INTRODUCTION
Hemispatial neglect is defined as a failure to report, respond, or orient to stimuli on the contralesional side of space in patients with damage to the brain (Heilman & Valenstein, Reference Heilman and Valenstein1979). Neglect is typically associated with right hemisphere damage, occurring frequently after stroke (Vallar & Perani, Reference Vallar and Perani1986). Despite being a common and debilitating syndrome, affecting up to two-thirds of right-hemisphere stroke patients (Parton et al., Reference Parton, Malhotra and Husain2004), neglect is poorly understood. This may be because neglect represents a complex syndrome with patients showing varied combinations and levels of impairment. While researchers generally agree that neglect is a heterogeneous disorder, the different categories that comprise the syndrome remain controversial. Different subtypes have been proposed (i.e., near/far neglect; perceptual/representational neglect, egocentric/allocentric neglect, and others), but there is a lack of agreement regarding subtype importance, definition, and assessment. Perhaps most challenging is distinguishing between allocentric and egocentric neglect, as the two subtypes are neither readily observable through everyday patient behavior, nor vastly different in their presentation. These subtypes do, however, have distinct and meaningful impacts on patients’ perceptual worlds. Egocentric neglect concerns a failure to perceive stimuli on the contralesional side of space relative to the individual’s body midline. Conversely, allocentric neglect refers to deficits in the perception of the contralesional side of individual objects, regardless of their position relative to the body (Chechlacz et al., Reference Chechlacz, Rotshtein, Bickerton, Hansen, Deb and Humphreys2010; Medina et al., Reference Medina, Kannan, Pawlak, Kleinman, Newhart and Davis2009). In a combined presentation, patients might show simultaneous egocentric and allocentric deficits, evidenced by failure to perceive both stimuli on the contralesional side of space, and the contralesional side of individual objects.
Though widely recognized, the relationship between egocentric and allocentric neglect is unclear. Some researchers have argued that allocentric neglect is simply a special form of egocentric neglect in which the patient’s attentional window has been limited to an individual stimulus (Driver & Pouget, Reference Driver and Pouget2000). Others propose that these presentations may not be distinct at all, or that either pattern can emerge, depending on the instructions or requirements of the particular neglect measure used (Baylis et al., Reference Baylis, Baylis and Gore2004; Driver & Pouget, Reference Driver and Pouget2000; Karnath et al., Reference Karnath, Mandler and Clavagnier2011; Mozer, Reference Mozer2002; Rorden et al., Reference Rorden, Hjaltason, Fillmore, Fridriksson, Kjartansson, Magnusdottir and Karnath2012; Yue et al., Reference Yue, Song, Huo and Wang2012). By contrast, a number of studies strongly support the dissociation between allocentric and egocentric neglect among stroke patients. Researchers have demonstrated that distinct patterns of deficit exist between egocentric and allocentric neglect patients, and that these differences are observable through behavioral assessments (Bickerton et al., Reference Bickerton, Samson, Williamson and Humphreys2011; Hillis et al., Reference Hillis, Newhart, Heidler, Barker, Herskovits and Degaonkar2005; Marsh & Hillis, Reference Marsh and Hillis2008; Medina et al., Reference Medina, Kannan, Pawlak, Kleinman, Newhart and Davis2009; Ota et al., Reference Ota, Fujii, Suzuki, Fukatsu and Yamadori2001). Additional investigation into anatomical correlates reveals that each subtype may be associated with distinct lesion types and locations (Khurshid et al., Reference Khurshid, Trupe, Newhart, Davis, Molitoris and Medina2012; Verdon et al., Reference Verdon, Schwartz, Lovblad, Hauert and Vuilleumier2010).
NEGLECT SUBTYPES AND FUNCTIONAL OUTCOME
The presence of neglect is a poor prognostic indicator for functional recovery following stroke (Buxbaum et al., Reference Buxbaum, Ferraro, Veramonti, Farne, Whyte, Ladavas and Coslett2004; Cherney et al., Reference Cherney, Halper, Kwasnica, Harvey and Zhang2001). Neglect is associated with prolonged hospitalization, increased risk for falls and injuries, poor return to independent living, and poor self-care (Czernuszenko, Reference Czernuszenko2006; Jehkonen et al., Reference Jehkonen, Laihosalo and Kettunen2006; Nijboer et al., Reference Nijboer, Kollen and Kwakkel2013; Wee & Hopman, Reference Wee and Hopman2008). While the general symptoms of neglect can reduce patients’ functional recovery, it is unclear whether the neglect subtypes differentially impact outcomes. The functional implications of egocentric neglect are well understood, as the inability to attend to an entire side of space results in a somewhat predictable pattern of impairment. However, significantly less is known about how allocentric neglect affects daily functioning. Given the distinct attentional patterns that appear to underlie each subtype, it is likely that the resulting functional implications are also distinct. Nevertheless, only one study to date has investigated the impacts of allocentric and egocentric neglect on functioning (Bickerton et al., Reference Bickerton, Samson, Williamson and Humphreys2011). Here it was observed that individuals with allocentric neglect were more impaired in everyday activities compared with those with egocentric neglect. In addition, patients with both types of neglect had worse functional outcomes than patients with egocentric or allocentric neglect alone. These results highlight the importance of distinguishing between neglect subtypes.
ASSESSMENT OF ALLOCENTRIC AND EGOCENTRIC NEGLECT
Most neuropsychological and functional assessments of neglect focus heavily on egocentric deficits. As such, allocentric neglect may be underrepresented and require specialized assessments. A recently developed tool of note is the Apples Test (Bickerton et al., Reference Bickerton, Samson, Williamson and Humphreys2011). In this task, patients must discriminate between complete apple shapes, and apples with a gap on either the left or right side. Patterns of performance on this task have been shown to reliably differentiate between neglect subtypes, with egocentric neglect patients failing to mark stimuli on the contralesional side of the page, and allocentric neglect patients attending to stimuli across the entire page, but incorrectly marking apples with a gap on the left as being intact.
Another robust method of measuring neglect subtype is tracking eye movements. Eye tracking systems continuously measure attention by sampling velocity and direction of eye movements, fixations, and gaze durations. If neglect is considered to be a bias in attention toward the ipsilesional side of space or objects, and movement of the eyes necessitates a corresponding shift in attention (Shepherd et al., Reference Shepherd, Findlay and Hockey1986), it follows that eye movements might be affected in neglect (Marshall & Robertson, Reference Marshall and Robertson2013). Indeed, research indicates that neglect patients’ eye movements are different from controls, with patients spending less time gazing at the neglected side (Delazer et al., Reference Delazer, Sojer, Ellmerer, Boehme and Benke2018). However, when patients’ eyes are free to perform visual search tasks, eye movements do not stop at an invisible barrier at the midline of a page or object. Rather, evidence from fixation durations and gaze time suggests that patients’ probability of prolonged fixation on the contralesional side decreases as distance from midline increases (Gainotti & Taicci, Reference Gainotti and Tiacci1971; Ishiai et al., Reference Ishiai, Sugishita, Mitani and Ishizawa1992; Marshall & Robertson, Reference Marshall and Robertson2013). This has important implications for the mechanisms of attentional control that underlie neglect, as well as measurement of the disorder through eye tracking versus paper-and-pencil tasks. Eye movement data can be used to calculate time spent looking at a particular location, which in turn reflects engagement of attention. Further, eye tracking has the unique ability to distinguish between early/automatic and later/controlled attentional processing. Total dwell time (the sum of all fixations and refixations on a target) is taken to reflect sustained or controlled attention and has been used to gain insights into attention and memory, reading, problem-solving, and learning (i.e., Grant & Spivey, Reference Grant and Spivey2003; Green et al., Reference Green, Lemaire and Dufau2007; Hannula et al., Reference Hannula, Althoff, Warren, Riggs, Cohen and Ryan2010; Libben & Titone, Reference Libben and Titone2009; Rayner, Reference Rayner1998). Conversely, first fixation duration (the length of time the eyes fixate on the target the first time they land on it) represents more initial attentional processes that are outside of overt cognitive control (Libben & Titone, Reference Libben and Titone2009; Rayner, Reference Rayner1998). Specifically, it appears that more superficial processing (e.g., preliminary scanning) is associated with earlier and shorter fixations while controlled, more deliberate processing is connected with longer, later, and repeated fixations (Glöckner & Herbold, Reference Glöckner and Herbold2011; Velichkovsky, Reference Velichkovsky1999; Velichkovsky et al., Reference Velichkovsky, Rothert, Kopf, Dornhofer and Joos2002). Consequently, eye tracking allows for dissociation between early/automatic, and later/controlled attention, providing a more direct measure than pencil-and-paper behavioral output, which occurs downstream of response selection and skeletomotor activity (Weierich et al., Reference Weierich, Treat and Hollingworth2008). As such, eye tracking may be used to uncover the attentional deficits that ultimately result in behavioral outcomes specific to either allocentric or egocentric neglect, potentially informing treatment targets for patients. To date, there has been a paucity of research investigating whether automatic, controlled, or both types of processing underlie attention deficits in neglect.
PRESENT STUDY
The goals of the current study were to (1) determine whether allocentric and egocentric neglect could be accurately and sensitively dissociated among stroke patients using figurative discrimination tasks in conjunction with eye tracking; (2) investigate the specific attentional patterns of neglect as they pertain to both the spatial and temporal characteristics of patients’ eye movements; and (3) investigate the relationship between neglect subtypes and functional outcome.
We hypothesized a distinct dissociation between patients in the sample who demonstrated allocentric, egocentric, or both neglect subtypes simultaneously, and that the eye tracking measure would be more sensitive in detecting neglect than a paper-and-pencil measure. To address possible over-diagnosis using the eye tracker, we included a control group of age- and education-matched, healthy adults, whom we expected would not show patterns of attention consistent with neglect. For neglect patients, we hypothesized that attention would differ between patients based on their neglect subtype classification. Specifically, we expected there to be no difference between global spatial allocation of attention for individuals with no neglect or allocentric neglect, and a bias against the left side of space in egocentric and combined neglect. We hypothesized that these attentional patterns would hold true for total dwell time, but not first fixations, indicating preserved initial processing with impaired sustained attention. Finally, we hypothesized that egocentric neglect patients would have better functional outcomes than those with either allocentric or both neglect subtypes.
METHOD
Participants
Stroke patients
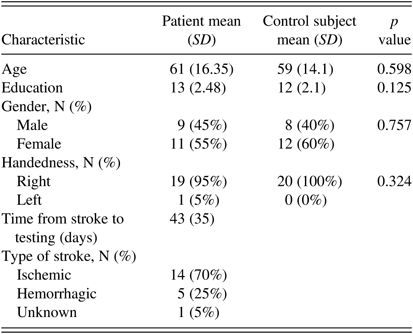
Twenty participants were recruited from the Rehabilitation Unit of a tertiary-care hospital. Inclusion criteria included a diagnosis of stroke within 60 days, probable contralesional neglect based on lesion location and observed behavior (e.g., occupational therapist or nurse notes a failure to attend to left side), absence of prior psychiatric history, absence of abnormal eye movement or vision as indicated by neurology and other medical reports, and ability to follow directions (assessed during clinical interview with neuropsychologist). Demographic information was collected from patients. Information regarding date of stroke, lesion location, and lesion nature was gathered from patient charts. Data were successfully collected from all participants. Our sample had a mean education level of 13 years (SD=2.48), mean age of 61 years (SD=16.35), was largely right-handed (N=19, 95%), and contained 9 males (45%) and 11 females (55%).
Control subjects
Twenty healthy adults were recruited as controls. Control subjects were age- and education-matched to patients (mean age=59 years, SD=14.1; mean education=12 years, SD=2.1) and completed only the eye tracking measure of neglect. Demographic data are presented in Table 1.
Table 1 Demographic and stroke information (N=20 per group)

Note: Independent samples t-tests were used to compare group means and derive p values.
Apparatus
Eye movements were tracked using an SMI RED-m remote system sampling at 120Hz and SMI Experiment Suite™360° software. The system has high tolerance for variables such as age, eye color, head position, glasses, and contact lenses. Stimuli were presented on a 343-mm (horizontal) x 190.5-mm screen (vertical; Dell Precision-M4800). Screen resolution was set at 1920x1080 pixels with 60-Hz refresh rate. The screen was centered on the mid-sagittal plane of the subject’s head and was viewed binocularly from a distance of 60 cm. We used 5-point calibration and 4-point validation routines for each participant. Fixations within 0.4° on the x- and y-axes were deemed acceptable. Software specific to the SMI system (BeGazeTM) was used to identify and extract fixations in the areas of interest and remove blink data. Fixations were defined with a maximum dispersion of 2° visual angle and a minimum duration of 80 ms.
Measures
Neglect assessment
Apples test
The Apples Test (Bickerton et al., Reference Bickerton, Samson, Williamson and Humphreys2011) is a paper-and-pencil assessment of neglect that is sensitive to allocentric and egocentric responses. To allow for measurement of eye movements, we modified the paper-and-pencil Apples Test for display on a computer screen. The total number of stimuli was reduced from 150 to 90 so each apple occupied the minimum size for accurate eye movement detection. In this short-form Apples Test, original ratios of whole, left-gap, and right-gap apples were retained. For comparison, both a paper-and-pencil and computerized version of the short-form Apples Test were administered in addition to the original paper-and-pencil Apples Test. The computerized version was programmed using CancellationTools (Dalmaijer et al., 2015; www.cancellationtools.org). Responses were made via mouseclick, and a red X appeared when participants clicked on an item. The mouse cursor was centered on screen at task onset.
Eye tracking
Interest areas were defined around the left and right halves of each apple in the Apples Test (3x3 cm; approximately 2.9° visual angle). Additionally, areas of interest were defined to separate the stimulus page into four sections: a far-left, middle-left, middle-right, and far-right section (8.5x19 cm). Finally, two interest areas encompassed the left and right sides of the screen (17x19 cm). Data of interest included total dwell time and first fixation duration. Total dwell time was used as it represents the total length of time a participant spends gazing at an area of interest. Given that the attentional biases underlying neglect appear to be related to fixation duration rather than relative location of stimuli (Gainotti & Taicci, Reference Gainotti and Tiacci1971), dwell time is an appropriate measure for investigating the patterns of sustained/late attention present in neglect. As a means of assessing early attention, we included the first fixation duration measure, which is considered to be largely automatic and independent of cognitive control (Rayner, Reference Rayner1998).
Scoring
Paper-and-pencil Apples Test scores were transformed to categorize each participant as having one of the following: egocentric neglect, allocentric neglect, both, or none. This categorical variable was computed based on asymmetry cutoff values described by the author/publisher of the measure (Bickerton et al., Reference Bickerton, Samson, Williamson and Humphreys2011).
For eye tracking neglect scores, a single repeated measures analysis of variance (ANOVA) with the factors of Page (left side, right side) and Apple (left side, right side) was conducted for each individual participant’s total dwell time and first fixation duration data. For each participant, significant main effects of total dwell time for Page and/or Apple indicated the presence of egocentric or allocentric neglect, respectively (i.e., the time spent on the right side of the page or apple was significantly different than the time spent on the left). A significant main effect for both Page and Apple indicated the presence of both subtypes. In the absence of established diagnostic eye tracking cutoff scores similar to Bickerton et al. (Reference Bickerton, Samson, Williamson and Humphreys2011), intra-individual statistical analyses may provide preliminary diagnostic utility (Gonzales et al., Reference Gonzalez, Schaefer, Buonanno, Schwamm, Budzik and Rordorf1999; Shine, Reference Shine1973).
Neuropsychological assessment
Test selection for the neuropsychological battery was based on the standardized stroke neuropsychological test protocol proposed by NINDS-CSN (Hachinski et al., Reference Hachinski, Iadecola, Petersen, Breteler, Nyenhuis, Black and Leblanc2006). Memory, language, visuospatial-constructional skills, and executive function were assessed using measures outlined in Table 2.
Table 2 Neuropsychological domains, corresponding tests, and standardization sources

Functional outcome assessment
Mayo-Portland Adaptability Inventory-4 (MPAI-4)
The MPAI-4 (Malec, Reference Malec, Kean, Altman and Swick2012) is a comprehensive assessment of patient functional outcome consisting of four indices: ability, adjustment, participation, and pre-existing and associated conditions. The first three indices can be combined to create a ‘Total Score’ of functional outcome. Raw scores were converted to standardized T-scores using normative data. For accuracy, the manual outlines criteria for scoring each item, based on an ordinal scale from 0 (None) to 4 (Severe problems). See Malec et al. (Reference Malec, Kragnessm, Evans, Finlay, Kent and Lezak2003) for MPAI-4 psychometric data.
Procedure
The study was conducted in accordance with the Declaration of Helsinki and was approved by the University of British Columbia Okanagan and Interior Health Harmonized Research Ethics Board. Neglect scores, MPAI-4 scores, demographic information, and stroke information based on radiology reports and therapists’ progress reports (i.e., occupational, physical, and/or speech-language) were collated for analyses.
Neuropsychological evaluation lasted approximately four hours, across two sessions to mitigate fatigue. Assessment was completed within two weeks to reduce impacts of rehabilitation and natural recovery on performance. Minor substitutions were made from the NINDS-CSN neuropsychological test battery to provide more robust and current measures of cognition (Table 2).
Evaluation of neglect lasted approximately thirty minutes. Each participant completed paper-and-pencil versions of the original Apples Test and the short-form Apples Test without eye tracking, as well as the short-form computerized Apples Test with eye tracking. Order of administration was randomized across participants.
Evaluation of patients’ functional outcomes via the MPAI-4 was conducted by trained examiners who were blind to patients’ neglect scores. MPAI-4 scores were based on semi-structured interview with the participant and review of therapists’ progress notes.
RESULTS
Descriptive Analyses
Means, standard deviations, skew, and kurtosis for Apples Tests, MPAI-4, and cognitive variables are presented in Table 3. All primary variables of interest (i.e., neglect, functional outcome, cognitive composite scores) were within an acceptable range of normality in terms of data distribution (i.e., within the cutoff values of ±2X SE; Gravetter & Wallnau, Reference Gravetter and Wallnau2014). See Supplementary Material: Appendix 1 for additional preliminary analyses.
Table 3 Neglect patient performance on independent and dependent variables

Note: COWAT=Controlled Oral Word Association Test; CVLT-II=California Verbal Learning Test-Second Edition; MPAI-4=Mayo-Portland Adaptability Inventory – 4; ROCF=Rey-Osterrieth Complex Figure Test; WAIS-IV Block Design=Weschler Adult Intelligence. Scale - Fourth Edition Block Design; CT2=Color Trails 2; CCC=Consonant Trigrams (Brown Peterson Task); WCST=Wisconsin Card Sort Test.
Equivalency of Paper-and-Pencil Apples Tests
Results from two-tailed paired samples t-tests revealed no significant difference in paper-and-pencil egocentric asymmetry scores between the original (M=3.82, SD=5.88) and short-form (M=3.33, SD=4.51) of the Apples Tests (t(19)=0.855, p=.31, d=0.094). Similarly, for allocentric asymmetry scores, there was no significant difference between scores on the original version (M=3.78, SD=4.69) and the short-form (M=3.23, SD=3.94) of the Apples Test (t(19)=1.46, p=.216, d=0.127). As the two measures were statistically equivalent, subsequent analyses used neglect scores derived from the short-form version of the Apples Test.
Equivalency of Paper-and-Pencil Versus Computerized Apples Tests
Results from two-tailed paired samples t-tests revealed no significant difference for egocentric asymmetry scores between the paper-and-pencil (M=3.40, SD=4.21) and computerized Apples Test (M=3.38, SD=4.33; t(19)=-0.228, p=.836, d=0.005). Similarly, for allocentric asymmetry scores, there was no significant difference between scores on the paper-and-pencil Apples Test (M=4.01, SD=4.62) and the computerized Apples Test (M=3.32 , SD=4.32; t(19)=-0.872, p=.303, d=0.154). Finally, no significant difference was found between total number of targets crossed out on the paper-and-pencil (M=21.63, SD=1.98) and computerized (M=22.69, SD=1.88) Apples Tests [t(19)=-0.194, p=.849, d=0.509]. Correlational analyses between total scores on the two test versions indicated that the measures were highly correlated, r(18)=0.973, p<.01.
Neglect Scores
Information on neglect subtypes among the sample and comparisons between classifications based on the pencil-and-paper Apples Test, computerized Apples Test, and eye tracking measure is presented in Tables 4 and 5. Based on the pencil-and-paper Apples Test, two patients (10%) displayed egocentric neglect, one (5%) displayed allocentric neglect, six (30%) displayed combined egocentric and allocentric neglect, and 11 (55%) displayed no neglect. Based on eye tracking, four patients (20%) displayed egocentric neglect, five (25%) displayed allocentric neglect, six (30%) displayed combined egocentric and allocentric neglect, and five (25%) displayed no neglect. For control participants, information on computerized Apples Test performance is summarized in Table 6. Only two control participants (10%) scored as neglectful on the eye tracking measure: one displaying leftward allocentric neglect, and the other showing rightward allocentric neglect.
Table 4 Classification based on Apples Test and eye tracking measures of neglect for stroke patients

Note: Grey box denotes differences in scores differ between versions of Apples Test or between Apples Test and eye tracking.
Table 5 Neglect subtype information for stroke patients

Table 6 Neglect subtype information for control participants

Neuropsychological Variable Reduction
Scores on the cognitive variables of interest (Table 2) were used to compute a single independent variable for cognitive functioning. Raw scores were converted into t-scores and were averaged across measures for each participant to create a composite for cognitive functioning.
Regression Analysis
Only variables demonstrating significant correlations at the a priori criterion of p<.01 were retained for analysis (Supplementary Material: Appendix 2). To assess the predictive value of eye movements, beyond both cognitive variables and the paper-and-pencil Apples Test, hierarchical multiple linear regression was used. We entered cognitive composite scores in Step 1, Apples Test scores in Step 2, and eye tracking scores in Step 3. Results are presented in Table 7. At Step 1, cognitive functioning contributed significantly to the regression model [F (1, 19)=8.276, p<.05], with poorer performance on cognitive testing predicting worse functional outcomes. Cognition accounted for 37.2% of the variation in functional outcome (R 2=.372). Introducing the paper-and-pencil Apples Test variable explained an additional 21.2% of variation in functional outcome; however, this did not significantly add to the prediction of functional outcome [ΔR 2=.212; F(3,16)=1.872, ns]. Adding the eye tracking variable to the regression model in Step 3 explained an additional 25% of variation in functional outcome and this change was significant [ΔR 2=.252; F(3,13)=4.099, p<.05]. Together the three independent variables accounted for 83.6% of the variance in function [R 2=.836; F(3,13)=5.826, p<.05].
Table 7 Results of hierarchical multiple linear regression analysis

Note: *p<.05, **p<.01.
Analysis of Variance
To determine the relative influence of neglect subtype on patients’ functional outcomes, one-way ANOVA was conducted using MPAI-4 total score as the dependent variable, and eye tracking neglect classification (i.e., egocentric, allocentric, both, none) as the independent variable. As small sample size would limit interpretation of the stability of results obtained via ANOVA, bootstrap analysis with 2000 resamples and corresponding bias-corrected and accelerated confidence intervals (CIs) were used to determine probability of another sample replicating the observed results. The CIs reported should be considered over reported p-values given the limited sample. Results revealed a statistically significant difference between neglect subtype groups [F(3,16)=24.148, p<.001, d=0.796]. Tukey post hoc testing revealed that MPAI-4 scores for patients without neglect (M=42.3, SD=2.63) were significantly better than egocentric neglect patients (M=49.3, SD=2.62, p<.05, CI: -10.128, -3.500), allocentric neglect patients (M=50.75, SD=3.40, p<.01, CI: -12.167, -4.500), and patients with combined egocentric and allocentric neglect (M=55.7, SD=.81, p<.001, CI: -15.628, -10.870). In addition, patients with combined neglect showed significantly worse functional outcome scores than both the pure egocentric (p<.05, CI: 3.821, 9.000) and pure allocentric (p<.05, CI: 1.667, 8.000) patients. Differences between egocentric and allocentric patients were not significant (p=.842, CI: -5.500, 2.333).
In order to contrast early/automatic versus later/controlled attentional patterns we first separated the page area into four quadrants (far-left, middle-left, middle-right, far-right) to investigate graded effects of attention across space. Next, nonparametric Wilcoxon signed-rank tests were conducted using this Page Area variable and patients’ eye tracking neglect classification. Results are presented in Table 8 and Figure 1. As expected based upon our definition of neglect subtypes, patients with no neglect, egocentric neglect, or allocentric neglect, demonstrated no significant differences in total dwell time between any two sections of the page. For patients with simultaneous egocentric and allocentric neglect, significantly less time was spent on the far left of the page (M=10.72, SD=13.81) than the far right (M=53.79, SD=29.33, Z=-5.01, p<.05), and on the middle-left of the page (M=21.56, SD=26.61) than each of the middle-right (M=43.06, SD=41.92, Z=-2.43, p<.05) and far right of the page (M=53.79, SD=29.33, Z=-3.79, p<.05). Conversely, Wilcoxon signed-rank testing for first fixation duration revealed no significant comparisons.

Fig 1 Graphical representation of means for total dwell time (ms) data by neglect subtype.
Table 8 Wilcoxon signed-rank test means of total dwell time (milliseconds) by page section and neglect classification

Note: *=difference significant at the p<.05 level. Difference value=absolute value of difference (subtraction) between page sections.
DISCUSSION
The aim of the current pilot study was to assess neglect subtypes among stroke patients using eye tracking, and to determine the relationship between these subtypes and functional outcomes. We sought to determine whether eye tracking measures could detect neglect subtypes and add predictive value to patient functioning beyond a pencil-and-paper measure. The computerized version of the Apples Test used in this study closely aligned with the original Apples Test (Bickerton et al., Reference Bickerton, Samson, Williamson and Humphreys2011). We found preliminary evidence suggesting that eye tracking may be more sensitive in detecting neglect symptoms than paper-and-pencil results. In line with this, previous research indicates that neglect deficits may go undetected when paper-and-pencil tasks are used for diagnosis, as these tools do not allow for direct monitoring of attention and gaze patterns (e.g., Azouvi et al., Reference Azouvi, Samuel, Louis-Dreyfus, Bernati, Bartolomeo and Beis2002; Bonato, Reference Bonato2012; Karnath & Niemeier, Reference Karnath and Niemeier2002). Our results also showed that patterns of attention exhibited by neglect patients were markedly different from patients without neglect and control participants, with respect to controlled attention processes (total dwell time) but not for early/automatic attention (first fixation duration). We also found preliminary results suggesting that neglect subtype classification, based on eye tracking performance, was more predictive of patients’ functional outcomes than subtype classification based on the paper-and-pencil Apples Test. Finally, results suggested that patients with either allocentric or egocentric neglect may have better functional outcomes than patients with both subtypes, and that all three neglect groups fared worse than those with no neglect.
Hemispatial neglect is a bias in attention against the contralesional side of space. As such, researchers have suggested that eye movements might follow a similarly biased pattern (Marshall & Robertson, Reference Marshall and Robertson2013). Expanding on previous research (see Gainotti & Taicci, Reference Gainotti and Tiacci1971), we hypothesized that these differences would manifest in terms of total dwell time, but not first fixation duration. In other words, we hypothesized that patients with neglect would initially fixate on stimuli across the page (in a similar manner to those without neglect) but would have a lower probability of prolonged fixation on the neglected side. Significant interactions between page area and neglect subtype were observed for total dwell time data, but not first fixation duration. These findings suggest that patients with neglect were initially fixating on stimuli across both the left and right sides of space but were less able to sustain attention on the left side. When investigating these effects as they pertain to neglect subtypes, a small sample size and low statistical power did not enable us to find significant results for group comparisons; however, the general trends observed are worth discussing. For participants with no neglect or allocentric neglect, sustained attention across sensory space was relatively equal (Figure 1). In contrast, for patients with egocentric neglect, probability of prolonged fixation across the page decreased in the far-left quadrant. A similar pattern was observed in patients with combined egocentric and allocentric neglect, with the probability of prolonged fixation significantly decreasing from right to left. These patterns of attention may be indicative of preserved initial/automatic processing in concert with impaired sustained attention in patients with an egocentric component to their neglect symptomology. While it has been suggested that automatic attention is more heavily impacted in neglect than controlled attention (e.g., Bartolomeo and Chokron, Reference Bartolomeo and Chokron2002; Gainotti, Reference Gainotti1996; Làdavas et al., Reference Làdavas, Carletti and Gori1994) our findings agree with a growing body of literature suggesting a relative sparing of very early attention in this population (e.g., Marshall & Robertson, Reference Marshall and Robertson2013; Mevorach et al., Reference Mevorach, Tsal and Humphreys2014; Vuilleumier & Landis, Reference Vuilleumier and Landis1998). To build upon these temporally based findings, future research should consider using tasks that can tease apart temporal and spatial patterns in early versus late stages of attentional processing. Spatially based eye tracking variables such as saccade position may also prove useful in this regard.
Previous studies have linked neglect to poor functional outcomes (Buxbaum et al., Reference Buxbaum, Ferraro, Veramonti, Farne, Whyte, Ladavas and Coslett2004; Cherney et al., Reference Cherney, Halper, Kwasnica, Harvey and Zhang2001). Results of the current study align with this. Based on our eye tracking measure, the presence of neglect was associated with both cognitive and functional impairment. To our knowledge, this is the first study to assess the specific contributions of neglect subtypes to functional outcome using both a comprehensive measure of global functioning and controlling for cognitive performance. Preliminary results indicate that patients with no neglect were the least functionally impaired. Patients with either egocentric or allocentric neglect, while not significantly different from each other, demonstrated poorer functional outcomes than those without neglect. Finally, those with both egocentric and allocentric neglect evidenced the poorest functioning of the four groups. While these results are based on a small sample size with unequal groups, our ability to detect categorical differences in such a restricted sample could represent a large and meaningful effect. We believe that future research investigating functional outcome is both clinically important and warranted given the current results.
Limitations
This pilot study presents with some limitations. Results of the current study may only generalize to patients referred to rehabilitation, and not to those who would not meet criteria for such a referral. Additionally, patient selection was based on observed behavior, potentially resulting in a truncated sample of neglect patients that was biased toward egocentric manifestations (which are behaviorally more detectable). Future research should evaluate all right-hemisphere stroke patients to identify subtle neglect that might otherwise go unnoticed. Another limitation to consider is the possibility that the eye tracking task increased the rate of false positives, indicating neglect when the disorder was not truly present. Results demonstrating that the eye tracking task has predictive superiority, in terms of functional outcomes over-and-above that of the Apples Test, suggest that this measure is detecting real and meaningful differences among participants. Furthermore, with an overall value of 90%, the eye tracking measure’s specificity (i.e., true negative rate) can be considered good (Parikh et al., Reference Parikh, Mathai, Parikh, Sekhar and Thomas2008). These results suggest that eye tracking tasks show promise in enhancing detection of neglect subtypes and have the potential to be clinically useful diagnostic tools. Nevertheless, our diagnostic procedure for classifying neglect subtype was preliminary, as intra-individual ANOVA and subsequent comparison to previously established normative data are not ideal. Future research should consider developing more specific cutoff scoring values for eye tracking based on normative data in order to improve measure accuracy and enhance clinical applicability. Finally, the small sample size had detrimental impacts on statistical power and the ability to detect true and meaningful differences between groups in the sample. While overall trends in the data provide rationale for screening for neglect in order to better predict functional outcomes, these results are preliminary in nature and future research is warranted.
CONCLUSION
The current study provides preliminary support for the use of eye tracking in evaluating subtypes of neglect. Results suggest that subtype identification, via eye tracking, can account for a significant amount of variation in patients’ functional outcomes, beyond traditional paper-and-pencil measures of neglect. The present study highlights the importance of identifying neglect subtypes as risk factors for poorer functional outcome among stroke patients, and specifically the need to measure and distinguish between allocentric, egocentric, and both neglect subtypes in the care of these individuals. Further investigation of the assessment, frequency, and functional implications of these subtypes is an important step toward improving our understanding of this prevalent and debilitating condition.
ACKNOWLEDGMENTS
This research was conducted with funding from Canadian Foundation for Innovation: John R. Evans Leaders Fund (awarded to M. Libben – Grant #32942) and Canadian Institutes of Health Research: Frederick Banting and Charles Best Canada Graduate Scholarships (awarded to J. Upshaw – Grant #146201). The authors have no conflicting interests or activities to report.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1355617719000110