Introduction
The larynx cancer is the second commonest site of primary malignant tumours in the head and neck region: 500 000 new cases of laryngeal squamous cell carcinoma (SCC) are diagnosed globally each year, 75 per cent of which are confined to the glottis.Reference Rucci, Romagnoli and Scala 1 – Reference Marcotullio, de Vincentiis, Iannella, Bigelli and Magliulo 3 Owing to the numerous intricate functions of the larynx, including phonation, deglutition and respiration, these cancers have a marked impact on quality of life (QoL).Reference Gallo, de Vincentiis, Manciocco, Simonelli, Fiorella and Shah 4 Fortunately, these lesions are often detected at an early stage owing to clear symptoms, primarily hoarseness.Reference Marcotullio, de Vincentiis, Iannella, Bigelli and Magliulo 3 Patients are predominantly men in their sixth decade of life, often with a high exposure to cigarette smoking. These malignancies often have a favourable outcome due to early diagnosis and a low risk of metastasis because of the low density local lymphatic network.
Since transoral laser microsurgery was first described for early glottic cancer (T1–2) by Strong and Jako in 1972,Reference Sigston, de Mones, Babin, Hans, Hartl and Clement 5 , Reference Lee, Buchanan, Riffat and Palme 6 its popularity has grown owing to the increasing number of experienced surgeons and published reports of its efficacy. As a consequence of the recent shift in focus towards functional preservation, transoral laser microsurgery is often a primary treatment for these malignancies. There is now a strong body of evidence supporting similar oncological outcomes between transoral laser microsurgery and radiotherapy (RT) for managing early laryngeal cancer,Reference Spielmann, Majumdar and Morton 7 , Reference To, Qureishi, Mortimore and De 8 with some studies reporting greater local control, laryngeal preservation and overall survival rates with transoral laser microsurgery.Reference Huang, Yu, Fang, Chen, Chen and Huang 9 – Reference Roh, Kim and Park 11 Although controversy remains about voice outcomes,Reference Cohen, Garrett, Dupont, Ossoff and Courey 12 these interventions are now generally believed to yield equivalent results.Reference Marcotullio, de Vincentiis, Iannella, Bigelli and Magliulo 3 , Reference Markou, Nikolaou, Nalbadian, Petridis, Nicolaidis and Daniilidis 13
However, patients with tumour–node–metastasis (TNM) stage T1b glottic cancer represent a particularly challenging cohort. The TNM classification describes these glottic tumours as limited to the vocal folds and involving both vocal folds without affecting mobility.Reference Marcotullio, de Vincentiis, Iannella, Bigelli and Magliulo 3 Outcomes are worse for T1b stage vocal fold carcinomas that deeply penetrate the anterior commissure or Broyle's ligament.Reference Gallo, de Vincentiis, Manciocco, Simonelli, Fiorella and Shah 4 , Reference Taylor, Kerr, Fung, Aneeshkumar, Wilke and Jiang 14 The present study investigated oncological and voice outcomes in a cohort of T1b glottic cancer patients.
Materials and methods
From January 2002 to June 2014, patients undergoing transoral laser microsurgery for TNM stage T1bN0M0 SCC of the glottis were prospectively enrolled into the study. Tumours were staged using flexible or rigid endoscopy and all patients underwent pre-operative computed tomography imaging. Only patients with biopsy-confirmed diagnoses were enrolled. Exclusion criteria were recurrent tumours, tumours not classified as T1b and any previous intervention. Prior to laser treatment, all cases were discussed by a multidisciplinary head and neck oncology team to determine the appropriate management. All transoral laser microsurgery procedures were performed by a single surgeon (SMT) within the Division of Otolaryngology Head and Neck Surgery at the Queen Elizabeth II Health Sciences Centre, Halifax.
Approval for data collection was provided by the local research ethics board prior to study commencement. Oncological and functional outcome data were collected prospectively during routine follow up. Post-operative voice changes were quantified using the Voice Handicap Index-10 scale (Table I) and maximum phonation time. The latter was measured by instructing the patient to produce the ‘a’ vowel sound on a full breath of air until phonation could no longer be sustained, with the longest of three attempts being recorded. Pre-operative scores were collected during appointments at which patients were scheduled for surgery. Post-operative scores were collected prospectively during routine follow-up consultations at approximately three months, one year and two years. A schematic diagram of the study method is shown in Figure 1.

Fig. 1 Flow chart showing the study methods. MPT = maximum phonation time; TLM = transoral laser microsurgery; VHI-10 = Voice Handicap Index-10
Table I Voice handicap index-10 scoring sheet

No = number; 0 = never; 1 = almost never; 2 = sometimes; 3 = almost always; 4 = always.
The oncological parameters evaluated were the five-year local control rate, overall survival, disease-specific survival and a laryngeal preservation rate conforming to the recommendations of the Larynx Preservation Consensus Panel.Reference Lefebvre and Ang 15 A Kaplan–Meier five-year survival analysis with censoring was performed for each variable. Absence of local control was defined as local recurrence or tumour reappearance within five years of laser treatment on the same vocal fold or on the contralateral fold with anterior commissure involvement. Thus, the definition did not include secondary glottic primary tumours defined as local recurrence at more than five years after treatment or tumours contralateral to the original cancer that did not cross the midline or involve the anterior commissure. Analysis of disease-specific survival included data on death related to laryngeal cancer or its treatment.
Patient demographics, Voice Handicap Index-10 scores, morbidities and subsequent treatment interventions were recorded for descriptive analysis. All statistical analyses were performed using IBM SPSS Statistics software version 22.0 (Armonk, New York, USA) and an intention to treat analysis was performed. Local control and overall survival data were analysed by smoking status (based on a telephone enquiry). Patients who continued to smoke were designated positive. Fisher's exact test was used to assess nominal variables and Student's t-test was used for continuous, normally distributed variables.
Results
A total of 21 male patients (12 smokers and 9 non-smokers) with an average age of 66.8 years (range 51–79 years) were enrolled. The mean follow up of 56.5 months (8–102 months; demographic information is shown in Table II). According to the European Laryngological Society classification of endoscopic cordectomy, 1 of the 21 initial transoral laser microsurgery cordectomy resections was type I, 9 were type II and 11 were type III. Four of the six recurrent tumours were treated by type II cordectomy resection, one was treated by type III resection and one was treated by type VI resection. No patients required tracheostomy or a gastrostomy tube.
Table II Demographic information

Y = years; M = male; F = female; mon = months
One patient (4.8 per cent) suffered respiratory distress requiring reintubation as a complication of transoral laser microsurgery. The patient was extubated after 24 hours in intensive care and had no further complications. Five patients (24 per cent) developed an anterior glottic web during follow up, and one patient had a second primary tumour that was successfully treated with salvage transoral laser microsurgery.
One patient had positive surgical margins following transoral laser microsurgery to treat a second primary tumour; the tumour was successfully re-resected, with negative surgical margins. Ultimately, the patient developed recalcitrant disease in the subglottis and underwent intensity modulated RT. He was disease free at the most recent follow-up examination.
During the follow-up period, six patients had recurrent tumours (two were primary recurrences, three were second primary recurrences and one was a regional recurrence): all underwent a second transoral laser microsurgery procedure. Of the two primary recurrent tumours, one was successfully managed and the other recurred again and was treated with salvage transoral laser microsurgery and external beam RT. Two patients with second primary tumours had second recurrences, which were treated with salvage external beam RT and intensity modulated RT. The patients with regional recurrence with neck nodal metastasis underwent salvage transoral laser microsurgery, chemoradiotherapy and selective neck dissection. Kaplan–Meier survival analysis revealed a five-year local control rate of 82 per cent (Figure 2). When further divided by smoking status, the local control rate was 75 per cent for smokers and 89 per cent for non-smokers (Figure 3). Likewise, the five-year overall survival rate (including all causes of death) was 88 per cent (Figure 4). Further, the overall survival by smoking status was 90 per cent for smokers and 83 per cent for non-smokers (Figure 5). Both the laryngeal preservation and disease-specific survival rates were 100 per cent (data not shown).

Fig. 2 Kaplan–Meier survival analysis showing the overall five-year local control rate.

Fig. 3 Kaplan–Meier survival analysis showing the five-year local control rate stratified by smoking status.

Fig. 4 Kaplan–Meier survival analysis showing the overall survival rate.

Fig. 5 Kaplan–Meier survival analysis showing the overall survival rate stratified by smoking status.
Overall, four patients (19 per cent) underwent successful salvage RT or chemoradiotherapy after the initial transoral laser microsurgery. In total, 15 patients (71 per cent) were successfully managed with a single transoral laser microsurgery procedure, and 17 patients (81 per cent) had their laryngeal cancer managed solely with transoral laser microsurgery.
Voice Handicap Index-10 scores and maximum phonation times were available for 10 and 13 patients, respectively. Prior to laser treatment, the mean values for these measures were 19.1 (range 7–37) and 16.23 seconds (range 7–24 seconds), respectively. Two years following the initial transoral laser microsurgery, the mean values fell to 5.8 (range 1–13) and 14.33 (range 9–26), respectively (Table III; voice outcomes are shown in Figure 6).
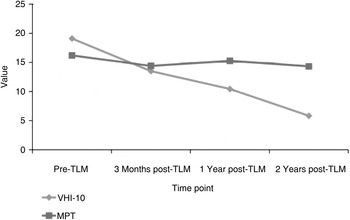
Fig. 6 Graph showing Voice Handicap Index-10 scores and maximum phonation times in seconds at different time points. TLM = transoral laser microsurgery; VHI-10 = Voice Handicap Index-10; MPT = maximum phonation time
Table III Voice handicap index-10 scores and maximum phonation times

TLM = transoral laser microsurgery; SD = standard deviation; VHI-10 = Voice Handicap Index-10; MPT = maximum phonation time; s = seconds
Discussion
Transoral laser microsurgery has several advantages compared with RT in managing early glottic cancer. Radiotherapy may cause higher morbidity due to radiation-induced mucosal oedema which, in addition to causing different tumour growth patterns and fibrosis, can delay the detection of persistent or recurrent tumours.Reference Khan, Koyfman, Hunter, Reddy and Saxton 16 – Reference Han, Lee, Kim, Hong, Kim and Park 19 Chronic laryngeal oedema is reported to occur in 18 per cent of early glottic cancer patients treated with RT, possibly resulting in chronic dysphagia, chondronecrosis, tracheostomy and more patients needing total laryngectomy.Reference Taylor, Kerr, Fung, Aneeshkumar, Wilke and Jiang 14 , Reference Remmelts, Hoebers, Klop, Balm, Hamming-Vrieze and van den Brekel 17 , Reference Steiner, Ambrosch, Rodel and Kron 18 However, an advantage of RT is that it does not require general anaesthesia.
Transoral laser microsurgery has disadvantages such as requiring general anaesthesia, possible unclear tumour margins caused by specimen shrinking, difficult access to the anterior commissure and possible worsened voice outcomes after anterior commissure resection.Reference Taylor, Kerr, Fung, Aneeshkumar, Wilke and Jiang 14 , Reference Peretti, Piazza, Bolzoni, Mensi, Rossini and Parrinello 20 , Reference Rioja, Blanch, Bores, Bernal-Sprekelsen and Vilaseca 21 According to Lee and colleagues, general anaesthesia caused complications after carbon dioxide laser surgery in 1 out of 270 early glottic cancer patients.Reference Lee, Buchanan, Riffat and Palme 6 Despite these limitations, transoral laser microsurgery provides greater precision and control for resection, shorter hospitalisation, minimal bleeding and absence of post-operative oedema.Reference Gallo, de Vincentiis, Manciocco, Simonelli, Fiorella and Shah 4 , Reference Vilaseca, Huerta, Blanch, Fernandez-Planas, Jimenez and Bernal-Sprekelsen 22 Furthermore, it is highly cost-effective: a single treatment provides both a biopsy to confirm diagnosis and an endoscopic view to confirm staging, both of which are essential for determining the appropriate management. Most importantly, laser treatment does not exclude subsequent treatment options, which is particularly useful for populations with increasing life expectancy and cancers prone to late recurrence.Reference Steiner, Ambrosch, Rodel and Kron 18 , Reference Vilaseca, Huerta, Blanch, Fernandez-Planas, Jimenez and Bernal-Sprekelsen 22 , Reference Hartl, Laoufi and Brasnu 23
All patients in the present study were men, consistent with previous reports that 97 per cent of laryngeal cancer patients are male.Reference Marcotullio, de Vincentiis, Iannella, Bigelli and Magliulo 3 , Reference Markou, Nikolaou, Nalbadian, Petridis, Nicolaidis and Daniilidis 24 Recurrence rates for T1b stage glottic cancers vary widely from 16 to 50 per cent.Reference Roh, Kim and Park 11 , Reference Markou, Nikolaou, Nalbadian, Petridis, Nicolaidis and Daniilidis 13 , Reference Lee, Chun, Kim, Kim, Oh and Hong 25 The success of laser treatment is attributable to the specific set of parameters in study participants, with anterior commissure involvement, oncological margins and endoscopic accessibility all having a larger impact on smaller samples.Reference Steiner, Ambrosch, Rodel and Kron 18 Recurrence rates in the present study fall within the reported limits.
Laser resection involving the anterior commissure may result in scarring, synechiae or anterior glottic webs.Reference Lee, Buchanan, Riffat and Palme 6 , Reference Roh, Kim and Park 11 Anterior glottic webs have been reported in approximately 20 per cent of patients, with recurrence rates of nearly 50 per cent reported in these patients.Reference Lee, Buchanan, Riffat and Palme 6 , Reference Taylor, Kerr, Fung, Aneeshkumar, Wilke and Jiang 14 This is understood to be due to Broyle's ligament, which initially acts as an anatomical barrier to obstruct the spread of cancer, but after invasion becomes a conduit for supraglottic or subglottic extension.Reference Gallo, de Vincentiis, Manciocco, Simonelli, Fiorella and Shah 4 , Reference Lee, Buchanan, Riffat and Palme 6 , Reference Steiner, Ambrosch, Rodel and Kron 18 This route of possible oncological expansion, in conjunction with difficult endoscopic access hindering comprehensive excision, means that an increased depth of involvement leads to an increasing likelihood of perichondrium perforation.Reference Lee, Buchanan, Riffat and Palme 6 Previous studies have thus shown greater rates of recurrence, lower rates of local control and worse voice outcomes in these patients.Reference Taylor, Kerr, Fung, Aneeshkumar, Wilke and Jiang 14 , Reference Steiner, Ambrosch, Rodel and Kron 18 , Reference Hoffmann, Cornu, Hans, Sadoughi, Badoual and Brasnu 26 , Reference Charbonnier, Thisse, Sleghem, Mouawad, Chevalier and Page 27 This trend was not seen in the present cohort, as only one out of five patients with anterior glottic webs developed recurrence.
While local control rates for early glottic cancer range from 80 to 100 per cent, significant variations in the post-operative local control rate for T1b stage malignancies have been reported: from 95 per cent after two years, 91 per cent after three years and 83 per cent after five years.Reference Gallo, de Vincentiis, Manciocco, Simonelli, Fiorella and Shah 4 , Reference Taylor, Kerr, Fung, Aneeshkumar, Wilke and Jiang 14 , Reference Khan, Koyfman, Hunter, Reddy and Saxton 16 , Reference Steiner, Ambrosch, Rodel and Kron 18 , Reference Lee, Chun, Kim, Kim, Oh and Hong 25 The present study recorded the expected local control rate. The greater local control rate seen in non-smokers relative to smokers may represent the established influence of cigarette smoking on glottic cancer recurrence, but this difference did not reach statistical significance (p = 0.66, log rank = 0.19). Similarly, the five-year overall survival rates were 92 per cent for early glottic cancer patients and 87–93 per cent for T1b stage patients.Reference Marcotullio, de Vincentiis, Iannella, Bigelli and Magliulo 3 , Reference Lee, Buchanan, Riffat and Palme 6 , Reference Lee, Chun, Kim, Kim, Oh and Hong 25 Hence, the present study centre provides the expected standard of successful transoral laser microsurgery treatment. The higher overall survival rate in patients with positive smoking status post-laser treatment may be attributed to sampling error or to a more extensive clinical history of smoking in the non-smoking group prior to transoral laser microsurgery. Regardless of the cause, this finding did not reach statistical significance (p = 0.24, log rank = 1.41).
Current reports, including a meta-analysis by Abdurehim et al. which identifies laryngeal preservation as the sole oncological parameter associated with more favourable outcomes for transoral laser microsurgery over RT, support the finding of a high laryngeal preservation rate in early glottic cancer patients in the current study.Reference To, Qureishi, Mortimore and De 8 , Reference Fink, Sibley, Kunduk, Schexnaildre, Kakade and Sutton 10 , Reference Abdurehim, Hua, Yasin, Xukurhan, Imam and Yugin 28 , Reference Kim, Ahn, Nam, Chung, Jeong and Son 29 More specifically, the present findings match the five-year anterior commissure involvement rates for early glottic cancer and T1b of approximately 96 per cent and 88–100 per cent, respectively.Reference Lee, Buchanan, Riffat and Palme 6 , Reference Steiner, Ambrosch, Rodel and Kron 18 Likewise, a retrospective analysis by Lee et al. of transoral laser microsurgery as a one-stage modality for early glottic cancer found a 91 per cent laryngeal preservation rate in 19 T1b participants.Reference Lee, Chun, Kim, Kim, Oh and Hong 25 Lastly, the reported five-year disease-specific survival rates for early glottic cancer patients of 97–99 per cent are highly comparable with the present findings.Reference Remmelts, Hoebers, Klop, Balm, Hamming-Vrieze and van den Brekel 17 , Reference Peretti, Piazza, Bolzoni, Mensi, Rossini and Parrinello 20 , Reference Mortuaire, Francois, Wiel and Chevalier 30 Given the mean patient age of 66.8 years, it is possible that many patients in the present study died from co-morbidities or a natural death before they could develop a late recurrence.
The Voice Handicap Index-10 is a validated questionnaire for assessing emotional and physical indications during post-laser recovery that captures particular aspects of QoL impairment such as telephone use.Reference Remmelts, Hoebers, Klop, Balm, Hamming-Vrieze and van den Brekel 17 , Reference Makeieff, de la Breteque, Guerrier and Giovanni 31 The present results are highly comparable to those of a multicentre study by Taylor et al. comparing transoral laser microsurgery and RT in T1b SCC patients.Reference Taylor, Kerr, Fung, Aneeshkumar, Wilke and Jiang 14 These authors reported a median Voice Handicap Index-10 score of 6 (range 0–11) at two years post-laser surgery. However, Remmelts et al. found that 8 T1b stage tumour patients had an average score of 16.7 in the Voice Handicap Index-10 questionnaire at a median of 51 months post-laser surgery.Reference Remmelts, Hoebers, Klop, Balm, Hamming-Vrieze and van den Brekel 17 Although the present study plotted data only until two years after transoral laser microsurgery, the evident plateau of the curve indicates that large fluctuations in scores are unlikely. Furthermore, Remmelts and colleagues’ participants may have been exposed to more extensive resection because there is a greater tendency towards permanent dysphonia with type III–IV cordectomies.Reference Remmelts, Hoebers, Klop, Balm, Hamming-Vrieze and van den Brekel 17 A study by Vilaseca and colleagues supports this possibility: voice quality correlated with the resection extent in T1 stage tumour patients as excision of more of the vocal muscle or anterior commissure led to persistent dysphonia in a third of patients.Reference Vilaseca, Huerta, Blanch, Fernandez-Planas, Jimenez and Bernal-Sprekelsen 22 Alternatively, as poorer QoL scales and higher Voice Handicap Index-10 scores have been associated with younger ages, it is possible that older patients in the present study (mean age 66.8 years) may have given higher scores on the subjective questionnaire.Reference Vilaseca, Huerta, Blanch, Fernandez-Planas, Jimenez and Bernal-Sprekelsen 22 , Reference Hartl, Laoufi and Brasnu 23
Previous studies assessing aerodynamic measures after laser treatment reported that the maximum phonation time decreases without returning to baseline despite near-normal subjective voice recovery.Reference Hartl, Laoufi and Brasnu 23 Aaltonen et al. explained this outcome as oval or incomplete glottic closure detected by videostroboscopy at two years after transoral laser microsurgery.Reference Aaltonen, Rautiainen, Sellman, Saarilahti, Mäkitie and Rihkanen 33 As comparable maximum phonation times were obtained pre-operatively and at two years after transoral laser microsurgery in the present study, this change is likely to be clinically insignificant.
A meta-analysis of six studies by Cohen et al. found comparable Voice Handicap Index-10 scores after laser treatment and RT for T1 glottic carcinoma.Reference Cohen, Garrett, Dupont, Ossoff and Courey 34 Likewise, Luo et al. reported no significant difference in Voice Handicap Index-10 evaluation when comparing RT and transoral laser microsurgery.Reference Luo, Fang, Lin, Chang, Liao and Chen 35 The same study found the transoral laser microsurgery group to have better communication scores on the Functional Assessment of Cancer Therapy-Head and Neck survey. Moreover, a study into the overall impact of transoral laser microsurgery using the Washington University QoL questionnaire found minimal impairment in early glottic cancer patients, who scored 1139 out of 1200 on average.Reference Hartl, Laoufi and Brasnu 23 Hence, the utility of transoral laser microsurgery as a primary treatment modality for T1b stage glottic cancers should not be limited by concerns of post-laser voice outcomes or a reduced QoL.
-
• Oncological and voice outcomes were evaluated in 21 T1b stage glottic cancer patients treated with transoral laser microsurgery
-
• At five years, the local control rate was 82 per cent, the overall survival rate was 88 per cent, and the disease-specific survival and laryngeal preservation rates were 100 per cent
-
• The pre-operative Mean Voice Handicap Index-10 score and maximum phonation time were 19.1 and 16.23 seconds, respectively, decreasing to 5.83 and 14.33 seconds, respectively, at two years
-
• Transoral laser microsurgery is a safe, efficacious primary treatment for T1b glottic cancer
Study limitations
The main limitation of the study was the small number of participants who met the inclusion criteria. Furthermore, pre-laser Voice Handicap Index-10 scores and maximum phonation times were not available for all 21 participants (i.e. universal voice outcome data was not available). However, this was mitigated by the lengthy prospective data collection period and Kaplan–Meier survival analysis of all available records. Additionally, the gradually improving trend in voice outcome scores seen in this cohort is consistent with the current literature.
Conclusion
Transoral laser microsurgery for managing T1b glottic cancer in a tertiary care head and neck cancer centre had successful oncological and voice recovery outcomes, consistent with the growing body of reported evidence. As a functional larynx is necessary for communication and breathing, the importance of functional preservation in laryngeal cancer therapy cannot be understated. Thus, transoral laser microsurgery is an efficacious primary treatment modality for these malignancies.











