Mammary epithelial cells (MEC) proliferate and differentiate during mammary gland development to form the central component of secretory alveoli. Alveoli contain a hollow sphere of MEC with apical-basal polarity, which secrete milk into the central lumen. Lumen formation and maintenance throughout lactation is achieved by a balance between MEC proliferation and apoptosis (Debnath et al. Reference Debnath, Mills, Collins, Reginato, Muthuswamy and Brugge2002). The milk-producing capacity of the mammary gland is determined by the MEC population and its biosynthetic capacity; thus the balance between the rates of proliferation and apoptosis impacts on milk production and lactational persistency in dairy cattle. After peak lactation the secretory MEC population is reduced in parallel with a decrease in milk production (Boutinaud et al. Reference Boutinaud, Guinard-Flament and Jammes2004). Milk accumulation at weaning triggers involution of the mammary gland, which is associated with an increase in the rate of apoptosis in a proportion of the remaining MEC (Wilde et al. Reference Wilde, Addey, Li and Fernig1997). Since secretory MEC are in direct contact with milk, it is conceivable that milk contains pro-apoptotic factors that are involved in MEC death. It has been postulated that an autocrine suppressor of milk secretion, FIL, is present in milk (Wilde et al. Reference Wilde, Addey, Boddy and Peaker1995).
A potential pro-apoptotic protein in milk is α-lactalbumin (α-la) which has been demonstrated to enhance apoptosis under certain conditions. A molten globule form of human α-la, complexed to oleic acid, termed HAMLET (human α-lactalbumin made lethal to tumour cells) induces apoptotic activity in a number of tumour cell lines (Hallgren et al. Reference Hallgren, Gustafsson, Irjala, Selivanova, Orrenius and Svanborg2006) and also in mouse MEC (Baltzer et al. Reference Baltzer, Svanborg and Jaggi2004). HAMLET-like species derived from bovine (BAMLET), equine and porcine α-la also reduce tumour cell viability; however, active HAMLET has been found naturally only in human milk (Pettersson et al. Reference Pettersson, Mossberg and Svanborg2006). HAMLET acts in two ways: it binds to histones in the nucleus, disrupting chromatin structures; and it interacts with mitochondria in the cytoplasm, causing the release of cytochrome c and subsequent caspase activation (Baltzer et al. Reference Baltzer, Svanborg and Jaggi2004). Studies in vitro indicate that α-la can bind histones without oleic acid bound to it (Permyakov et al. Reference Permyakov, Pershikova, Khokhlova, Uversky and Permyakov2004). However, other studies suggest that complexing to oleic acid is necessary for transport into the cell (Svensson et al. Reference Svensson, Sabharwal, Hakansson, Mossberg, Lipniunas, Leffler, Svanborg and Linse1999).
Expression of α-la occurs from the onset of lactation, when the gland is fully differentiated, suggesting that its regulation is controlled via a different mechanism to other milk proteins (Goodman & Schanbacher, Reference Goodman and Schanbacher1991). α-La is found in relatively low concentrations in milk (1·2 mg/ml) compared with caseins, and the concentration remains relatively constant throughout lactation (Swaisgood, Reference Swaisgood and Jensen1995). Hence α-la may require modification to become apoptotic during involution via conversion to BAMLET, or an oligomeric form that has displayed apoptotic effects in some cell types (Svensson et al. Reference Svensson, Sabharwal, Hakansson, Mossberg, Lipniunas, Leffler, Svanborg and Linse1999; Xu et al. Reference Xu, Sugiura, Nagaoka and Kanamaru2005). Oligonucleotides in milk can also interact with α-la to modify its activity (Permyakov & Berliner, Reference Permyakov and Berliner2000).
α-La may also influence milk production through its role in lactose synthesis. It is one of the two components of lactose synthase, which catalyses the final step in lactose biosynthesis in the lactating mammary gland (Permyakov & Berliner, Reference Permyakov and Berliner2000). Lactose controls the osmolarity of milk and hence may affect milk production by controlling milk volume. Several studies have shown that reducing α-la decreases milk production, while milk production was increased in transgenic sows over-expressing α-la (Wheeler, Reference Wheeler2003).
The role of α-la in controlling milk volume, and its potential role in apoptosis and proliferation of MEC, makes it a molecule of interest for dairy production. The ability to increase milk protein levels or to increase lactational persistency in dairy cattle may have a significant economic benefit. In this study we investigated the effect of α-la on casein gene expression in vitro, and compared the role of the native and a fold variant conformation of bovine α-la in proliferation and apoptosis of primary bovine mammary epithelial cells (BMEC).
Materials and Methods
All reagents and oligonucleotides were from Sigma-Aldrich, Castle Hill, NSW 2154, Australia, unless stated otherwise.
Primary bovine mammary epithelial cell isolation
Mammary biopsies were taken from multiparous Holstein-Friesian cows as previously described (Sheehy et al. Reference Sheehy, Della-Vedova, Nicholas and Wynn2004). Mammary tissue was washed in phosphate-buffered saline (PBS, pH 7·4), extraneous tissue excised and mammary tissue placed into 1× Hanks Balanced Salt Solution (HBSS) containing 4·2 mm-NaHCO3, 50 U/ml penicillin/50 μg/ml streptomycin (Invitrogen, Mount Waverley, VIC 3149, Australia), 50 μg/ml kanamycin (Invitrogen) and 625 ng/ml fungizone (Invitrogen), pH 7·4. Typically, 8–10 g of mammary tissue was collected.
Tissue was weighed, minced with scissors and incubated in 10 ml/g predigest medium [1× HBSS, 1× minimal essential medium (MEM), 5·5 mm-glucose, 4% bovine serum albumin, 20 μm-CaCl2, 20 μm-MgSO4, 5 mm-4-(2-hydroxyethyl)piperazine-1-ethanesulphonic acid (HEPES), 200 μm-glutamine, 10 U/ml penicillin/10 μg/ml streptomycin, 10 μg/ml kanamycin, 250 ng/ml fungizone, 1 μg/ml insulin, 1 μg/ml cortisol, 50 U/ml DNase I (Roche Molecular Biochemicals, Castle Hill, NSW 2154, Australia), pH 7·4] at 200 rpm at 37°C for 10 min. Media was aspirated and replaced by digest media (predigest media+200 U/ml collagenase Type II, 250 U/ml hyaluronidase) and incubated at 37°C at 225 rpm for 2 h. Digested tissue was filtered through a 150-μm nylon mesh. Retained tissue was further incubated in fresh digest media. The filtrate was centrifuged at 200 g for 5 min and the cell pellet was washed three times in 20 ml wash media (1× HBSS, 1× MEM, 5·5 mm-glucose, 20 μm-CaCl2, 20 μm-MgSO4, 5 mm-HEPES, 2 mm-glutamine, 100 U/ml penicillin/100 μg/ml streptomycin, 100 μg/ml kanamycin, 2·5 μg/ml fungizone, 100 U/ml DNase I, 100 μg/ml trypsin inhibitor, 5 μg/ml insulin, 1 μg/ml cortisol, pH 7·4). The final wash was filtered through a 50-μm nylon mesh and the filtrate centrifuged at 200 g for 5 min. The cell pellet was resuspended in 2·5 ml 1× HBSS and overlayed onto a 17·5-ml 1·01–1·07 g/ml Percol-Ficoll step gradient. The gradient was centrifuged at 800 g for 20 min. Cells were subsequently collected from the 1·03/1·04 density layers and washed in 20 ml 1× HBSS. Cells were then cryopreserved. Cells were passaged at least twice on tissue culture plastic prior to plating experiments to ensure that single cell suspensions were used.
Cell viability assays
To investigate the effect of bovine α-la on the viability of BMEC monolayers and mammospheres, cells were plated in 96-well plates at 1×104 cells/well in proliferation media (4·75 g/l M199, 5·32 g/l Hams F12, 2·38 g/l HEPES, 1·875 g/l NaHCO3, 0·164 g/l sodium acetate, 20% horse serum (Invitrogen), 5% FCS (Invitrogen), 100 U/ml penicillin/100 μg/ml streptomycin, 100 μg/ml kanamycin, 1·25 μg/ml fungizone, 5 μg/ml insulin, 1 μg/ml cortisol, 10 ng/ml EGF) to form monolayers or on plates coated with matrigel (25 μl/cm2; BD Biosciences, North Ryde, NSW 2113, Australia) at 1×105 cells/well in attachment media [4·75 g/l M199, 5·32 g/l Hams F12, 2·38 g/l HEPES, 1·875 g/l NaHCO3, 0·164 g/l sodium acetate, 20% horse serum, 5% FCS, 100 U/ml penicillin/100 μg/ml streptomycin, 100 μg/ml kanamycin, 1·25 μg/ml fungizone, 5 μg/ml insulin, 1 μg/ml cortisol, 3 μg/ml prolactin (NHPP, Torrance CA 90509, USA), 10 μg/ml transferrin] to form mammospheres (Schmidhauser et al. Reference Schmidhauser, Bissell, Myers and Casperson1990; German & Barash, Reference German and Barash2002) and incubated at 37°C under 5% CO2. Bovine holo-α-la (Type I, Ca2+ bound), apo-α-la (Type III, Ca2+ free) or BAMLET was added to cell monolayers at 1·2 mg/ml in proliferation media 24 h after plating. BAMLET was formed by incubating apo-α-la with oleic acid (1:10 molar ratio) in water at 37°C for 1 h until the solution became cloudy (Wehbi et al. Reference Wehbi, Perez, Sanchez, Pocovi, Barbana and Calvo2005). Holo-α-la and apo-α-la and oleic acid alone (0·85 μm) were also incubated at 37°C for 1 h. Remaining media components were then added and the treatments were incubated with the cells for 48 h. A 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) cell proliferation assay (Promega Corporation, Annandale, NSW 2038, Australia) was then performed as per manufacturer's instructions. For cells on matrigel, 1·2 mg/ml bovine holo-α-la, 1·2 mg/ml apo-α-la, 1·2 mg/ml BAMLET, or oleic acid in differentiation media (4·75 g/l M199, 5·32 g/l Hams F12, 2·38 g/l HEPES, 1·875 g/l NaHCO3, 0·164 g/l sodium acetate, 100 U/ml penicillin/100 μg/ml streptomycin, 100 μg/ml kanamycin, 1·25 μg/ml fungizone, 5 μg/ml insulin, 1 μg/ml cortisol, 3 μg/ml prolactin, 10 μg/ml transferrin) was added 24 h after plating and a proliferation assay performed 4 d later. Absorbances were read at 560 nm and 690 nm in a plate reader (POLARstar OPTIMA, BMG Labtech, VIC 3931, Australia). Setting the control to 100% cell viability normalized data from multiple experiments.
Caspase assays
BMEC were cultured on matrigel in 96-well white-walled plates at 1×105 cells/well to form mammospheres. On day 4 of culture 1·2 mg/ml bovine holo-α-la, 1·2 mg/ml apo-α-la, 1·2 mg/ml BAMLET, or oleic acid (0·85 μm) was added. Etoposide (5 μm) was used as a positive control. Mammospheres were incubated with treatments in differentiation media overnight and then assayed for caspase activity using the Caspase Glo 3/7 Assay (30 min incubation, Promega) and Caspase Glo 9 assay (2 h incubation; Promega). Luminescence was measured on a plate reader (POLARstar OPTIMA, BMG Labtech). Data from multiple experiments were normalized by expressing the control for each experiment as having a relative luminescence of 1.
Effect of α-lactalbumin on casein gene expression
BMEC were plated onto matrigel-coated 6-well plates at 2×106 cells/well in attachment media containing bovine holo-α-la at 0·12 mg/ml and 1·2 mg/ml, to form mammospheres. After 24 h media was changed to differentiation media containing bovine holo-α-la at 0·12 mg/ml and 1·2 mg/ml. Media was replaced after 2 d. Cells were harvested in the RNA extraction kit lysis buffer after 4 d in differentiation media.
RNA was extracted using an RNeasy Mini kit (QIAGEN, Doncaster, 3018 VIC, Australia) with some modifications. Following homogenization, samples were made up to 800 μl in RNase-free water and incubated with 0·2 mg proteinase K (Roche Molecuar Biochemicals) at 55°C for 10 min. Then 400 μl of 100% ethanol was added prior to addition to the column. An on-column DNase I treatment was included. RNA yield and purity were determined by spectrophotometry and 1·2% agarose/formaldehyde gel electrophoresis.
cDNA was generated for real-time PCR analysis using 1 μg of purified and DNase-treated RNA, 0·7 mm-dNTPs (Promega) and 100 ng of oligo (dT)15 primers (Promega) in a 14·5 μl reaction volume. The reaction was incubated for 5 min at 65°C, then cooled on ice for at least 1 min. The reaction volume was then brought to 20 μl in 1× First Strand Buffer (50 mm-Tris–HCl, pH 8·3; 75 mm-KCl, 3 mm-MgCl2) with 40 U of RNAsin (Promega) and 100 U of SuperScript III MMLV RT (Invitrogen), and incubated at 50°C for 60 min. The enzyme was then inactivated by heating to 70°C for 15 min.
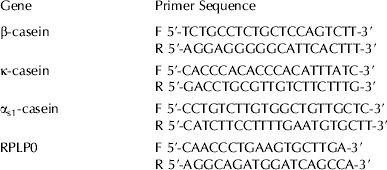
Expression levels of β-casein, αs1-casein, κ-casein, and a housekeeping gene, ribosomal protein, large, P0 (RPLP0) were determined by real-time PCR analysis using gene-specific primer sets (Table 1). Each gene was amplified in a separate reaction and each reaction performed in quadruplicate. Each reaction contained 20 ng cDNA for the casein genes or 10 ng cDNA for the housekeeping gene, 0·3 μm each primer, 0·2 mm-dNTPs, 0·075 U Platinum Taq polymerase (Invitrogen), 1× Platinum Taq buffer, 3 mm-MgCl2 and 0·4× SYBR green. Appropriate amounts (0·5 fg to 50 pg) of purified β-casein, αs1-casein, κ-casein and RPLP0 PCR product were also amplified under the same conditions to generate standard curves from which the copy number of each gene was determined. Reactions were incubated at 95°C for 2 min then 35 cycles were performed as follows: 95°C for 20 s, 60°C for 20 s, 72°C for 20 s (Rotor-Gene 3000; Corbett Life Science, Mortlake, NSW 2137, Australia). Finally a 72–99°C gradient at 5 s per degree was performed for a melt curve analysis. RPLP0 results were used to normalize the casein gene results. Data from multiple experiments were then expressed as relative normalized expression by setting the control in each experiment to 1-fold expression.
Table 1. Primers used for real-time PCR analyses
Non-denaturing PAGE
α-La treatments (12 μg of each) in differentiation media were analysed by 15% non-denaturing polyacrylamide gel electrophoresis (ND-PAGE). Gels were run according to the method Laemmli (Reference Laemmli1970) except that sodium dodecyl sulphate (SDS) was omitted from the gel, running buffer and loading buffer and loading buffer did not contain β-mercaptoethanol and samples were not boiled prior to loading. Protein was visualized by staining with Coomassie blue.
Statistical analyses
All data were analysed using restricted maximum likelihood (REML) procedures in GenStat (Release 10, VSN Intl., Hemel Hempstead, UK) using the following model:
where Yij is the measure being modelled (% cell viability, normalized luminescence or relative normalized gene expression). Treatment (either α-la type or dose) was designated as a fixed effect while Experiment was a random effect, with εij being the residual random error. A natural logarithm was applied to the gene expression data to normalize the variance, while cell viability and caspase data did not require transformation. Model-based means were calculated and presented in the graphs and in instances where the data were log-transformed, were back-transformed (exponentiated) to facilitate interpretation of the results. Standard errors shown in tables and figures are in the form mean±se, using model-based se, with approximate se calculated for the back-transformed means.
Results
As HAMLET has been reported to induce death of certain cell types (Baltzer et al. Reference Baltzer, Svanborg and Jaggi2004; Hallgren et al. Reference Hallgren, Gustafsson, Irjala, Selivanova, Orrenius and Svanborg2006) we investigated the effect of BAMLET, holo-α-la and apo-α-la on BMEC viability. All forms of α-la tested decreased the viability of BMEC (Table 2). Holo-α-la and apo-α-la had similar effects on BMEC monolayers, decreasing viability by 9–11% although this was not significantly different from the control. BAMLET exerted a significantly greater effect on monolayer viability, reducing it by 59% (P<0·001). However, there was no significant difference between BAMLET and oleic acid, with oleic acid decreasing the proliferation rate of monolayers by 46%. Mammosphere viability was reduced by holo-α-la and apo-α-la by 36% and 28%, respectively (P<0·001). Holo-α-la was significantly more effective at reducing mammosphere viability than apo-α-la. BAMLET and oleic acid reduced mammosphere viability by 99% and 98%, respectively (P<0·001).
Table 2. Effect of α-lactalbumin treatments and no treatment controls on BMEC viability. Values are predicted mean±se (n=9 for each treatment)

† Different letter superscripts in the same column denote significant differences between treatments (P<0·001)
Gel electrophoresis of the various α-la forms was performed to look for differences in conformation that could explain the different levels of efficacy in the viability assays and to test the purity of the commercial preparations. No difference in mobility on SDS-PAGE (not shown) or ND-PAGE (Fig. 1) was detected between holo-α-la and apo-α-la in cell culture media. BAMLET ran at a lower position on native PAGE than holo-α-la and apo-α-la (Fig. 1). There was no evidence of oligomers of α-la on SDS-PAGE (not shown) or native PAGE (Fig. 1), nor of contaminating proteins in the commercial preparations.

Fig. 1. 15% non-dentaturing PAGE of α-lactalbumin in cell culture media. Lane 1: holo-α-lactalbumin; Lane 2: apo-α-lactalbumin; Lane 3: BAMLET; Lane 4: oleic acid.
Apoptosis assays were conducted to help elucidate the mechanism by which the α-la forms were able to reduce mammosphere viability. All forms of α-la tested induced caspases 3/7 to a similar level (Table 3). Caspase 3/7 activity was 3–4-fold higher in α-la-treated mammospheres compared with control cells (P<0·001). At 1·2 mg/ml, the α-la forms tested induced Caspase 3/7 to a similar level as 5 μm-etoposide, a known inducer of apoptosis. Caspase 3/7 activity in oleic acid-treated mammospheres was not significantly different from the activity seen in mammospheres treated with BAMLET.
Table 3. Effect of α-lactalbumin (1·2 mg/ml) treatments and no treatment controls on caspase 3/7 and caspase 9 activity in mammospheres. Values are predicted mean±se (n=6)
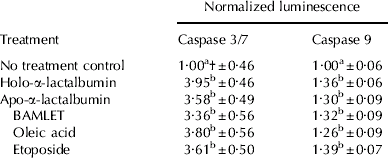
† Different letter superscripts in the same column denote significant differences between treatments (P<0·001)
Activation of caspase 9, an upstream initiator of caspases 3 and 7, was also tested. Caspase 9 activity was induced by all forms of α-la tested (Table 3). Activity was 1·3–1·4-fold higher in α-la-treated mammospheres compared with controls (P<0·001). There was no significant difference in caspase 9 activity between BAMLET- and oleic acid-treated mammospheres nor were there any significant differences between any other treatments.
To investigate the potential of α-la to affect milk protein yield, casein mRNA expression was measured in α-la-treated mammospheres. Holo-α-la at 0·12 mg/ml had no effect on β-casein mRNA expression in mammospheres while 1·2 mg/ml holo-α-la decreased β-casein mRNA expression 25-fold (P<0·001; Fig. 2). Expression of κ-casein mRNA was 2·4-fold that of the control (P<0·001; Fig. 2) in mammospheres treated with 0·12 mg/ml α-la and 3·1 fold greater (P<0·001) in mammospheres treated with 1·2 mg/ml α-la. Expression of αs1-casein mRNA was 4·8 fold greater in mammospheres treated with 0·12 mg/ml α-la (P<0·001; Fig. 2) and 7·3 fold greater in mammospheres treated with 1·2 mg/ml α-la (P<0·001; Fig. 2). αs2-Casein mRNA levels were too low to be measured by real-time PCR.

Fig. 2. Effect of native α-lactalbumin at 0 mg/ml (white bars), 0·12 mg/ml (grey bars) and 1·2 mg/ml (black bars) on β-casein, αs1-casein and κ-casein mRNA levels in mammospheres. Values are predicted mean±se (n⩾6 for each treatment). *** Denotes that values are significantly different from their respective controls (P<0·001).
Discussion
There have been several reports of cell death induced by folding variants of α-la. HAMLET causes cell death in numerous tumour cell lines (Hallgren et al. Reference Hallgren, Gustafsson, Irjala, Selivanova, Orrenius and Svanborg2006) and murine MEC (Baltzer et al. Reference Baltzer, Svanborg and Jaggi2004). Others have found that oligomeric forms of α-la induced cell death in an intestinal cell line and tumour cells, with monomeric forms being inactive (Svensson et al. Reference Svensson, Sabharwal, Hakansson, Mossberg, Lipniunas, Leffler, Svanborg and Linse1999; Xu et al. Reference Xu, Sugiura, Nagaoka and Kanamaru2005). Thompson (Reference Wehbi, Perez, Sanchez, Pocovi, Barbana and Calvo1992) observed growth inhibitory effects of α-la on MEC proliferation in primary culture in monolayers that may have been due to native α-la.
In the current study, we found that at concentrations normally found in milk, native α-la and possibly BAMLET reduced the viability of BMEC monolayers and mammospheres. Surprisingly, we found that α-la did not need to be in the form of BAMLET since holo-α-la and apo-α-la alone reduced viability of both proliferating and differentiated cells. The similar effects observed for these treatments may be as a result of apo-α-la converting to holo-α-la upon contact with cell culture medium, as this conversion is known to take place in the presence of calcium under physiological conditions (Pettersson et al. Reference Pettersson, Mossberg and Svanborg2006). Analysis of the preparations by gel electrophoresis supports this conclusion, since there was no difference in mobility on ND-PAGE, whereas apo-α-la may be expected to migrate further than holo-α-la (Thompson et al. Reference Thompson, Groves, Brower, Farrell, Jenness and Kotts1988). BAMLET migrated further than holo-α-la and apo-α-la, confirming that a change in hydrodynamic volume or shape had taken place, consistent with the effect of binding of oleic acid which allows α-la to maintain a partially unfolded state under physiological conditions (Mok et al. Reference Mok, Pettersson, Orrenius and Svanborg2007). There was no evidence that either oligomeric forms or proteolytic fragments of α-la were responsible for the effects of native α-la, although these possibilities cannot be entirely discounted. The BAMLET treatment was more effective than holo-α-la, almost completely eliminating mammosphere viability. However, there was no significant difference in the effectiveness of BAMLET and oleic acid. The study precluded determining whether the effect was due to BAMLET or oleic acid alone. Oleic acid, which has been reported to be cytostatic in urothelial cells (Southgate et al. Reference Southgate, Pitt and Trejdosiewicz1996), reduced the viability of BMEC.
HAMLET induces cell death by binding to histones thereby preventing transcription and replication (Svanborg et al. Reference Svanborg, Agerstam, Aronson, Bjerkvig, Duringer, Fischer, Gustafsson, Hallgren, Leijonhuvud, Linse, Mossberg, Nilsson, Pettersson and Svensson2003). The molten globule state is believed to be important for transport into the cell. Svensson (Reference Svensson, Sabharwal, Hakansson, Mossberg, Lipniunas, Leffler, Svanborg and Linse1999) found that the oligomeric and native α-la-containing fractions both bound to the cell surface but only the oligomeric fraction was transported to the nucleus. However, Permyakov (Reference Permyakov, Pershikova, Khokhlova, Uversky and Permyakov2004) observed that monomeric α-la without oleic acid could still bind histones, and theorized that the interaction of negatively charged α-la with basic histones stabilizes apo-α-la and destabilizes holo-α-la. According to this model, it may take several days for native α-la to have an effect, relying on passive diffusion to reach the nucleus (Permyakov et al. Reference Permyakov, Pershikova, Khokhlova, Uversky and Permyakov2004). Thus it is possible that the anti-proliferative effects of native α-la observed in the present study were due to the extended exposure time of cells to treatments compared with most previous studies. α-La was stable in cell culture media for at least 48 h (data not shown) and there have been reports of molecules of up to 40 kDa in size gaining access to cells in the luminal space of alveoli (Debnath et al. Reference Debnath, Mills, Collins, Reginato, Muthuswamy and Brugge2002). Therefore native α-la may gain access to MEC over a sufficient time interval to induce cell death through a similar mechanism to HAMLET. A delay in gaining entry to the cell may explain why native α-la was not as effective as BAMLET in reducing cell viability in the current study.
HAMLET induced caspase 3 activity in tumour cells, MEC and involuting mouse mammary gland in previous studies (Baltzer et al. Reference Baltzer, Svanborg and Jaggi2004; Hallgren et al. Reference Hallgren, Gustafsson, Irjala, Selivanova, Orrenius and Svanborg2006). However, there have not been any reports of caspase activation by native α-la. In the present study, activation of the effector caspases 3/7 were observed in mammospheres treated with BAMLET or native α-la as well as oleic acid alone. Caspase 9 activity, an upstream initiator caspase, was also induced, implying that the intrinsic cell death pathway plays a role in α-la-induced cell death (Jin & El-Deiry, Reference Jin and El-Deiry2005). This pathway involves mitochondria, which is consistent with previous findings on the mechanism of action of folding variants of α-la (Kohler et al. Reference Kohler, Gogvadze, Hakansson, Svanborg, Orrenius and Zhivotovsky2001; Svanborg et al. Reference Svanborg, Agerstam, Aronson, Bjerkvig, Duringer, Fischer, Gustafsson, Hallgren, Leijonhuvud, Linse, Mossberg, Nilsson, Pettersson and Svensson2003). In the mammary gland, apoptosis is involved in alveolar lumen formation and maintenance, and also during involution. Caspase 3 activity increases during involution (Motyl et al. Reference Motyl, Gajkowska, Zarzynska, Gajewska and Lamparska-Przybysz2006) and both caspases 3 and 9 are involved in apoptotic clearing of the lumen (Debnath et al. Reference Debnath, Mills, Collins, Reginato, Muthuswamy and Brugge2002). Given that the concentration of α-la in milk remains constant during lactation, one potential role of its apoptosis-inducing capacity may be in maintenance of the alveolar luminal space throughout lactation, as a balance between proliferation and apoptosis of MEC is required to maintain the lumen throughout lactation (Debnath et al. Reference Debnath, Mills, Collins, Reginato, Muthuswamy and Brugge2002). Alternatively, α-la may initiate the cascade of apoptotic events associated with involution that are then perpetuated by other regulatory molecules, since α-la mRNA abundance decreases 3 d after cessation of milking and is negligible by day 7 (Wilde et al. Reference Wilde, Knight and Flint1999).
While α-la can influence total milk volume through its role in lactose synthesis, which regulates the osmolarity of milk, it has not been reported to regulate milk protein expression; indeed, there are no previous reports of it influencing the level of casein expression. In the present study α-la affected the level of casein mRNA expression. Interestingly, β-casein mRNA expression was decreased and αs1-casein and κ-casein mRNA expression increased relative to a housekeeping gene, RPLP0, with increasing α-la concentration. Caseins are genetically linked and are considered to be coordinately regulated; however, there are known differences in the promoter regions of each gene (Alexander et al. Reference Alexander, Stewart, Mackinlay, Kapelinkskaya, Tkach and Gorodetsky1988; Rijnkels et al. Reference Rijnkels, Kooiman, Krimpenfort, de Boer and Pieper1995; Rijnkels et al. Reference Rijnkels, Kooiman, de Boer and Pieper1997). In several studies, changes in the level of one milk protein led to compensatory changes in other milk proteins to help maintain total protein concentration. In β-casein-deficient mice, production of other caseins and whey proteins increased to help compensate for the loss of β-casein (Kumar et al. Reference Kumar, Clarke, Hooper, Horne, Law, Leaver, Springbett, Stevenson and Simons1994) and casein micelles were still formed, albeit they were smaller than normal. However, in κ-casein-deficient mice, lactation failed and there was no change in expression of β-casein mRNA (Shekar et al. Reference Shekar, Goel, Rani, Sarathi, Alex, Singh and Kumar2006). From these results we suggest that there is some redundancy in the calcium-sensitive caseins whereas there is no substitute for κ-casein, and that there are differences in their regulation. Even within the calcium-sensitive caseins there is evidence of differences in regulation with another study in rat mammary cells showing that α- and β-caseins were up-regulated when grown on basement membrane whereas γ-casein was unaffected (Blum et al. Reference Blum, Zeigler and Wicha1989). In dairy cattle carrying a high expressing β-lactoglobulin variant, milk contained lower levels of caseins and α-la (Hill, Reference Hill1993). Overexpression of α-la in a transgenic sow was found to increase milk production (Wheeler, Reference Wheeler2003). However, this study did not look directly at the level of milk protein production, and the results may have been related to the role of α-la in lactose synthesis and its function in controlling milk volume. The effects of α-la on casein mRNA levels observed in the present study may be due to changes in the mRNA expression level and/or changes in mRNA stability. It is unclear how α-la alters casein mRNA levels or what the functional role of this might be in vivo, but its ability to affect expression levels of individual caseins may have potential applications in the dairy industry. Effects on the differential expression of caseins by α-la requires further investigation.
Bovine α-la can induce apoptotic cell death in BMEC and affect casein mRNA expression in vitro. Further work needs to be conducted to investigate the specificity of the proposed role of native α-la. Previous studies in our laboratory suggest that another bovine whey protein, lactoferrin, does not initiate the apoptotic caspase 3/7 cascade when incubated with BMEC (Riley et al. Reference Riley, Williamson, Wynn and Sheehy2008). The influence of native α-la on other cell types could also be investigated to ascertain whether the observed effect on cell viability is MEC specific or whether there may be a regulatory role for the protein in other tissues, presumably in the neonate. The role of α-la in bovine mammary gland also needs to be determined. α-La may play a role in maintaining the lumen during lactation and/or be involved in the early stages of involution. In vivo, the majority of MEC proliferation occurs during gestation, while the increase in milk production from parturition to peak lactation in dairy cattle is due to differentiation of MEC (Capuco et al. Reference Capuco, Wood, Baldwin, Mcleod and Paape2001). Following peak lactation, the decline in milk production is largely due to loss of secretory cells through apoptosis. Manipulation of α-la expression may extend lactation by reducing apoptosis of MEC; however, the effects of α-la on milk volume would also have to be considered. The ability of α-la to reduce BMEC viability and effect casein mRNA levels in vitro warrants further investigation of the autoregulatory effects of milk proteins on lactogenesis.
We thank Peter Thomson for advice on statistics and Kevin Nicholas for helpful discussions.







