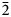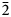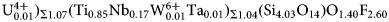Introduction
Rinkite-(Y) is a new mineral of the rinkite group in the seidozerite supergroup (Sokolova and Cámara, Reference Sokolova and Cámara2017). It is an Y-analogue of rinkite-(Ce), ideally Na2Ca4REETi(Si2O7)2OF3, where Ce is the dominant rare-earth element (Table 1). Rinkite-(Y) is also isostructural with nacareniobsite-(Ce) and mosandrite-(Ce) (Table 1). On formation of the seidozerite supergroup of TS-block minerals, Sokolova and Cámara (Reference Sokolova and Cámara2017) changed the name rinkite to rinkite-(Ce). Hence, elsewhere in the paper we will refer to rinkite-(Ce) instead of just rinkite. The new mineral and its name have been approved by the Commission on New Minerals, Nomenclature and Classification, International Mineralogical Association (IMA2017-043, Pautov et al. Reference Pautov, Agakhanov, Karpenko, Uvarova, Sokolova and Hawthorne2017). The holotype material is deposited in the collection of the Fersman Mineralogical Museum, Moscow, Russia, catalogue number 5043/1. The current paper reports the description and refinement of the crystal structure of rinkite-(Y).
Table 1. The rinkite group of the seidozerite supergroup of TS-block minerals*, Ti (+ Nb + Zr) = 1 apfu.

* Structure types, B (basic), and structural formula are from Sokolova and Cámara (Reference Sokolova and Cámara2013) and Sokolova and Cámara (Reference Sokolova and Cámara2017), respectively; formulae are per (Si2O7)2, except per (Si2O7)4 for rosenbuschite.
MO and MH = cations of the O and H sheets, AP = cations at the peripheral (P) sites, ![]() ${\rm X}_4^{\rm O} $ = anions of the O sheet not bonded to Si:
${\rm X}_4^{\rm O} $ = anions of the O sheet not bonded to Si: ![]() ${\rm X}_{\rm M}^{\rm O} $ = anions at the common vertices of 3MO and MH polyhedra;
${\rm X}_{\rm M}^{\rm O} $ = anions at the common vertices of 3MO and MH polyhedra; ![]() ${\rm X}_{\rm A}^{\rm O} $ = anions at the common vertices of 3MO and AP polyhedra; atoms labelling is in accord with Sokolova (Reference Sokolova2006); composition of the M O1 site is shown in red.
${\rm X}_{\rm A}^{\rm O} $ = anions at the common vertices of 3MO and AP polyhedra; atoms labelling is in accord with Sokolova (Reference Sokolova2006); composition of the M O1 site is shown in red.
References (discovery of a mineral, the most recent structure work): (1) Lorenzen (Reference Lorenzen1884); (2) Sample 1991C, Cámara et al. (Reference Cámara, Sokolova and Hawthorne2011); (3) this work; (4) Petersen et al. (Reference Petersen, Rønsbo and Leonardsen1989); (5) Sokolova and Hawthorne (Reference Sokolova and Hawthorne2008); (6) Brögger (Reference Brögger1890); (7) Sokolova and Hawthorne (Reference Sokolova and Hawthorne2013); (8) Semenov et al. (Reference Semenov, Kazakova and Simonov1958); (9) Christiansen et al. (Reference Christiansen, Johnsen and Makovicky2003a); (10) Bellezza et al. (Reference Bellezza, Franzini, Larsen, Merlino and Perchiazzi2004); (11) Sahama and Hytönen (Reference Sahama and Hytönen1957); (12) Blumrich (Reference Blumrich1893); (13) Lyalina et al. (Reference Lyalina, Zolotarev, Selivanova, Savchenko, Zozulya, Krivovichev and Mikhailova2015); (14) Cámara et al. (Reference Cámara, Sokolova, Abdu, Hawthorne, Charrier, Dorcet and Carpentier2017); (15) Lyalina et al. (Reference Lyalina, Zolotarev, Selivanova, Savchenko, Krivovichev, Mikhailova, Kadyrova and Zozulya2016); (16) Christiansen et al. (Reference Christiansen, Gault, Grice and Johnsen2003b); and (17) Brögger (Reference Brögger1887).
Review of the relevant literature
Rinkite-(Ce) was originally described by Lorenzen (Reference Lorenzen1884). Slepnev (Reference Slepnev1957) considered the mineralogy and crystal chemistry of rinkite-(Ce) and related minerals. Semenov (Reference Semenov1963) and Mineev (Reference Mineev1969) considered variations in the distribution of REE in some rinkite-group minerals and showed that Ce dominance is characteristic for minerals of this group. Semenov and Dusmatov (Reference Semenov and Dusmatov1975) described Y-rich rinkite-(Ce) (under the name “Y-rinkolite”) from the Darai-Pioz massif. Based on chromatography analysis of “Y-rinkolite”, they reported the following composition for the REE (wt.% from ΣREE = 100): La 9.2, Ce 33.0, Pr 6.9, Nd 23.6, Sm 3.5, Gd 5.0, Dy 2.50, Ho 0.70 and Y 15.6. Chakrabarty et al. (Reference Chakrabarty, Mitchell, Ren, Sen and Pluseth2013) reported a Nd-rich rinkite from the Sushina Hill Complex, India, with the following composition for the rare-earth elements (wt.% from ΣREE = 100): La 6.8, Ce 12.1, Pr 5.8, Nd 39.6, Sm 7.4, Gd 9.0, Dy 9.0 and Y 10.3. The first determination of the crystal structure of the type rinkite-(Ce) from Kangerdluarssuk, Greenland, was done by Kheirov et al. (Reference Kheirov, Mamedov and Belov1963) (space group P ![]() $\bar{1}$); then followed structure refinements in space group P21 by Tê-yü et al. (Reference Tê-yü, Simonov and Belov1965), Simonov and Belov (Reference Simonov and Belov1968) and Rastsvetaeva et al. (Reference Rastsvetaeva, Borutskii and Shlyukova1991). Galli and Alberti (Reference Galli and Alberti1971) refined rinkite-(Ce) from Kangerdluarssuk, Greenland, in space group P21/c; the refinement of Sokolova and Cámara (Reference Sokolova and Cámara2008) was in good agreement with the work of Galli and Alberti (Reference Galli and Alberti1971). The most recent work on rinkite-(Ce) was done by Cámara et al. (Reference Cámara, Sokolova and Hawthorne2011). They refined the crystal structures of five rinkite-(Ce) crystals from three alkaline massifs (Ilímaussaq, Greenland; Khibiny, Kola Peninsula, Russia and Mt. St.-Hilaire, Canada) in space group P21/c as two components related by the TWIN matrix (
$\bar{1}$); then followed structure refinements in space group P21 by Tê-yü et al. (Reference Tê-yü, Simonov and Belov1965), Simonov and Belov (Reference Simonov and Belov1968) and Rastsvetaeva et al. (Reference Rastsvetaeva, Borutskii and Shlyukova1991). Galli and Alberti (Reference Galli and Alberti1971) refined rinkite-(Ce) from Kangerdluarssuk, Greenland, in space group P21/c; the refinement of Sokolova and Cámara (Reference Sokolova and Cámara2008) was in good agreement with the work of Galli and Alberti (Reference Galli and Alberti1971). The most recent work on rinkite-(Ce) was done by Cámara et al. (Reference Cámara, Sokolova and Hawthorne2011). They refined the crystal structures of five rinkite-(Ce) crystals from three alkaline massifs (Ilímaussaq, Greenland; Khibiny, Kola Peninsula, Russia and Mt. St.-Hilaire, Canada) in space group P21/c as two components related by the TWIN matrix (![]() $\bar{1}$ 0 0/ 0
$\bar{1}$ 0 0/ 0 ![]() $\bar{1}$ 0/ 1 0 1); the crystals were analysed with an electron microprobe subsequent to collection of the X-ray data. Transmission electron microscopy confirmed the presence of pseudomerohedral twinning in sample 2909 of rinkite-(Ce) (Cámara et al., Reference Cámara, Sokolova and Hawthorne2011). Cámara et al. (Reference Cámara, Sokolova and Hawthorne2011) concluded that the lower symmetry described by the space group P21 was not justified for rinkite-(Ce). They said that the pseudomerohedral twins correspond to two different cell choices for space group No. 14. Pseudomerohedral twinning results in the apparent loss of the glide plane and apparent reduction of symmetry to space group P21. Rønsbo et al. (Reference Rønsbo, Sørensen, Roda-Robles, Fontan and Monchoux2014) reported on the rinkite-(Ce)-nacareniobsite-(Ce) solid-solution series from the Ilímaussaq alkaline complex.
$\bar{1}$ 0/ 1 0 1); the crystals were analysed with an electron microprobe subsequent to collection of the X-ray data. Transmission electron microscopy confirmed the presence of pseudomerohedral twinning in sample 2909 of rinkite-(Ce) (Cámara et al., Reference Cámara, Sokolova and Hawthorne2011). Cámara et al. (Reference Cámara, Sokolova and Hawthorne2011) concluded that the lower symmetry described by the space group P21 was not justified for rinkite-(Ce). They said that the pseudomerohedral twins correspond to two different cell choices for space group No. 14. Pseudomerohedral twinning results in the apparent loss of the glide plane and apparent reduction of symmetry to space group P21. Rønsbo et al. (Reference Rønsbo, Sørensen, Roda-Robles, Fontan and Monchoux2014) reported on the rinkite-(Ce)-nacareniobsite-(Ce) solid-solution series from the Ilímaussaq alkaline complex.
Occurrence
Rinkite-(Y) occurs in the Darai-Pioz alkaline massif in the upper reaches of the Darai-Pioz River, Tajikistan [the Rasht (formerly Garm) district]. The area is near the junction of the Turkestansky, Zeravshansky and Alaisky ridges (39°30′N, 70°40′E).
The multiphase Darai-Pioz massif belongs to the Upper Paleozoic Alaysky (Matchaisky) intrusive complex. The area of outcrop of the massif is ~16 km2. Most of the massif rocks are covered by moraine or glaciers, and/or are difficult to access. In the north, the massif intrudes Silurian limestones and slates, and in the south, it intrudes terrigenous slates of Late Carboniferous age. The outer zone of the massif consists of subalkaline biotite granite, often tourmalinised (300–290 Ma), surrounding a discontinuous ring of biotite granites and granosyenites. The central part of the massif comprises quartz and aegirine syenites. In the northeast part of the massif, there is a stock of cancrinite and nepheline foyaite (247 ± 6 Ma). There are veins of syenite pegmatites and aegirine-potassium feldspar-quartz rocks containing polylithionite (286 ± 7 Ma) and various rare-metal and boron mineralisation. Veins of calcite carbonatites and syenite carbonatites (Faiziev et al., Reference Faiziev, Gafurov and Sharipov2010) are widespread. Much of the rock is fenitised to various degrees. Detailed descriptions of the petrography and mineralogy of the Darai-Pioz massif can be found in Moskvin (Reference Moskvin1937), Dusmatov (Reference Dusmatov1968, Reference Dusmatov1971), Semenov and Dusmatov (Reference Semenov and Dusmatov1975), Belakovskiy (Reference Belakovskiy1991) and Reguir et al. (Reference Reguir, Chakhmouradian and Evdokimov1999).
Rinkite-(Y) has been found in a pegmatite fragment from the moraine of the Darai-Pioz glacier. The main minerals in this fragment are quartz, which forms a white medium-grained granulated aggregate, and microcline, which occurs as large white grains. Minor minerals are as follows: brownish-red eudialite, which forms crystals up to 3.5 cm in diameter, dark and henna-red neptunite which occurs as grains up to 1 cm in diameter, dark green columnar aegirine crystals up to 2 cm long, and accumulations of white calcite up to 2 cm in diameter. Accessory minerals are fluorite, galena, albite, pyrochlore-group minerals, pectolite, titanite, turkestanite, kupletskite and rinkite-(Y). So far, rinkite-(Y) has been found in only one sample.
Primary pegmatites with eudialyte and neptunite are exposed in the eastern wall of the glacial valley in the central part of the massif; they intrude quartz-bearing aegirine syenites. The veins are irregular, up to 1.5 m thick, and are commonly branched and weakly zoned. Contacts with the country rocks are weak, with a melanocrate aegirine zone. Toward the central part of a vein, there is a coarse-grained feldspar–aegirine zone, comprising microcline, albite, aegirine with minor titanite, eudialyte, neptunite, fluorite and pyrochlore-group minerals. The central part of the pegmatitic body is comprised of 80% white translucent quartz with calcite, eudialyte, fluorite, albite, titanite, neptunite and galena. It is highly probable that the pegmatite fragment with rinkite-(Y) comes from these veins.
Physical properties
Rinkite-(Y) occurs as aggregates up to 1.5 cm long, formed of acicular crystals 0.1–1.0 mm thick, and as separate elongated columnar flattened-prismatic crystals up to 1 cm long with rectangular or rhombic sections up to 0.5 mm across (Fig. 1a). Crystals are generally colourless to white (Fig. 1b), partly due to the presence of inclusions, and with a vitreous lustre. Rinkite-(Y) has a white streak, an uneven conchoidal fracture and does not fluoresce under a cathode or ultraviolet light. Cleavage is very good on {100} good, no parting was observed. The microhardness of rinkite-(Y) is VHN100 = 569 kg/mm2 (547–659 range) which corresponds to a Mohs hardness of ~5; measurements were done on the PMT-3, calibrated on NaCl at a loading of 50 g. It is brittle, D meas. = 3.44(2) g/cm3 (determined by flotation in Clerici liquid); D calc. = 3.475 g/cm3 (using the empirical formula and the single-crystal unit cell).

Fig. 1. Back-scattered electron image of a large crystal with typical rhombic section and numerous small crystals of rinkite-(Y) (pale-grey) in microcline (dark-grey) (a) and a photomicrograph under crossed-polars showing an intergrowth of rinkite-(Y) crystals (white) in a granular quartz aggregate (b).
Macroscopically, individual crystals do not show twinning. Rinkite-(Y) is biaxial (+) with refractive indices (λ = 590 nm) α = 1.662(2), β = 1.666(2) and γ = 1.685(5). The optic-axial plane occurs at an acute angle to the direction of elongation of the grains, and the maximum extinction angle relative to the elongation (Y) is 29°. The sign of elongation can be either positive, or negative, though for acicular grains, negative elongation is more common. The optic axial angle, measured with a Fyodorov stage, is 50(3)°, and 2Vcalc. = 49.7°. Rinkite-(Y) is nonpleochroic. Dispersion is medium: v > r. The compatibility index (1 – K p/K c) = 0.035 (for D calc. = 3.475 g/cm3) is rated as excellent (Mandarino, Reference Mandarino1981).
Chemical analysis
A single crystal of rinkite-(Y) previously used for structure analysis was analysed using a Cameca SX-100 electron-microprobe operating in wavelength-dispersion mode with an accelerating voltage of 15 kV, a specimen current of 20 nA, a beam size of 2 µm and count times on peak and background of 20 and 10 s, respectively. The following standards were used: F: fluorite; Na: albite; Si, Ca: diopside; Nb: Ba2NaNb5O15; Mn: spessartine; Ce: CePO4; La: LaPO4; Nd: NdPO4; Pr: PrPO4; Sm: SmPO4; Gd: GdPO4; Dy: DyPO4; Ti: titanite; Sr: SrTiO3; Y: Y3Al5O12 and Ta: Mn(Ta1.7Nb0.3)O6. The elements Zr, Ba, Th, Hf, K, Mg, Fe and Al were sought but not detected. Data were reduced using the φ(ρZ) procedure of Pouchou and Pichoir (Reference Pouchou, Pichoir and Armstrong1985). The chemical composition of rinkite-(Y) is the mean of 10 determinations and is given in Table 2. The empirical formula calculated on 18 (O + F) is Na2.11(Ca3.74Sr0.03Mn0.03)Σ3.80(Y0.50Nd0.16Ce0.16Gd0.07Dy0.06Sm0.05Pr0.03La0.03U4+0.01)Σ1.07(Ti0.85Nb0.17W6+0.01Ta0.01)Σ1.04(Si4.03O14)O1.40F2.60 with Z = 2. For rinkite-(Y), the ideal structural formula of the form AP2MH2MO4(Si2O7)2(![]() ${\rm X}_{\rm M}^{\rm O} $)2(
${\rm X}_{\rm M}^{\rm O} $)2(![]() ${\rm X}_{\rm A}^{\rm O} $)2 is (Ca3Y)Na(NaCa)Ti(Si2O7)2(OF)F2 with Z = 2. The ideal formula is Na2Ca4YTi(Si2O7)2OF3.
${\rm X}_{\rm A}^{\rm O} $)2 is (Ca3Y)Na(NaCa)Ti(Si2O7)2(OF)F2 with Z = 2. The ideal formula is Na2Ca4YTi(Si2O7)2OF3.
Table 2. Chemical composition and unit formula for rinkite-(Y).

* Formula calculated on 18 (O + F)
** REE = (Nd0.16Ce0.16Gd0.07Dy0.06Sm0.05Pr0.03La0.03)Σ0.56
Infrared spectroscopy
The infrared (IR) spectrum in the range 400–1800 cm–1 was recorded from a KBr pellet and in the range 3000–4000 cm–1 from finely-ground rinkite-(Y) dispersed in Nujol with a Specord 75IR spectrometer (Carl Zeiss, Jena). The spectrum is shown in Fig. 2; the region from ~3000–1800 cm–1 is omitted as it contains the major peaks from Nujol. The observed bands (cm–1) are as follows: 1158, 1059, 965 and 871 (Si–O-stretching vibrations), 799, 775 and 663 (O–Si–O bending vibrations of Si2O7 groups), 587 and 561 (Ti–O-stretching vibrations) and 479 (Si–O–Si bending and stretching vibrations). There are no bands around 3500 and ~1630 cm–1, indicating the absence of H2O and OH– groups, in accord with the refined crystal structure.

Fig. 2. The IR-spectrum of rinkite-(Y): the region from 400 to1800 cm–1 was recorded from a KBr pellet and the region from 400 to 1800 cm–1, from finely-ground rinkite-(Y) dispersed in Nujol; the region from ~3000 to 1750 cm–1 is omitted as it contains the major peaks from Nujol in which the rinkite-(Y) was suspended.
Powder X-ray diffraction
Powder X-ray diffraction data for rinkite-(Y) were collected with a DRON-2.0 diffractometer with FeKα radiation and are given in Table 3. Unit-cell parameters refined from the powder data are as follows: a = 7.3934(5), b = 5.6347(4), c = 18.713(1) Å, β 101.415(2)° and V = 762.2(2) Å3.
Table 3. X-ray powder-diffraction data for rinkite-(Y)

The strongest lines are given in bold.
X-ray data collection and structure refinement
X-ray single-crystal data for rinkite-(Y) were collected from a twinned crystal with a single-crystal Bruker P4 four-circle diffractometer equipped with a graphite monochromator (MoKα radiation), multilayer optics and Smart 1K CCD detector. Details of data collection and structure refinement are given in Table 4. The intensities of reflections with –8 ≤ h ≤ 10, –7 ≤ k ≤ 7, –26 ≤ l ≤ 26 were collected with a frame width of 0.2° and a frame time of 30 s, and an empirical absorption correction (SADABS, Sheldrick, Reference Sheldrick2008) was applied. We observed 115 violations (> 3σF) of the c-glide extinction criterion and refined the structure as two components related by the TWIN matrix (![]() $\bar{1}$ 0 0/ 0
$\bar{1}$ 0 0/ 0 ![]() $\bar{1}$ 0/ 1 0 1) (see discussion on the nonmerohedral twinning in rinkite-(Ce) in Cámara et al., Reference Cámara, Sokolova and Hawthorne2011). There were few observed reflections at high 2θ, and those that do occur show splitting due to pseudomerohedral twinning, and refinement of the structure was carried out for 2θ ≤ 55°, –8 ≤ h ≤ 9, –7 ≤ k ≤ 7, –24 ≤ l ≤ 24. The crystal-structure refinement was undertaken with Bruker SHELXTL Version 5.1 (Sheldrick, Reference Sheldrick2015) in space group P21/c using the atom coordinates of rinkite-(Ce) (Cámara et al., Reference Cámara, Sokolova and Hawthorne2011). The crystal structure of rinkite-(Y) was refined to R 1 = 4.59%, the twin ratio being 0.558(3):0.442(3) (Table 4). The occupancies of five cation sites were refined with the following scattering curves: M H and AP sites: Y; M O1 site: Ti; M O2 and M O3 sites: Na and Ca, respectively (for the site-labelling see footnote of Table 1). The occupancies of the
$\bar{1}$ 0/ 1 0 1) (see discussion on the nonmerohedral twinning in rinkite-(Ce) in Cámara et al., Reference Cámara, Sokolova and Hawthorne2011). There were few observed reflections at high 2θ, and those that do occur show splitting due to pseudomerohedral twinning, and refinement of the structure was carried out for 2θ ≤ 55°, –8 ≤ h ≤ 9, –7 ≤ k ≤ 7, –24 ≤ l ≤ 24. The crystal-structure refinement was undertaken with Bruker SHELXTL Version 5.1 (Sheldrick, Reference Sheldrick2015) in space group P21/c using the atom coordinates of rinkite-(Ce) (Cámara et al., Reference Cámara, Sokolova and Hawthorne2011). The crystal structure of rinkite-(Y) was refined to R 1 = 4.59%, the twin ratio being 0.558(3):0.442(3) (Table 4). The occupancies of five cation sites were refined with the following scattering curves: M H and AP sites: Y; M O1 site: Ti; M O2 and M O3 sites: Na and Ca, respectively (for the site-labelling see footnote of Table 1). The occupancies of the ![]() ${ X}_{\rm M}^{\rm O} $ and
${ X}_{\rm M}^{\rm O} $ and ![]() ${X}_{\rm A}^{\rm O} $ anion sites were refined with the scattering curve of F; refinement of the
${X}_{\rm A}^{\rm O} $ anion sites were refined with the scattering curve of F; refinement of the ![]() ${\rm X}_{A}^{\rm O} $ site occupancy converged to an integer value (within 3 e.s.d.) and was subsequently fixed at full occupancy. Scattering curves for neutral atoms were taken from the International Tables for Crystallography (Wilson, Reference Wilson1992). Final atom coordinates and equivalent displacement parameters are given in Table 5, selected interatomic distances and angles in Table 6, refined site-scattering values and assigned site-populations in Table 7, and bond-valence values for selected anions in Table 8. A list of observed and calculated structure factors and crystallographic information file have been deposited with the Principal Editor of Mineralogical Magazine and are available as Supplementary material (see below).
${\rm X}_{A}^{\rm O} $ site occupancy converged to an integer value (within 3 e.s.d.) and was subsequently fixed at full occupancy. Scattering curves for neutral atoms were taken from the International Tables for Crystallography (Wilson, Reference Wilson1992). Final atom coordinates and equivalent displacement parameters are given in Table 5, selected interatomic distances and angles in Table 6, refined site-scattering values and assigned site-populations in Table 7, and bond-valence values for selected anions in Table 8. A list of observed and calculated structure factors and crystallographic information file have been deposited with the Principal Editor of Mineralogical Magazine and are available as Supplementary material (see below).
Table 4. Miscellaneous refinement data for rinkite-(Y).

* Data collection; **structure refinement; ***second component of the crystal is related to the first component by the twin matrix [![]() $\bar{1}$ 0 0/ 0
$\bar{1}$ 0 0/ 0 ![]() $\bar{1}$ 0/ 1 0 1].
$\bar{1}$ 0/ 1 0 1].
Table 5. Atom coordinates and anisotropic displacement parameters (Å2) for rinkite-(Y).

Table 6. Selected interatomic distances (Å) and angles (°) in rinkite-(Y).

φ = O or F
Symmetry operators (given in brackets): a: x–1, y, z; b: –x + 1, y–½, –z+½; c: –x + 1, y+½, –z+½; d: –x–1, –y + 1, –z; e: x, y–1, z; f: –x + 1, –y + 1, –z; g: x + 1, y, z.
Table 7. Refined site scattering and assigned site-populations for rinkite-(Y)

*Coordination numbers are shown for non-[6]-coordinated cation sites; **distances < Cation–φ> were calculated using ionic radii of Shannon (Reference Shannon1976), φ = O or F; ***REE = (Nd0.16Ce0.16Gd0.07Dy0.06Sm0.05Pr0.03La0.03)Σ0.56, with f av = 60.54 el.
Table 8. Bond-valence values* for ![]() ${\rm X}_{\rm M}^{\rm O} $ and
${\rm X}_{\rm M}^{\rm O} $ and ![]() ${\rm X}_{\rm A}^{\rm O} $ anions in rinkite-(Y).
${\rm X}_{\rm A}^{\rm O} $ anions in rinkite-(Y).

* Bond-valence parameters (vu) are from Brown (Reference Brown, O'Keeffe and Navrotsky1981).
Site-population assignment
There are seven cation sites in the crystal structure of rinkite-(Y): three M O sites of the O sheet and the M H, AP and two Si sites of the H sheet; site labelling is in accord with Sokolova (Reference Sokolova2006). Consider first the Ti-dominant M O1 site. We assign cations to this site based on our previous work on rinkite-(Ce) (Cámara et al., Reference Cámara, Sokolova and Hawthorne2011): Ti-dominant sites are always fully occupied. We assign Ti0.85Nb0.15 apfu to the M O1 site, with refined and calculated scattering values of 24.0 and 24.85 electrons per formula unit (epfu), respectively (Table 7). Such assignment is supported by matching values of observed and calculated mean bond lengths of 1.987 and 1.990 Å, respectively (Tables 6, 7).
Consider next the [8]M O2 and [6]M O3 sites in the O sheet occupied by alkali cations. In accord with structure-refinement results for rinkite-(Ce) (Cámara et al., Reference Cámara, Sokolova and Hawthorne2011), we assign Na0.96Sr0.03□0.01 to the M O2 site and Na1.15Ca0.79Mn0.03□0.03 to the M O3 site. These assignments are supported by close agreement between (1) refined and calculated scattering values of 11.7 and 11.70 epfu for the M O2 site and 29.1 and 29.20 epfu for the M O3 site, and (2) observed and calculated mean bond lengths of 2.494 and 2.550 Å for the M O2 site and 2.378 and 2.373 Å for the M O3 site (Tables 6, 7).
Consider next the two [7]-coordinated M H and AP sites in the H sheet. In rinkite-(Ce), these sites are fully occupied mainly by Ca and REE 3+ in the ratio ~3:1 (Cámara et al., Reference Cámara, Sokolova and Hawthorne2011). The cations to be assigned to the M H and AP sites are Ca2.95Y0.50REE 0.56U4+0.01, with a total calculated scattering of 113.32 epfu (Table 2). The < MH–φ> distance of 2.415 Å is shorter than the <AP–φ> distance of 2.458 Å (where φ = O or F) (Table 6) and hence we assign all (smaller) Y (radius = 0.96 Å, Shannon, Reference Shannon1976) to the M H site (Table 7). The scattering at the AP site is lower than that at the M H site, and we distribute Ca and REE at the M H and AP sites so their calculated site-scattering values are in the same ratio as the corresponding refined values. The two latter assignments are supported by close agreement between the observed and calculated mean bond lengths of 2.415 and 2.416 Å for the M H site and 2.458 and 2.440 Å for the AP site (Table 7).
There are nine anion sites in the crystal structure of rinkite-(Y). We assign O atoms to the O(1–7) sites that constitute the tetrahedral coordination of the Si1 and Si2 sites. Anions at two sites, ![]() ${X}_{\rm M}^{\rm O} $ and
${X}_{\rm M}^{\rm O} $ and ![]() ${X}_{\rm A}^{\rm O} $, receive bond valence from four cations, [MO1, 2MO3 and MH] and [MO2, 2MO3 and AP], respectively (Table 8). We expect the
${X}_{\rm A}^{\rm O} $, receive bond valence from four cations, [MO1, 2MO3 and MH] and [MO2, 2MO3 and AP], respectively (Table 8). We expect the ![]() ${\rm X}_{\rm M}^{\rm O} $ atom to receive a higher bond valence as it is bonded to Ti at the M O1 site, and the
${\rm X}_{\rm M}^{\rm O} $ atom to receive a higher bond valence as it is bonded to Ti at the M O1 site, and the ![]() ${\rm X}_{\rm A}^{\rm O} $ atom, a lower bond-valence as it is bonded to Na at the M O2 site. Moreover, the contribution to the
${\rm X}_{\rm A}^{\rm O} $ atom, a lower bond-valence as it is bonded to Na at the M O2 site. Moreover, the contribution to the ![]() ${\rm X}_{\rm M}^{\rm O} $ anion from the MH cation is higher than that to the
${\rm X}_{\rm M}^{\rm O} $ anion from the MH cation is higher than that to the ![]() ${\rm X}_{\rm A}^{\rm O} $ anion from the AP cation as the content of (Y + REE) is higher at the M H site (Tables 6, 7). Calculation of bond-valence sums at the
${\rm X}_{\rm A}^{\rm O} $ anion from the AP cation as the content of (Y + REE) is higher at the M H site (Tables 6, 7). Calculation of bond-valence sums at the ![]() ${\rm X}_{\rm M}^{\rm O} $ and
${\rm X}_{\rm M}^{\rm O} $ and ![]() ${\rm X}_{\rm A}^{\rm O} $ anions (using cation-oxygen parameters) gave a lower sum for the
${\rm X}_{\rm A}^{\rm O} $ anions (using cation-oxygen parameters) gave a lower sum for the ![]() ${\rm X}_{\rm A}^{\rm O} $ anion (Table 8). Therefore we assigned 2 F apfu to the
${\rm X}_{\rm A}^{\rm O} $ anion (Table 8). Therefore we assigned 2 F apfu to the ![]() ${X}_{\rm A}^{\rm O} $ site. Chemical analysis gives 2.6 F apfu (Table 2), hence we subtract 2 apfu from the total F (Table 2) and assign the remaining 0.60 F apfu to the
${X}_{\rm A}^{\rm O} $ site. Chemical analysis gives 2.6 F apfu (Table 2), hence we subtract 2 apfu from the total F (Table 2) and assign the remaining 0.60 F apfu to the ![]() ${X}_{\rm M}^{\rm O} $ site, which has the following composition: (O1.40F0.60).
${X}_{\rm M}^{\rm O} $ site, which has the following composition: (O1.40F0.60).
The crystal structure
The crystal structure of rinkite-(Y) is a framework of TS (Titanium-Silicate) blocks. The TS block consists of HOH sheets (H – heteropolyhedral, O – octahedral) (Sokolova, Reference Sokolova2006).
In the O sheet, there is one [6]-coordinated Ti-dominant M O1 site, with < MO1–φ> = 1.987 Å (Tables 6, 7). The M O1 site ideally gives 1 Ti apfu. There are [8]M O2 and [6]M O3 sites, ideally occupied by 1 Na apfu and (NaCa) apfu, respectively (Tables 6, 7; Fig. 3a). The ideal composition of the O sheet, MO4(![]() ${\rm X}_{\rm M}^{\rm O} $)2(
${\rm X}_{\rm M}^{\rm O} $)2(![]() ${\rm X}_{\rm A}^{\rm O} $)2, is [Na(NaCa)Ti(OF)F2]3+ or, in a shorter form, [Na2CaTiOF3]3+ apfu (Tables 1,7).
${\rm X}_{\rm A}^{\rm O} $)2, is [Na(NaCa)Ti(OF)F2]3+ or, in a shorter form, [Na2CaTiOF3]3+ apfu (Tables 1,7).

Fig. 3. The details of the TS (Titanium-Silicate) block in the structure of rinkite-(Y): the close-packed O sheet of [8]Na-polyhedra, (NaCa) octahedra and Ti octahedra (a); the H sheet of [7]-coordinated Ca-dominant polyhedra and Si2O7 groups (b); and linkage of O and H sheets in the TS block (c). SiO4 tetrahedra are orange, Ti-dominant, Ca-dominant and Na + (NaCa) polyhedra are pale yellow, pink and navy blue, respectively; F (![]() ${X}_{\rm A}^{\rm O} $ site) and (OF) (
${X}_{\rm A}^{\rm O} $ site) and (OF) (![]() ${X}_{\rm M}^{\rm O} $ site) anions are shown as yellow and orange spheres.
${X}_{\rm M}^{\rm O} $ site) anions are shown as yellow and orange spheres.
In the H sheet, there are two [7]-coordinated Ca-dominant sites: M H and AP, which ideally give (Ca3Y) apfu (Table 7). The two [4]Si sites are occupied solely by Si with <Si–O> = 1.623 Å. The MH and AP polyhedra and Si2O7 groups constitute the H sheet (Fig. 3b). The ideal composition of the two H sheets, AP2MH2(Si2O7)2, is [(Ca3Y)(Si2O7)2]3– or, in a shorter form, [Ca3Y(Si2O7)2]3– apfu (Tables 1,7).
Linkage of H and O sheets via common vertices of MH and AP polyhedra and Si2O7 groups with MO1–3 polyhedra results in a TS block (Fig. 3c). The TS block in rinkite-(Y) exhibits linkage 1 of H and O sheets and stereochemistry typical for the rinkite group (Ti = 1 apfu) (Sokolova, Reference Sokolova2006): two H sheets connect to the O sheet such that two Si2O7 groups link to the trans edges of an [8]Na polyhedron of the O sheet. In the crystal structure of rinkite-(Y), two adjacent TS blocks are related by the c y glide plane.
For rinkite-(Y), we write the ideal structural formula of the form AP2MH2MO4(Si2O7)2(![]() ${\rm X}_{\rm M}^{\rm O} $)2(
${\rm X}_{\rm M}^{\rm O} $)2(![]() ${\rm X}_{\rm A}^{\rm O} $)2 as the sum of the ideal compositions of the O sheet and two H sheets: [Na(NaCa)Ti(OF)F2]3+ + [(Ca3Y)(Si2O7)2]3– = (Ca3Y)Na(NaCa)Ti(Si2O7)2(OF)F2 with Z = 2. We write the ideal formula of rinkite as the sum of the ideal compositions of the O sheet and two H sheets in shorter forms: [Ca3Y(Si2O7)2]3– + [Na2CaTiOF3]3+ = Na2Ca4YTi(Si2O7)2OF3.
${\rm X}_{\rm A}^{\rm O} $)2 as the sum of the ideal compositions of the O sheet and two H sheets: [Na(NaCa)Ti(OF)F2]3+ + [(Ca3Y)(Si2O7)2]3– = (Ca3Y)Na(NaCa)Ti(Si2O7)2(OF)F2 with Z = 2. We write the ideal formula of rinkite as the sum of the ideal compositions of the O sheet and two H sheets in shorter forms: [Ca3Y(Si2O7)2]3– + [Na2CaTiOF3]3+ = Na2Ca4YTi(Si2O7)2OF3.
Summary
Rinkite-(Y) differs from rinkite-(Ce) in the dominant rare-earth element, Y versus Ce, respectively. In accord with Bayliss and Levinson (Reference Bayliss and Levinson1988), the mineral is named rinkite-(Y) as it is structurally identical to rinkite-(Ce). Table 9 lists comparative data for rinkite-(Y) and rinkite-(Ce).
Table 9. Comparison of rinkite-(Y) and rinkite-(Ce)*.

* Rinkite-(Ce): ideal formula, crystallographic data and D calc. for the six samples of rinkite-(Ce) are from Cámara et al. (Reference Cámara, Sokolova and Hawthorne2011) and Sokolova and Cámara (Reference Sokolova and Cámara2008); D meas. and optical data are from Anthony et al. (Reference Anthony, Bideaux, Bladh and Nichols1995), powder X-ray diffraction data, from JCPDS 71-0440 [powder diffraction file from the International Centre for Diffraction Data (http://www.icdd.com/)].
Acknowledgements
We thank reviewers F. Cámara and P. Leverett and Associate Editor I. Graham for their useful comments which helped to improve the manuscript. We are grateful to A.R. Faiziev, P.V. Kvorov and R.U. Sobirova for their help in organisation and carrying-out the fieldwork. This work was supported by a Canada Research Chair in Crystallography and Mineralogy and by a Discovery grant from the Natural Sciences and Engineering Research Council of Canada to FCH, and by Innovation Grants from the Canada Foundation for Innovation to FCH.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/mgm.2018.122.