Introduction
In recent years, obesity and type 2 diabetes mellitus have become increasingly prevalent in the USA. According to the National Center for Health Statistics Data brief released in 2017, 39.8% of adults are obese. Reference Hales, Carroll, Fryar and Ogden1 Poor cardiovascular health and insulin resistance are commonly associated with a high body mass index. Reference Hagg, Fall and Ploner2,Reference Reis, Allen and Gunderson3 Established obesity combined with a chronically poor diet and sedentary lifestyle increases an individual’s propensity to develop diabetes, leading to a 9.3% diabetes prevalence in the USA. 4 The financial consequences of obesity and diabetes are great; one in five healthcare dollars are spent caring for people with diabetes. 4
Obesity and its resulting metabolic disorders are largely due to behavioral patterns that include a persistent sedentary lifestyle and poor dietary choices. Besides the heavy financial burden precipitated by comorbidities associated with obesity, there is great potential impact regarding the health of future generations. Current research has shown that exposure to a suboptimal perinatal environment can predispose offspring to metabolic disorders later in life. Reference Dearden and Ozanne5 The Thrifty Phenotype Hypothesis proposed by Barker and Hales in 1990 suggests that poor nutrition in utero could promote type 2 diabetes mellitus and metabolic syndrome in adult life. Reference Hales and Barker6 Subsequent research suggests that maternal high-fat diet and obesity during pregnancy increase the susceptibility of offspring to metabolic disorders. Reference Samuelsson, Matthews and Argenton7
More recent work has shown this phenomenon to occur with both overnutrition and undernutrition in utero. Maternal overnutrition results in increased risk for diabetes later in the life of the offspring, while undernutrition during pregnancy leads to increased adiposity and renal issues in offspring at advanced ages. Reference Barker8,Reference Carolan-Olah, Duarte-Gardea and Lechuga9 Mouse and rat models of maternal diet-induced obesity have indicated that when the perinatal environment is altered by a hypercaloric maternal diet, offspring are more likely to develop a lifelong predisposition to obesity, hyperphagia, hypertension, and insulin resistance, even when on a normocaloric diet. Reference Samuelsson, Matthews and Argenton7,Reference Carolan-Olah, Duarte-Gardea and Lechuga9,Reference Joaquim, Coelho and Motta10
Previous research has focused on the impact of negative behaviors, such as high fat diet, smoking, and alcohol consumption during pregnancy, and the associated adverse offspring effects. Reference Ouellette, Rosett, Rosman and Weiner11,Reference Phelan12 Most research involving developmental origins of disease has documented the negative effects of such maternal environments on offspring health, while there is less information on positive interventional strategies that might mitigate negative impacts of unhealthy maternal behaviors on long-term offspring health. Reference Dior, Lawrence and Sitlani13,Reference Pereira, Moyce, Kereliuk and Dolinsky14 Exercise could be one such positive intervention strategy to promote improved glucose metabolism in offspring, decreased body fat, improved vascular health, and improved metabolic regulation and glucose metabolism. Reference Goedecke and Micklesfield15,Reference Mæhlum and Pruett16
In rodents, maternal exercise during pregnancy has improved glucose homeostasis and body composition in offspring. Reference Carter, Lewis and Wilkerson17,Reference Vega, Reyes-Castro, Bautista, Larrea, Nathanielsz and Zambrano18,Reference Stanford, Takahashi and So19 Voluntary perinatal exercise enhances long-term glucose homeostasis in offspring both in vivo (glucose and insulin tolerance testing) and ex vivo (soleus and adipose glucose uptake) Reference Carter, Lewis and Wilkerson17 and may even protect offspring from the effects of a maternal high-fat diet. Reference Vega, Reyes-Castro, Bautista, Larrea, Nathanielsz and Zambrano18 Our group found that maternal exercise improved glucose tolerance and insulin sensitivity in male and female offspring. Reference Carter, Lewis and Wilkerson17 Furthermore, offspring lean mass increased and fat mass decreased in male offspring born to exercising compared to sedentary mothers. Reference Carter, Lewis and Wilkerson17 We followed up this study in a rat model and found that maternal exercise improved insulin sensitivity during a hyperinsulinemic–euglycemic clamp test in female adult offspring. Reference Carter, Qi, De Cabo and Pearson20 We found significant differences in hepatic glucose production and glucose uptake in the skeletal muscle of the offspring born to exercised dams. Reference Carter, Qi, De Cabo and Pearson20
While maternal exercise has by-and-large been demonstrated to impart health advantages to the offspring, Reference Harris, Baer and Stanford21 it can be difficult to appreciate the translational relevance of many rodent studies involving exercise which relies on a free-choice exercise model. Rodents have a natural inclination to run great distances over long periods of time, unlike most humans. Reference Meijer and Robbers22 In addition, each mouse may run variable amounts from day to day, and the inter-mouse variability is high regarding both running speed and duration. The current study aims to understand the effects of maternal exercise on offspring health using a model of controlled maternal exercise. We have previously shown that this controlled exercise intervention does not negatively influence pregnancy outcomes. Reference Platt, Charnigo, Kincer, Dickens and Pearson23 This running wheel bed allows each dam to exercise at a specific speed for a set duration so all animals exercise the same amount each day. Because mice are nocturnal, their activity is highest at night, so we exposed the mice to exercise wheels during the dark phase. This study aims to analyze the effects of maternal exercise during pregnancy on offspring body composition and glucose tolerance using a mouse model of controlled exercise. HYPOTHESIS: Maternal running during pregnancy will improve long-term glucose homeostasis and body composition in offspring.
Methods
General husbandry
All animal experiments were carried out according to an approved Institutional Animal Care and Use Committee protocol at the University of Kentucky. The University of Kentucky Division of Laboratory Animal Resources is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC). Upon arrival from the vendor (Taconic Farms, Germantown, NY), the 3–4-month-old primiparous female mice were single housed in individually ventilated cages (ACE, Allentown, NJ) with Sani-Chip bedding (Harlan-Teklad, Madison, WI) and maintained on a chow diet (FormuLab Diet 5008, LabDiet, St. Louis, MO) for the duration of the study. Females were maintained on a 10:14 h (dark:light) reverse light cycle at temperatures between 21° and 24°C. Quarterly testing was completed on sentinel mice from related racks to verify the colony as being disease, virus and parasite-free.
Dams
Female Institute of Cancer Research (ICR/CD1) animals were separated into three cohorts: sedentary home cage, sedentary wheel, and exercise (n = 40 per group) such that body weight was approximately balanced between the groups (p > 0.9). The controlled exercise walking wheel apparatus (Mouse Forced Exercise–Walking Wheel System 80800A, Lafayette Instrument, Lafayette, IN) is described in detail in previous work. Reference Platt, Charnigo, Kincer, Dickens and Pearson23 Exercise and sedentary wheel group mice were exposed to the running wheels for their respective treatment only during their active phase (dark photoperiod, 7:30 am–5:30 pm) within 3 h of lights-off. Exercising the animals during the dark phase serves to follow the metabolic cycle of a mouse, which peaks during the active (dark) period and declines during the rest (light) period. The addition of a “sedentary home cage group” was meant to offer an additional control to ensure that study design did not induce stress in dams during pregnancy. The design for the maternal portion of the study is outlined in Fig. 1a.

Fig. 1. Study design. The study design is diagrammed for both the maternal portion of the study (A) as well as the offspring portion of the study (B). After arrival to the University, females were singly housed for a brief acclimation period. The light cycle was then switched such that the dark phase was during the daytime (time “−1” week in the schematic). Animals acclimated to this new light/dark cycle for 1 week, at which point exercise training began (time “0”) and persisted until the females delivered. During the training week, exercise speed was incrementally increased. Females were housed with males for 1 night of mating. As pregnant female mice naturally decrease their running habit as pregnancy progresses, the exercise speed was decreased stepwise beginning 11 d after mating (week 5.5 in the schematic). Offspring were weaned in week 10 (postnatal day 21) onto standard chow diet and subjected to glucose tolerance testing as well as body composition analysis as indicated (B). Both male and female offspring were analyzed across the course of the study.
Females in the sedentary and exercise groups were removed from their home cage daily and placed into the controlled exercise wheel system. The wheel was activated on day 1 at a slow speed (3 m/min for 30 min; 3.5 m/min for 30 min) and the speed was incrementally increased in this manner on each subsequent day (4–4.5, 5–5.5, 7–7.5, 8–8.5, 9–9.5 m/min) such that the experimental speed of 10 m/min was achieved on day 7. Each of the following days until 11 d after mating, 7 d/week, the wheel was activated at 10 m/min with the exception of the day after placing males in the female cages and the speed was decreased as the dams approached delivery as described below. The “sedentary wheel group” of females was removed from their home cage on a daily basis and placed into the wheel, but the motor was not activated. For the “sedentary home cage” group, the females remained in the cage throughout the duration of the study, and their food and water was removed for 1 h daily while the sedentary wheel group and the exercise group were in the wheel apparatus. Animals were weighed daily and mated after 4 weeks of intervention (1 training week plus 3 weeks at 10 m/min), subsequent to exposure to male bedding to stimulate the estrous cycle. Males remained housed with females for one night, and no exercise or wheel exposure occurred the day after placing the males in the cages. On the 11th day postmating (week 5.5 in Fig. 1a), the running speed was decreased to 9 m/min for 1 h and then decreased again by 1 m/min every subsequent day until reaching 5 m/min, at which point this speed was maintained until birth. On the day that females gave birth, the dams with pups were not removed from their cage or disturbed in any way. The exercise portion of the study concluded upon parturition. On postnatal day (PND) 2 and each subsequent day, lactating females were weighed.
Offspring
Pups were weighed and culled to 8/l on PND 2. If litters were born with less than eight pups, then pups were removed from litters of more than eight (within the same treatment group) and fostered into the litter with fewer pups. Cross-fostered pups were killed at weaning and not included in experimental cohorts. Offspring were also weighed on PNDs 7, 14, and 21, and they were weaned at 21 d of age. Weaned female offspring were housed five mice per cage and weaned male offspring were single-housed to eliminate fighting concerns. Offspring were given access to a quantified amount of standard chow diet such that they could eat ad libitum (Teklad Global 18% Protein Rodent Diet #2018) and kept on a standard 14:10 light:dark cycle for the duration of the study. Body weight and food remaining were taken weekly and diet was replaced biweekly. These data include all surviving offspring, including siblings of the same sex. The offspring portion of the study is outlined in Fig. 1b.
Glucose tolerance testing
Offspring were aged to 6 weeks and then subjected to oral glucose tolerance testing. One male offspring and one female offspring per dam were analyzed (i.e. siblings of the same sex were not included in the same experiment). Animals were fasted in a clean cage with no access to food. Fast times were 3 h (weeks 6, 24, 52), 4 h (week 20), or 6 h (weeks 8, 12). After fasting, a sharp blade was used to nick the tail and blood glucose was recorded as time zero using an Ascencia Breeze2 Glucometer (Bayer HealthCare, Tarrytown, NY). Subsequently, the animal received the bolus of USP-certified dextrose (Bimeda, Oakbrook Terrace, IL – 2 g/kg body weight, oral dosing), a timer was activated, and blood glucose was recorded at 15, 30, 60, and 120 min after glucose delivery.
Body composition
At 10 weeks of age, offspring body composition was analyzed by using EchoMRITM (Echo Systems, Inc., Houston, TX). This system is designed to analyze fat mass, lean mass, and water content of a conscious animal in only ∼2 min. Some parts of the animal are unaccounted for bone, fur, and nails. The animal is placed into a long cylindrical tube with a plunger that is gently compressed to restrain the animal. The tube is inserted into the machine which then provides the animal’s body composition. After testing, the animal was immediately returned to its home cage. EchoMRI was repeated at 24 and 52 weeks of age.
Statistics and sample size considerations
Data were analyzed using SAS version 9.3 or later (SAS Institute, Cary, NC) and SigmaPlot version 13.0 (SigmaPlot Software, San Jose, CA). For all analyses, significance was defined by a p-value <0.05. Maternal body weight was analyzed by linear mixed modeling (Fig. 2a). Chi-squared test was used to analyze the number of pregnancies per group (Fig. 2b). The number of pups per litter and body weight at PND2 were analyzed using one-way analysis of variance (ANOVA) after natural log transformation to reduce non-normality of the data (Fig. 2c, 2d). One-way ANOVA was used to analyze the offspring body weights at 24 and 52 weeks of age (Fig. 3). Area under the curve (AUC; “area below curves” function in SigmaPlot) was used to summarize glucose tolerance test results at the individual time points. Subsequently, linear mixed modeling was used to analyze the AUC values across multiple time points at various ages (Fig. 4a, 4b). Lean-to-fat mass ratio was calculated at each of the time points, and subsequently natural log was transformed to reduce non-normality of the data. The trajectory for each group was analyzed using linear mixed modeling in a repeated measures fashion (essentially calculating the AUC of the trajectory) (Fig. 4c, 4d). When applicable, Bonferroni-adjusted post-hoc testing was applied to identify between-group differences.
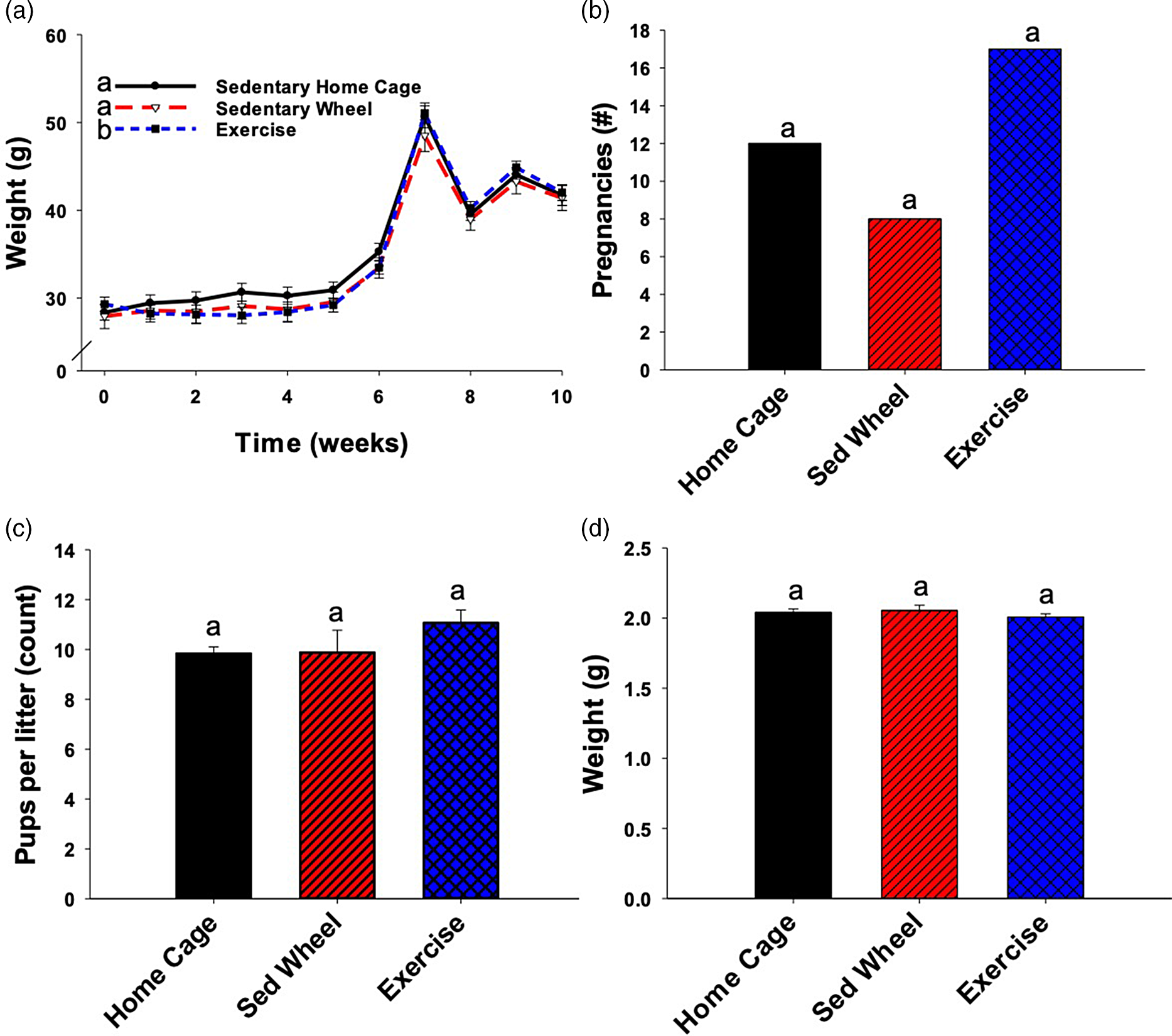
Fig. 2. Maternal body weight, number of pregnancies, pups per litter, and pup weight at postnatal day (PND) 2. The body weight of the female mice was taken upon arrival and the animals were split into three groups (n = 40 per group) such that body weight was approximately balanced between groups. Animals were weighed weekly until mating, then daily thereafter (A). Only the data for the animals that delivered and reared offspring to weaning is presented (B) (n = 12 for sedentary home cage, n = 8 for sedentary wheel, and n = 16 for exercise). The body weight was significantly lower for the exercise group when compared with both the sedentary home cage group, as well as the sedentary wheel group. There was no significant difference in the number of pregnancies per group due to the interventions. On PND2, the pups were counted (n = 95 in home cage group, n = 60 in sedentary group, n = 123 in exercise group) and culled (or fostered) to eight per litter. The number of pups born per litter was not significantly altered due to either of the maternal interventions (C). The pups were weighed on PND2 and there were no significant differences between groups because of the maternal exercise or sedentary wheel interventions (D). Groups not sharing a common letter ("a" or "b") in the legend of the graph are significantly different. Error bars indicate S.E.M.

Fig. 3. Offspring body weight. Body weight was taken weekly but the data at 24 (A, B) and 52 (C, D) weeks of age are shown. There were no significant differences in male offspring body weight as a consequence of either the maternal exercise intervention or the maternal sedentary wheel intervention at either time point (A, C). There were also no significant differences noted among the female offspring at either time point (B, D) (n = 28–70 per sex per group). Error bars indicate S.E.M.

Fig. 4. Offspring glucose tolerance and body composition. At several offspring time points (as indicated), animals were fasted prior to recording fasting blood glucose. Animals were dosed with an oral bolus of dextrose, followed by blood glucose readings at 15, 30, 60, and 120 min after bolus delivery (n = 8 per group at all time points for both males and females). Only one pup per sex per litter was analyzed. These data were summarized as area under the curve (AUC) and presented longitudinally across the length of the study (A, B). For male offspring (A), both maternal sedentary wheel exposure and the maternal exercise intervention resulted in significantly increased AUC over the course of the experiment. For females, only the offspring born to sedentary wheel exposure dams had significantly increased AUC compared to sedentary home cage offspring (B). Offspring body composition data were collected at three time points (C, D) and summarized as lean-to-fat mass ratio. Neither male (C) nor female (D) offspring had any significant change in lean-to-fat mass ratio as a consequence of the maternal interventions. n = 23–35 per group at each time point. Groups not sharing a common letter ("a" or "b") in the legend of the graph are significantly different. Error bars indicate S.E.M.
The following remarks on power and sample size refer to the analyses of offspring glucose tolerance, for which results appear in Fig. 4a, 4b. These analyses are central to this study and also feature a smaller sample size than other analyses described in this article. With a three-group design, and under typical assumptions for the parametric statistical analysis of a quantitative outcome, group sizes of 8 yield 80% power to detect a pairwise difference between two groups at a Bonferroni-adjusted 5% significance level, if the effect size (absolute value of the difference between group means, divided by within-group standard deviation) is 1.73. This is a very large effect size for a single outcome. However, the analyses of offspring glucose tolerance entailed repeated measurements on six occasions, which spanned nearly a full year. Thus, if between-group differences in glucose tolerance appeared early and were consistently sustained over the time course, the differences might be detectable even if they were less pronounced than might be suggested by the aforementioned effect size. Indeed, significant differences were found in the analyses of offspring glucose tolerance, although not between the maternal exercise and sedentary wheel groups. While the sample sizes were small, the lack of a significant difference between these two groups may reflect actual similarity in offspring glucose tolerance. Similarity is suggested by the fact that these two groupsʼ means were not consistently ordered across time points; this is evident from Fig. 4a, 4b by noting that the red bars are taller than the blue bars at some time points and vice versa. This pattern does not provide a very strong signal that significant differences between the two groups would have been exposed, if only the sample sizes had been slightly larger.
Supplemental statistical analysis
We also examined the joint contribution of sex and intervention group to selected outcomes. For offspring body weight, two-way ANOVA was performed with intervention group (sedentary wheel, sedentary home cage, or exercise) and sex as the two factors, at both 24 and 52 weeks of age (Fig. 3). For offspring glucose tolerance and (log-transformed) lean-to-fat mass ratio, a linear mixed model was fit in which the outcome’s trajectory over time was allowed to depend on both sex and intervention group (Fig. 4). Bonferroni-adjusted pairwise comparisons were completed and needed to clarify the nature of significant differences.
Results
Overall, maternal body weight was steady over the time course of the exercise intervention prior to pregnancy (Fig. 2a). After conception, (weeks 4–7 in Fig. 2a), weight rose steadily until delivery. Dam weight was impacted by the wheel intervention. While the sedentary wheel group did not have significantly different body weight compared to the sedentary home cage group (p = 0.146), the exercise group body weight was significantly different from both the sedentary home cage group (p < 0.001) and the sedentary wheel group (p < 0.001). The mating window was only one night in this study so not every animal delivered a litter. The number of litters born per group (p = 0.092) and weight of the pups at PND2 (p = 0.669) were not significantly different between groups (Fig. 2b, 2d, respectively). There was an overall trend toward differences in the number of pups born per litter between the groups (p = 0.046) (Fig. 2c), but upon Bonferroni-adjusted post-hoc testing, the pairwise comparisons showed no significant differences. The exercise group did deliver more than twice as many litters as the sedentary wheel group (17 vs 8), though one litter in the exercise group did not survive.
The body weights of the offspring were analyzed at the 24 and 52-week time points (Fig. 3). By one-way ANOVA, there was no significant difference in male or female offspring body weight at 24 weeks (Fig. 3a, 3b, p = 0.103 and p = 0.086, respectively). The same lack of significant difference was still apparent at 52 weeks for both male and female offspring (Fig. 3c, 3d, p = 0.891 and p = 0.238, respectively).
Figure 4 displays the AUC for the glucose tolerance tests in the offspring at multiple ages collectively. The AUCs over the course of 52 weeks in the offspring are presented for both males and females (Fig. 4a, 4b). There were significant differences between the three groups (p = 0.0084 and 0.026 for males and females, respectively) when analyzing the measures over the first year of life (essentially, taking an AUC of the AUC). Male offspring born to both sedentary wheel dams and exercise dams had significantly increased AUC over the course of the experiment when compared with sedentary home cage offspring (p = 0.0088 and p = 0.0057, respectively). Interestingly, only the female offspring of sedentary wheel dams had significantly increased AUC compared to sedentary home cage offspring (p = 0.0086) (Fig. 4b). While trending toward significance, the female offspring born to exercised dams did not have significantly impaired glucose disposal compared to sedentary home cage offspring (p = 0.067). Neither the male nor female offspring of sedentary wheel dams had an AUC significantly different from the offspring born to the running dams (p = 0.873, p = 0.346, respectively).
Lean-to-fat mass ratio was analyzed in a similar manner over the course of the study. Neither male nor female offspring exhibited a significant difference in lean-to-fat mass ratio when comparing the three groups (p = 0.891, p = 0.075, respectively) (Fig. 4c, 4d).
Results specific to supplemental statistical analysis
Regarding offspring body weight at 24 weeks (Fig. 3a, 3b), there was no significant interaction effect between intervention group and sex (p = 0.851). There was a highly significant main effect for sex (p < 0.001), as the male mice were heavier than females. There was also a significant main effect for intervention group (p = 0.020), triggering Bonferroni-adjusted pairwise comparisons. Exercise mice were heavier than sedentary home cage mice (p = 0.028), but there were no significant differences neither between sedentary wheel and exercise mice (p = 0.144) nor between sedentary wheel and sedentary home cage mice (p = 1.000). There were no significant main or interaction effects at 52 weeks (all p > 0.3) (Fig. 4c, 4d).
Regarding offspring glucose tolerance test results (Fig. 4a, 4b), there was no significant interaction effect between intervention group and sex (p = 0.178), but there were highly significant main effects for both intervention group (p < 0.001) and sex (p < 0.001). Males had greater AUC than females, and sedentary home cage animals had lesser AUC than exercise animals (Bonferroni-adjusted p = 0.001) and sedentary wheel animals (Bonferroni-adjusted p < 0.001); exercise animals were not distinguished from sedentary wheel animals (Bonferroni-adjusted p = 1.000). For offspring lean-to-fat mass ratio (Fig. 4c, 4d), there was no significant interaction neither between sex and intervention group (p = 0.155) nor any significant main effect for intervention group (p = 0.283). There was, however, a highly significant main effect for sex (p < 0.001), with males having a greater lean-to-fat mass ratio than females.
Discussion
Exercise during pregnancy has been shown to offer multiple advantages for the offspring throughout their lifespans including improved glucose metabolism, favorable changes in body composition, and improved hepatic steatosis. Reference Carter, Lewis and Wilkerson17,Reference Vega, Reyes-Castro, Bautista, Larrea, Nathanielsz and Zambrano18,Reference Sheldon, Nicole Blaize and Fletcher24 However, many rodent studies exploring the offspring benefit of maternal exercise utilize a voluntary exercise model. Reference Carter, Lewis and Wilkerson17,Reference Stanford, Takahashi and So19,Reference Carter, Qi, De Cabo and Pearson20,Reference Sheldon, Nicole Blaize and Fletcher24–Reference Stanford, Lee, Getchell, So, Hirshman and Goodyear29 There are some potential drawbacks to this experimental model that are worth considering including the inter-mouse variability in speed and distance ran as well as the long distances ran overall. This has prompted exploration of nonvoluntary exercise interventions to control the dose of the physical activity in dams. Reference Fidalgo, Falcao-Tebas and Bento-Santos30 The controlled exercise model described herein offers several potential advantages to explore the offspring consequences of very specific, prescribed amounts of activity for the dam.
Voluntary exercise in rodent dams has been shown to improve a number of physiologic parameters in offspring, including improved glucose tolerance, decreased cancer incidence, and decreased Alzheimer’s pathology in a transgenic mouse model. Reference Carter, Lewis and Wilkerson17,Reference Camarillo, Clah and Zheng31,Reference Herring, Donath and Yarmolenko32 These outcomes are very encouraging and lend further support to the advantages of maintaining an active lifestyle, especially during pregnancy, to impart long-term advantages to offspring. The current recommendation of the American College of Obstetrics and Gynecology suggests women without complications complete 30 min of moderate-intensity physical activity most days of the week. 33 The goal of our study was to pursue a controlled exercise intervention with more distinct translational relevance because of the shorter distances run by the female mice (600 m or less per day). Regardless, it is difficult to correlate rodent exercise bouts to comparable amounts of physical activity for humans, and future studies should carefully consider ways to translate rodent exercise to humans.
Our past work using the controlled exercise wheel intervention during pregnancy employed two groups: the exercise group, and a control group that stood in the idle wheel apparatus for the same duration that the intervention group was exercising. Reference Platt, Charnigo, Kincer, Dickens and Pearson23 While this model did not reveal maternal stress due to the intervention (i.e. no change in pups per litter, number of pregnancies, number successfully reared to weaning), it is possible that the wheel exposure in general has some impact on the dams. In this study, the controlled exercise intervention was completed in parallel with two control groups—a sedentary group that was not removed from the home cage and a sedentary wheel group that was placed into an inactive exercise wheel apparatus. The sedentary home cage group allowed for determining whether or not the sedentary wheel exposure itself resulted in any perturbation of the maternal outcomes, which it did not. For example, there was no significant change in number of mice to deliver litters, the number of pups per litter, or the weight of the pups at PND2. However, both the sedentary wheel male and female offspring differed from the sedentary home cage offspring in their glucose tolerance over their first year of life, suggesting that there was some sort of developmental programming caused by the removal of the dam from her home cage. Perhaps time spent in the inactivated wheel is stressful. Moreover, glucose tolerance was impaired in the male offspring of the exercise group compared to the home cage controls, and there was some suggestion of the same for female offspring (via the marginal p-value in the sex-specific analysis as well as results from the supplemental analysis). Thus, the stress of removing the dams from the home cage may cause unfavorable changes in glucose tolerance. This is a critical consideration for future controlled exercise experiments, though further investigation is not within the scope of the current project. No significant differences existed in the body composition in the offspring from any of the groups over the course of the study.
Several studies report that interruption of the light/dark cycle is stressful and has the potential to be metabolically disruptive. Reference Bailey, Udoh and Young34 Indeed, maternal sleep deprivation negatively influences offspring cardiovascular health, as well as learning and memory. Reference Lima, Rodrigues and Bergamaschi35,Reference Peng, Wang and Tan36 The current study was designed such that the dams exercised during the dark phase of the light:dark cycle. This was in an attempt to minimize the potential stress that may be generated by disruption of the light cycle, although previous work using the controlled exercise model during pregnancy did not indicate any evidence of the intervention being markedly stressful. Reference Platt, Charnigo, Kincer, Dickens and Pearson23 Future studies of a similar nature should weigh the advantages of employing a reversed light cycle.
The function of an oral glucose tolerance test is to assess glucose tolerance (or impaired glucose tolerance), and AUC was used to summarize the data over the time course of the experiment. Based on our results, the exercise group did not show the anticipated improvement in glucose tolerance compared to either the sedentary home cage group or the sedentary wheel group. Despite these results, numerous studies have shown positive long-term effects of exercise on offspring glucose metabolism. Reference Carter, Lewis and Wilkerson17,Reference Vega, Reyes-Castro, Bautista, Larrea, Nathanielsz and Zambrano18,Reference Carter, Qi, De Cabo and Pearson20 Hyperinsulinemic–euglycemic clamping, a more sensitive measure of glucose handling, may be able to detect more subtle differences in offspring glucose disposal. Reference Kim37 Future studies should consider additional more sensitive measurements. Alternatively, future work could consider different mechanistic approaches to determining whether maternal controlled exercise is protective to offspring in other ways. For example, recent work suggests that maternal voluntary exercise in mice protects male offspring from nonalcoholic fatty liver disease. Reference Bae-Gartz, Kasper and Grossmann38 Another recent study found histological changes in offspring beta cell morphology as a result of maternal voluntary exercise, Reference Zheng, Alves-Wagner, Stanford and Prince39 and future studies could look at offspring pancreatic histology using controlled exercise models.
Results from the EchoMRI body composition analysis show no significant differences in lean-to-fat mass ratio between the offspring of the three groups. The same is true for both male and female offspring. Other maternal exercise studies, including voluntary exercise studies, have reported a lack of difference in offspring outcomes. Reference Platt, Charnigo, Kincer, Dickens and Pearson23,Reference Kelly, Hua, Wallace, Wells, Nehrenberg and Pomp27 Using the same stock of mice in our voluntary exercise study, we detected significant improvements in only the male offspring by 1 year of age, Reference Carter, Lewis and Wilkerson17 though the females showed a strong trend toward improvement at ∼16 months of age. Reference Carter, Tamashiro, Pearson, Ho and Domann40 Importantly, the maternal intervention applied herein was mild in terms of speed and duration of exercise compared to free-choice running studies, and a longer duration or more intense exercise might have greater impact on offspring glucose tolerance and body composition. Future studies using more aggressive exercise stimuli are warranted.
A burgeoning field of research has investigated whether maternal exercise during pregnancy can help mitigate the effects of adverse maternal environments (such as a high-fat diet) in mice. Both Raipuria et al. and Stanford et al. analyzed metabolic outcomes in offspring born to exercising dams exposed to high-fat or normocaloric (chow) diets. Reference Stanford, Takahashi and So19,Reference Raipuria, Bahari and Morris26 Raipuria et al. found that maternal exercise significantly reduced insulin concentrations in male offspring of dams consuming either chow or high-fat diet as compared to sedentary groups, and reduced glucose in males in the high-fat exercise group as compared to the high-fat sedentary group. One future approach could therefore combine maternal high-fat diet consumption with the controlled exercise intervention to elucidate offspring outcomes. Other studies demonstrate that maternal exercise protects offspring from the consequences of maternal high-fat diet consumption. Reference Stanford, Lee, Getchell, So, Hirshman and Goodyear29 A third study design indicates that maternal exercise may be protective for offspring consuming a high fat diet. For example, Wasinski et al. subjected C57BL/6 dams to swim exercise, which protected adult male offspring from deleterious effect when fed a high-fat diet. Reference Wasinski, Bacurau and Estrela41 Perhaps if the offspring in this study had been fed a high-fat diet, protective effects of the maternal intervention would have been made apparent. Future studies could therefore use the controlled exercise model in combination with high-fat diet feeding.
Importantly, our controlled exercise mice ran much less (≤600 m/d) than mice voluntarily exercising on running wheels in their home cage (≥5000 m/d) Reference Carter, Lewis and Wilkerson17 . Thus, the lack of differences could be because the running distance, speed, and time (1 h/d) were not long or fast enough to provide benefits. This low dose of exercise is one of the possible limitations of a controlled exercise model such as the one employed in this study. However, future studies could use additional doses of exercise (time, speed, distance) to determine whether offspring benefits are observed. It could be especially interesting to design a dose–response study to determine not only the minimum amount of exercise needed to impart protective benefits to the offspring, but also to address whether greater time and/or intensity of exercise leads to enhanced protection.
In addition, this study used a low dose of exercise which offered translational relevance: less than one-third of pregnant women meet physical activity guidelines as it is, Reference Hesketh and Evenson42 so expecting that they will run 5 km daily—as mice tend to do when given access to a voluntary exercise wheel—is unrealistic. Other possible limitations of such a forced/controlled exercise model include the aforementioned potential for stress to the animal (being removed from her home cage daily), or perhaps lack of animal choice (regarding dose of exercise). Future studies will then need to continue to test whether exercise is a useful, positive lifestyle intervention during pregnancy in humans.
Acknowledgements
We thank two anonymous peer reviewers for their insights.
Financial Support
This study and core services for this study were supported by US NIH grant (National Center for Research Resources, 5P20 RR021954-05) as well as The National Institute of Diabetes and Digestive and Kidney Diseases, R01 DK090460, and the National Institute of Environmental Health Sciences, P42 ES007380. K.M.P. was supported by an NIH training grant (DK07778).
Conflicts of Interest
None.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guides on the care and use of laboratory animals, herein mice, and all procedures have been approved by the University of Kentucky Institutional Animal Care and Use Committee (IACUC).
Authors’ Contributions
K.M.P. and K.J.P. designed research. K.M.P., S.Y.N.T., J.P., L.J.R., and K.J.P. collected or analyzed data. R.J.C. and K.M.P. performed statistical analyses. K.M.P., J.P., R.J.C. S.Y.N.T., L.J.R., and K.J.P. wrote and edited the manuscript. K.J.P. is the guarantor and takes responsibility for the contents of this article. The authors are responsible for the scientific content of this paper and all have read and approved the final version of the manuscript.






