Historically, there have always been two congenital cardiac defects that strike fear into the hearts of paediatricians. Transposition of the great arteries was highly feared because of its association with early death unless associated with a ventricular septal defect. Similarly, common arterial trunk was a defect to be dreaded because of a poor natural and unnatural history. A baby would face progressive congestive heart failure, and a guarded prognosis after surgery with significant mortality, possible arrhythmias, and multiple conduit replacements over a lifetime.
For transposition of the great arteries, this picture has changed dramatically, with the improvements in the Jatene or arterial switch operation yielding anatomical and functional results in some cases close to a “cure”. For common arterial trunk, however, there is a less rosy picture with persistent death before surgery from haemodynamic instability and surgical mortality especially with an abnormal truncal valve; the problems mentioned above are far from having been eliminated from the clinical course.
As part of our 12th Annual International Symposium on Congenital Heart Disease, held from February 18 to 21, 2012 at All Children's Hospital in Saint Petersburg, Florida, we discussed the foetal recognition, diagnosis, and foetal–perinatal management of transposition of the great arteries and common arterial trunk. From humble beginnings in the late 1970s around the time that surgery for these conditions became available, foetal echocardiography has progressed. Today, a complete diagnosis is possible by 18 weeks’ gestation with 95% confidence. Both conditions present with normal four-chamber views, and an analysis of the great arteries and outflow tracts is necessary for the diagnosis. Today, not only can the anatomical diagnosis be made, but a functional analysis can be achieved to develop predictions of pre- and postnatal prognosis information for the family. Prenatal treatment of congestive heart failure is also possible.
As in all congenital heart disease discovered in utero, an initially optimistic prediction of outcome can be ruined by a preterm delivery for whatever reason. A critical goal in managing these patients in the next decade is the early diagnosis and prevention of preterm labour and the delivery of a fully grown, mature neonate at term.
Transposition of the great arteries
Transposition of the great arteries is a conotruncal abnormality characterised by the presence of concordant atrioventricular and discordant ventriculoarterial connections, with the aorta arising from the right ventricle and the pulmonary artery arising from the left ventricle. Transposition of the great arteries is a common form of cyanotic congenital heart disease and one in which prenatal diagnosis has an important impact on neonatal outcomes.Reference Simpson 1
Prenatal detection
Several studies support the benefits of prenatal diagnosis of transposition of the great arteries by documenting a decrease in postnatal morbidity and mortality.Reference Bonnet, Coltri and Gianfranco 2 Transposition of the great arteries is not detected on prenatal ultrasound when the foetal cardiac evaluation is limited to a four-chamber view. As a result, the detection rate for isolated transposition of the great arteries remains disappointingly low (around 19%).Reference Friedberg, Silverman and Moon-Grady 3 Up to 72.5% of cases of transposition of the great arteries were detected when the screening examination was modified to include visualisation of the outflow tracts.Reference Khoshnood, De Vigan and Vodovar 4 Complex transposition, associated with ventricular septal defect and pulmonic stenosis, has a higher foetal detection rate because discovery of these more easily detectable lesions spurs a more in-depth evaluation of the heart. Efforts are being made to improve prenatal detection of transposition of the great arteries, by increasing education and adding the extended outflow tract view to the four-chamber view during a sonogram when “technically feasible”.
Prenatal diagnosis can greatly alter the postnatal outcomes in foetal transposition, especially associated with restriction of the foramen ovale and/or ductal restriction. Adequate postnatal mixing of the circulations is integral for survival in transposition of the great arteries. Therefore, prenatal identification of these patients allows for appropriate planning for delivery and postnatal management, including available immediate balloon septostomy and prostaglandin infusion. In addition, in-depth prenatal evaluation will differentiate simple transposition from those cases with outflow tract obstruction that require a different surgical approach, such as a Rastelli or Nikaido operation.
Anatomical considerations
In transposition of the great arteries, the pulmonary trunk arises from the morphologic left ventricle and the aorta from the morphologic right ventricle. The pulmonary valve is not “wedged” as deeply between the mitral and tricuspid valve as the aortic valve is in the normal heart, making the offsetting of the leaflets of the atrioventricular valves less marked. In addition, abnormal resorption of the subpulmonary conus and persistence of the subaortic conus leads to pulmonary-mitral fibrous continuity. In the majority of patients, the aortic root is positioned to the right of the pulmonary trunk. However, in some cases the great vessels are side by side with the aortic valve to the right of the pulmonary artery. Rarely, the aorta can be positioned leftward or posterior to the pulmonary artery. The most common associated lesion found in transposition of the great arteries is a ventricular septal defect, present in 40–45% of cases.Reference Allen, Driscoll, Shaddy and Feltes 5 Less commonly seen is a ventricular septal defect with left ventricular outflow tract obstruction, which occurs in 10% of cases. Other associated lesions include valve and valve apparatus abnormalities, aortic obstructive lesions, and anomalies of the coronary arteries.
Foetal echocardiography
Clues for the diagnosis of transposition of the great arteries include:
-
• the presence of parallel great arteries on extended outflow tract views;
-
• the presence of two vessels seen in the three-vessel-trachea view; and
-
• posterior branching of the pulmonary artery arising from the left ventricle and superiorly branching aorta arising from the right ventricle.
As previously mentioned, the four-chamber view of the foetal heart is deceptively normal, except in patients with an associated ventricular septal defect. Of importance is the evaluation of the patency of the foramen ovale, which may be assessed in this view by two-dimensional imaging and colour flow mapping. Colour flow mapping should demonstrate the presence of laminar, bidirectional flow. Several authors have reported that an aneurysmal septum primum, abnormal septum primum angle, and abnormal motility of the septum primum may all be associated with potential restriction of the foramen ovale. A hypermobile septum, in conjunction with reverse diastolic flow in the patent ductus arteriosus, has been associated with the need for urgent balloon atrial septostomy postnatally.Reference Maneo, Kamenir, Sinclair, van der Velde, Smallhorn and Hornberger 6 , Reference Punn and Silverman 7
The outflow tract view is crucial in making the diagnosis of transposition of the great arteries. Typically, this demonstrates the two great vessels running in parallel, with the aorta anterior and to right of the pulmonary artery. In addition, the great vessels fail to cross each other, which is also demonstrated by colour flow mapping (Figs 1 and 2). Colour flow mapping and spectral Doppler evaluation are useful in diagnosing outflow tract obstruction.

Figure 1 Colour Doppler evaluation in a foetus with transposition of the great arteries depicting a parallel course of the great vessels with the aorta (Ao) arising from the anterior right ventricle and the pulmonary artery (PA) arising from the posterior left-sided ventricle. The patent arterial duct (PDA) is also seen.
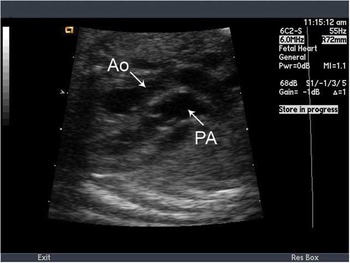
Figure 2 Parallel origin of the great vessels in greyscale. The patent arterial duct is shown to connect the aorta (Ao) to the pulmonary artery (PA).
In the three-vessel-trachea view, only two vessels – the transverse aortic arch and the superior vena cava, located to the right of the aorta – are identified (Fig 3). The pulmonary artery is not readily visible in this view in transposition of the great arteries secondary to its inferior position to the aorta. However, other forms of congenital heart disease also display only two vessels in the three-vessel view, such as common arterial trunk and some forms of double-outlet right ventricle.

Figure 3 The three-vessel-trachea view in greyscale in a foetus with transposition of the great arteries demonstrating the aorta (Ao) as a single large vessel with the superior caval vein (SVC) to the right.
The aortic arch arises anteriorly from the right ventricle as the most cranial arch, and assumes a “hockey-stick” orientation as it curves posteriorly (Fig 4). The pulmonary artery in transposition of the great arteries arises more centrally from the heart.
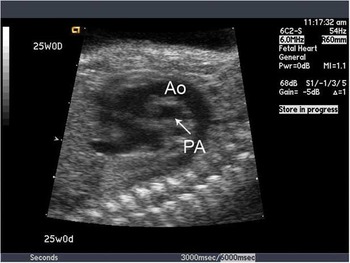
Figure 4 The aortic arch is depicted in greyscale in a foetus with transposition of the great arteries adopting a “hockey-stick configuration” as it curves posteriorly. Ao = aorta; PA = pulmonary artery.
The arterial duct forms the more caudal arch. It is important to evaluate for ductal constriction, as this is associated with a risk of developing pulmonary hypertension postnatally. The diameter of the arterial duct should be measured at the pulmonary end – the narrowest end. The Doppler flow pattern is abnormal when there is continuous, high-velocity antegrade flow or bidirectional flow.
Associated findings: Any type of ventricular septal defect can be seen in association with transposition of the great arteries; about 33% are perimembranous, 30% malalignment type, 25% muscular, and 5% canal type. Anterior malalignment ventricular septal defects may result in right ventricular (systemic) outflow tract obstruction. Posterior malalignment of the infundibular septum is associated with left ventricular outflow tract obstruction, including valvar and subvalvar stenosis, and may also be secondary to redundant tricuspid valve tissue. Anterior malalignment of the outlet septum may lead to subaortic obstruction and is associated with coarctation of the aorta. The coronary anatomy may be difficult to discern prenatally.
Differential diagnosis of transposition of the great arteries
Double-outlet right ventricle and congenitally corrected transposition of the great arteries are the two most common congenital lesions that are misdiagnosed as transposition of the great arteries because the great arteries fail to cross in all three of these lesions. However, in double-outlet right ventricle both great arteries arise from the right ventricle with a lack of aortic-mitral fibrous continuity, and usually accompanied by a ventricular septal defect. In congenitally corrected transposition of the great arteries, the aorta is anterior and leftward to the pulmonary artery and the ventricles are inverted with atrioventricular and ventriculoarterial discordance.
Family counselling
Amniocentesis is not indicated in transposition of the great arteries, as it is rarely associated with an abnormal karyotype. However, the finding of extracardiac anomalies should prompt further analysis of chromosomes. Counselling depends on associated intracardiac anomalies and any evidence of Patent Foramen Ovale/arterial duct restriction prenatally. Any abnormality of the cardiovascular profile score should prompt a search for an extracardiac cause of foetal heart failure.Reference Wieczorek, Hernandez-Robles, Ewing, Leshko, Luther and Huhta 8 Monitoring during foetal life should focus on following the degree of pulmonary stenosis and evaluating the patency of the arterial duct and the foramen ovale. Delivery should be planned in a tertiary care centre where access to procedures such as balloon atrial septostomy and extracorporal mechanical circulation exists.
Common arterial trunk
Common arterial trunk is a congenital cardiac lesion characterised by a single great artery exiting the heart, with branches to the pulmonary, systemic, and coronary circulations. There is typically an associated large, malaligned outlet ventricular septal defect. The spectrum of disease in common arterial trunk is largely related to the anatomy of the branch pulmonary arteries. These variations have been classified into types, and include:
-
• The main pulmonary artery arising from the trunk and then branching into the left and right pulmonary arteries.
-
• Separate left and right branch pulmonary arteries arising from the trunk in close proximity to one another.
-
• Separate left and right branch pulmonary arteries arising from the trunk at a distance from one another.
-
• A single branch pulmonary artery arising from the trunk and the other from a patent arterial ductus or an aortopulmonary collateral vessel.
-
• Any branch pulmonary artery configuration with an associated interrupted aortic arch.
Foetal echocardiography
Skilled foetal echocardiography increases prenatal diagnosis of common arterial trunk and accurately delineates the constellation of defects in that foetal patient.
The four-chamber view of the foetal heart with common arterial trunk can appear deceptively normal, especially when the two ventricles are balanced in size. Common arterial trunk can be missed if the sonographer does not interrogate the great arteries. Tilting the transducer to the five-chamber view will reveal a large, single great vessel that straddles an outlet ventricular septal defect. Occasionally, the great artery will originate more from the right or left ventricle, although this is a less common finding. Further anterior angulation of the transducer confirms that there is only a single great vessel arising from the heart (Figs 5a, b and 6). The leaflets of the truncal valve are typically thickened and may show poor excursion. Doppler and colour Doppler assessment of the valve is essential. Truncal valve Doppler velocities above the normal aortic range have been shown to be predictive of postnatal truncal valve stenosis.Reference Duke, Sharland, Jones and Simpson 9 In neonates with common arterial trunk, one-third will have truncal valve stenosis and half will have truncal valve insufficiency.Reference Lai, Mertens, Cohen and Geva 10 Therefore, this finding in the foetal patient makes the diagnosis of common arterial trunk more likely over pulmonary atresia with ventricular septal defect and tetralogy of Fallot.

Figure 5 ( a ) Foetal echocardiography of common arterial trunk in utero at 26 weeks’ gestation. Note the overriding truncal root (Tr) arising from the right (RV) and left (LV) ventricles. The pulmonary arterial trunk (PA) arises posteriorly and branches to the right and left pulmonary arteries. ( b ) Foetal echocardiography of common arterial trunk in the same foetus showing the origin of the pulmonary arterial trunk from the truncal root (arrow).

Figure 6 Foetal Common Arterial Trunk showing the pulmonary trunk (TP) arising from the ascending truncal root (Ao). LV = left ventricle; RV = right ventricle.
The parasternal long-axis view of the foetal heart is the preferred view to assess the ventricular septal defect and the degree of truncal valve override.
The short-axis view of the heart shows the truncal valve en face. The valve is most often tricuspid, but may be quadracuspid – as seen in 22% of cases – or bicuspid – as seen in 9% of cases.Reference Fuglestad, Puga and Danielson 11 In fact, the number of valve leaflets can range from a single cusp to as many as six.
The three-vessel-trachea view differentiates a left versus right aortic arch. Arch sidedness is diagnosed by following the arch as it passes either to the right or to the left of the trachea. A right aortic arch is seen in 33% of cases of common arterial trunk.Reference Calder, Van Praagh and Van Praagh 12 The three-vessel-trachea view is also important in the assessment of the branch pulmonary arteries in the common arterial trunk. This view reveals a single, large trunk with the main pulmonary artery branching off to the left of the posterior-lateral aspect of the common trunk. The truncal artery and the main pulmonary artery form a “V” that points towards the anterior foetal chest wall. Additional views to look at the branch pulmonary arteries include the four-chamber view, the short-axis view, and the aortic arch view. Both pulmonary arteries should be identified, if possible, as this will confirm the diagnosis and differentiate it from pulmonary atresia with ventricular septal defect and tetralogy of Fallot. It is reported that 16% of patients with common arterial trunk have only a single branch pulmonary artery. Interestingly, the missing branch pulmonary artery is typically on the same side as the aortic arch.Reference Calder, Van Praagh and Van Praagh 12
The aortic arch view will diagnose interruption of the aortic arch, which is seen in 19% of patients with common arterial trunk.Reference Mair, Ritter and Davis 13 Type B interruption, between the left common carotid artery and the left subclavian artery, is the most common type seen. Both the three-vessel-trachea view and the arch view will diagnose the absence of the arterial ductus, which is seen in 50% of foetuses with common arterial trunk.Reference Butto, Lucas and Edwards 14 Persistent left superior caval vein and aberrant subclavian artery are additional anomalies seen in the common arterial trunk, which are best viewed from the three-vessel-trachea view and the aortic arch view.
Differential diagnosis of common arterial trunk
It is challenging to differentiate among common arterial trunk, pulmonary atresia with ventricular septal defect, and tetralogy of Fallot. Visualisation of the branch pulmonary arteries off of the common trunk is diagnostic of common arterial trunk. In contrast, the branch pulmonary arteries of pulmonary atresia with ventricular septal defect are located in the normal anatomic position and are supplied by the arterial duct via retrograde flow. The presence of a pulmonary valve with a normal-appearing aortic valve points towards a variant of tetralogy of Fallot. A rare lesion that is especially difficult to differentiate in the foetus is tetralogy of Fallot with pulmonary atresia and an aortopulmonary window. The branch pulmonary arteries of pulmonary atresia with ventricular septal defect and tetralogy of Fallot are often diminutive in size. The branch pulmonary arteries of common arterial trunk, in contrast, are normal to supernormal in size. Additional echocardiographic findings that favour the diagnosis of common arterial trunk over pulmonary atresia with ventricular septal defect include a thickened, dysplastic, truncal valve with stenosis and/or insufficiency, a markedly dilated aortic root, an absent arterial duct, and multiple truncal valve leaflets.
Family counselling
The overall neonatal survival rate following a foetal diagnosis of common arterial trunk in patients with intention to treat, was reported to be in the range of 42Reference Punn and Silverman 7 –68%.Reference Swanson, Selamet Tierney, Tworetzky, Pigula and McElhinney 15 Prenatal diagnosis has been associated with a higher rate of elective termination and a younger age of surgical repair, but is not associated with improved neonatal survival.Reference Swanson, Selamet Tierney, Tworetzky, Pigula and McElhinney 15
The specifics of the anatomy of this lesion play an important part in the counselling of families. Significant truncal valve stenosis or insufficiency can progress over the course of the pregnancy and result in foetal hydrops and foetal demise. The cardiovascular profile score can be useful in assessing prognosis, where a score of 7 out of 10 or less is associated with increased postnatal mortality.Reference Wieczorek, Hernandez-Robles, Ewing, Leshko, Luther and Huhta 8 Transplacental therapy with digoxin has been shown to improve the foetal condition and may prolong gestation.Reference Patel, Cuneo, Viesca, Rasanen, Leshko and Huhta 16 Associated cardiac lesions, such as interrupted aortic arch and single ventricle physiology, will make the surgical interventions and long-term prognosis more complicated.
It is important to review with families the association of microdeletion 22q11 in up to 40% of cases of truncus arteriosus.Reference Wieczorek, Hernandez-Robles, Ewing, Leshko, Luther and Huhta 8 Foetal echocardiogram assessment for the presence and size of a thymus, a right aortic arch, and aortic arch anomalies will help with a presumptive diagnosis, but definitive diagnosis of deletion 22q11 is made by amniocentesis with FISH analysis. Additional chromosomal anomalies have been reported in association with common arterial trunk, but are rare.
Summary
A careful cardiac screening assessment by skilled sonographers with an understanding of conotruncal lesions will not only increase the detection of foetal transposition of the great arteries and common arterial trunk, but will also improve the diagnosis of variations in anatomy and associated anomalies. This will lead to improved family counselling and possibly improved postnatal outcomes over time.








