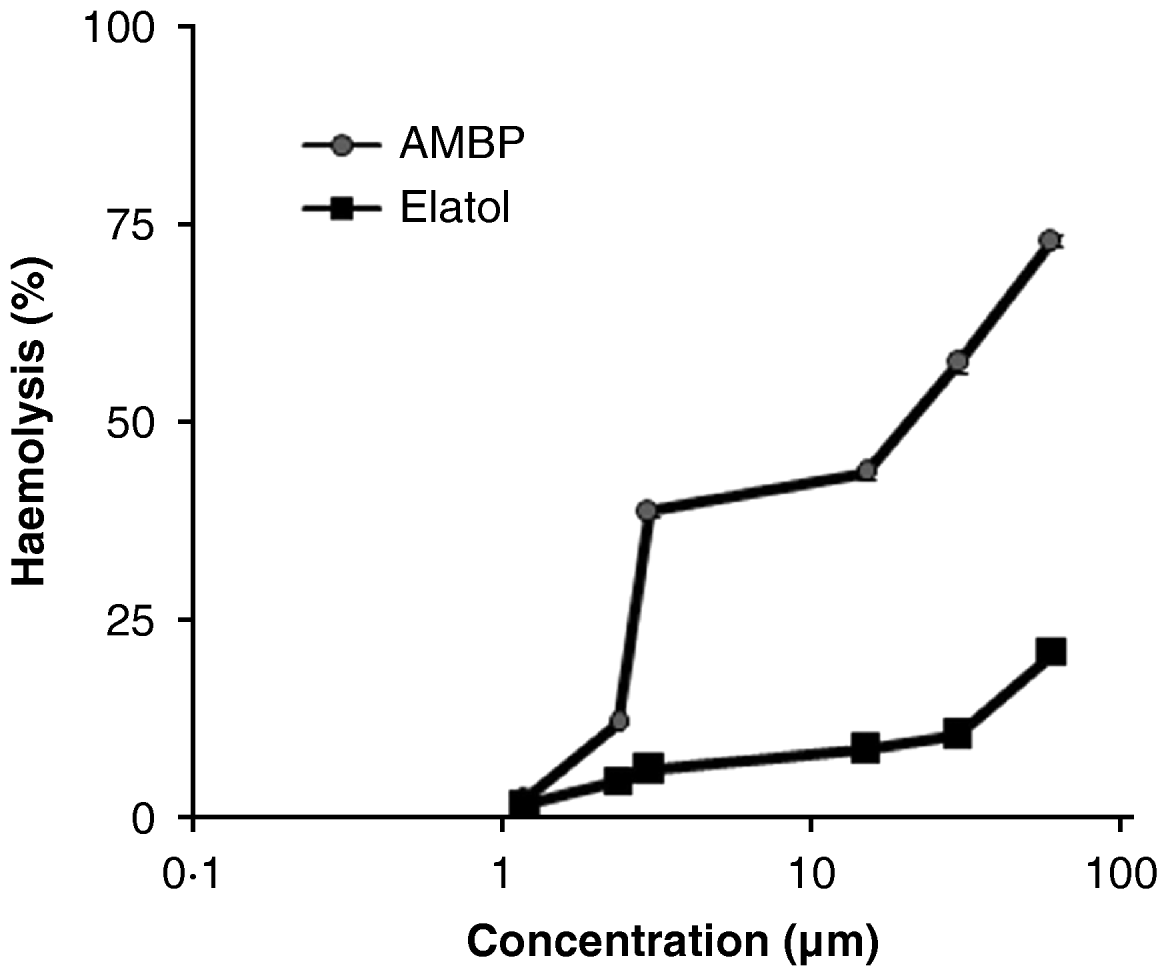
Fig. 5. Haemolytic properties of elatol obtained from Laurencia dendroidea. Amphotericin B (AMPB) was included in the assay as a reference drug. The data represent the mean values from 3 independent experiments. All the assays were carried out in duplicate.
Table 2. Comparison of values of CC50 for LLCMK2 cells with IC50 trypomastigote and intracellular amastigote forms of T. cruzi, and their respective selectivity indices (SI)

* SI, CC50 LLCMK2 cells/EC50 of T. cruzi forms.
Effect of elatol on the morphology and ultrastructure of T. cruzi
Figure 2 C and D and Fig. 3 C and D show morphological alterations in epimastigotes and trypomastigotes, respectively, treated with concentrations corresponding to the IC50 and IC90 value of elatol for each form. Untreated control epimastigotes (Fig. 2 B) and trypomastigotes (Fig. 3 B) showed the typical elongated shape. However, when the parasites were treated with elatol, we observed notable morphological changes, such as the appearance of aberrant-shaped cells. Ultrastructural changes in the 3 forms of T. cruzi treated with elatol are illustrated in Fig. 6. Untreated epimastigotes, trypomastigotes, and intracellular amastigotes showed no plasma membrane alterations and organelles with normal morphology (Fig. 6 A, E and I). Similar ultrastructural alterations were observed in all forms of the parasite treated with elatol. The most prominent effects observed in treated parasites were swollen mitochondria (Fig. 6 B-D, J-L), and extensive formation of cytoplasmic vacuoles in all the treated cells.

Fig. 6. Elatol caused marked ultrastructural changes in Trypanosoma cruzi epimastigote (A–D), trypomastigote (E–H), and intracellular amastigote forms (I–L); TEM images of (A) epimastigote control, kinetoplast (k), mitochondrion (m), and nucleus (n); (B–D) cell treated with IC50; (E) trypomastigote control, kinetoplast (k) and flagellum (f); (F–H) cells treated with IC50; (I) amastigote control, kinetoplast (k), mitochondrion (m), nucleus (n), Golgi complex (g), acidocalcisome (a), and flagellum; (J–L) cells treated with IC50. Scale bar=1 μm.
DISCUSSION
The undesirable side-effects associated with classical trypanocidal drugs, as well as the development of resistance, are encouraging research for alternative synthetic (Tonin et al. Reference Tonin, Barbosa, Bocca, Ramos, Nakamura, Costa, Basso, Ueda-Nakamura and Sarragiotto2009; Valdez et al. Reference Valdez, Tonin, Ueda-Nakamura, Dias-Filho, Morgado-Díaz, Sarragiotto and Nakamura2009) or natural (Luize et al. Reference Luize, Tiuman, Morello, Maza, Ueda-Nakamura, Dias-Filho, Cortez, Mello and Nakamura2005, Reference Luize, Ueda-Nakamura, Dias-Filho, Cortez and Nakamura2006; Izumi et al. Reference Izumi, Morello, Ueda-Nakamura, Yamada-Ogatta, Dias-Filho, Cortez, Ferreira, Morgado-Días and Nakamura2008; Moreira et al. Reference Moreira, Leite, Santos and Soares2009) compounds that are effective for the treatment of Chagas' disease. In this investigation, we demonstrated that the sesquiterpene elatol, the major constituent of the Brazilian red seaweed L. dendroidea (Hudson) J. V. Lamouroux, showed important activity against epimastigote, trypomastigote, and amastigote forms of T. cruzi. Our data showed that elatol had a dose-dependent activity against the epimastigote form after 96 h of treatment, exhibiting an IC50 of 45·4±1·9 μm. Moreover, elatol was effective in killing trypomastigotes with a concentration of 1·38±0·15 μm (IC50), and intracellular amastigotes with an IC50 value of 1·01±0·65 μm. The effect of the reference drug for the trypomastigote form, crystal violet, showed an IC50 of 12·8±2·6 μm. In addition, the IC50 value of the reference drug used for intracellular amastigotes, benznidazole, was 24·3 μm. Therefore, elatol showed better activity against trypomastigote and intracellular amastigote forms than did the reference drug. These results are especially interesting because trypomastigotes and intracellular amastigotes are the forms that are present in the vertebrate host, and pose a challenge for treatment of Chagas' disease. A previous report described significant inhibitory action of 2 other marine algae, Fucus evanescens and Pelvetis babingtonii, on the infection rate and the amastigote growth of T. cruzi in HeLa cells, with a weak inhibitory effect on epimastigotes (Nara et al. Reference Nara, Kamei, Akiko, Annoura, Hirota, Iizumi, Dohmoto, Ono and Aoki2005).
The search for bioactive compounds originating from the sea is recent. The red alga Laurencia microcladia has been reported to have properties against Plasmodium falciparum (Mendiola-Martínez et al. Reference Mendiola-Martínez, Hernández, Acuña, Esquivel, Scull, Lizama and Abreu-Payrol2005). In addition, the sesquiterpenes ((8R)-8-bromo-10-epi-beta-snyderol) and aromatic compounds (p-hydroxybenzaldehyde and p-methoxybenzyl) isolated from Laurencia sp. show anti-malarial activity (Wright et al. Reference Wright, König, Angerhofer, Greenidge, Linden and Desqueyroux-Faundez1996; Topcu et al. Reference Topcu, Anydoqmus, Imre, Goren, Pezzuto, Clement and Kingston2003). Studies with members of the Phaeophyta demonstrated anti-trichomonal activity and activity against Trypanosoma brucei rhodesiense and Leishmania donovani (Orhan et al. Reference Orhan, Sener, Atici, Brun, Perozzo and Tasdemir2006).
An important criterion in the search for compounds active against T. cruzi with therapeutic potential is that they are not toxic to the mammalian host cells. Elatol showed promising parasite inhibition at dosages that did not show cytotoxicity to mammalian LLCMK2 cells, and this resulted in a good selective index against the forms of the parasite that are present in the vertebrate host. Additionally, elatol showed lower haemolytic activity.
Observation by SEM of elatol-treated epimastigotes revealed swelling of the parasite body and shortening of the flagellum, when compared to control cells. Elatol-treated trypomastigotes showed distortion in the cell body and loss of integrity of the membrane. Several other studies have also demonstrated ultrastructural alterations in T. cruzi treated with synthetic or natural compounds (Salas et al. Reference Salas, Tapia, Ciudad, Armstrong, Orellana, Kemmerling, Ferreira, Maya and Morello2008; Valdez et al. Reference Valdez, Tonin, Ueda-Nakamura, Dias-Filho, Morgado-Díaz, Sarragiotto and Nakamura2009). Transmission electron microscopy indicated that epimastigotes treated with elatol showed intensely swollen mitochondria and the matrix became less electron dense, containing myelin-like figures, and damage to the plasma membrane also occurred. The treatment of trypomastigotes and intracellular amastigotes caused mitochondrial swelling and the formation of small vesicles within organelles, especially in the mitochondrion. Mitochondria of trypanosomatid parasites exhibit unique structural and functional features which are remarkably different from mammalian mitochondria, making this organelle an exceptionally attractive chemotherapeutic target (Menna-Barreto et al. Reference Menna-Barreto, Gonçalves, Costa, Silva, Pinto, Oliveira and de Castro2009). In fact, through the years, several trypanocidal compounds have been designed that target parasite mitochondrial function. Mitochondrial disorganization and dysfunction have been described after treatment with different drugs for T. cruzi (Van-Hellemond et al. Reference Van-Hellemond, Opperdoes and Tielens2005; Luize et al. Reference Luize, Ueda-Nakamura, Dias-Filho, Cortez and Nakamura2006; Menezes et al. Reference Menezes, Valentim, Oliveira and Vannier-Santos2006; Menna-Barreto et al. Reference Menna-Barreto, Corrêa, Pinto, Soares and Castro2007, Reference Menna-Barreto, Gonçalves, Costa, Silva, Pinto, Oliveira and de Castro2009).
This is the first report of the in vitro anti-trypanosomal effect of the sesquiterpene elatol. Although the mode of action likely includes a specific metabolic pathway of the parasites, it still remains to be elucidated, which will be the subject of our further studies as well as in vivo studies. Molecular identification and characterization of enzymes and metabolic pathways that are essential and distinct in T. cruzi show the greatest potential as primary targets for screening bio-resources in vitro, in the search for a new generation of chemotherapies.
ACKNOWLEDGEMENTS
This study was supported through grants from DECIT/SCTIE/MS and MCT by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Financiadora de Estudos e Projetos (FINEP), Programa de Núcleos de Excelência (PRONEX/Fundação Araucária), and Programa de Pós-graduação em Ciências Farmacêuticas da Universidade Estadual de Maringá.










