Published online by Cambridge University Press: 19 April 2005
Studies using antibodies to immunolocalize the Toxoplasma gondii dense granule protein GRA3, have shown that this protein associates strongly with the parasitophorous vacuole membrane (PVM). However, as there was no predicted membrane-spanning domain this highlighted an unanswered paradox. We demonstrate that the previously published sequence for GRA3 is actually an artificial chimera of 2 proteins. One protein, of molecular weight 65 kDa, shares the C-terminus with published GRA3 and possesses no significant sequence similarity with any protein thus far deposited in Genbank. The second, with a predicted molecular weight of 24 kDa shares the N-terminal region, is recognized by the monoclonal antibody 2H11 known to react with the dense granules of T. gondii and is therefore the authentic GRA3. The corrected GRA3 has an N-terminal secretory signal sequence and a transmembrane domain consistent with its insertion into the PVM. Antibodies to recombinant GRA3 recognize a protein of 24 kDa in T. gondii excretory–secretory antigen preparations. The signal peptide is necessary and sufficient to target GFP to the dense granules and parasitophorous vacuole. A homologue was identified in Neospora caninum. Finally, GRA3 possesses a dilysine ‘KKXX’ endoplasmic reticulum (ER) retrieval motif that rationalizes its association with PVM and possibly the host cell ER.
Toxoplasma gondii is an obligate intracellular parasite capable of infecting a wide variety of cells in numerous animal species. It uses a number of surface proteins and secretory products from specialized organelles (micronemes, rhoptries and dense granules) to facilitate active invasion and establishment within host cells. Surface proteins including members of the SAG protein families, in conjunction with a number of proteins secreted from the micronemes, mediate attachment of the parasite to the host cell. The parasite then orientates its apical end in close proximity with the host cells and the microneme proteins are discharged. Within the host cells, tachyzoites of T. gondii reside and multiply in a specialized compartment called the parasitophorous vacuole (PV). The PV fails to fuse with other endocytic vesicles, including both early and late endosomes, or to lower its internal pH (Jones & Hirsch, 1972; Sibley, Weidner & Krahenbuhl, 1985; Joiner et al. 1990). This vacuole is delimited by the parasitophorous vacuole membrane (PVM), which functions as a molecular sieve (Schwab, Beckers & Joiner, 1994), forms a critical interface between the growing parasites and the host cell cytoplasm, and is crucial for nutrient acquisition and evasion of host endocytic processing. The PVM forms associations with the host cell mitochondria and the endoplasmic reticulum (ER) (Sinai, Webster & Joiner, 1997).
It has been shown that host cell proteins are selectively excluded from the PVM. Thus, while those with GPI anchors, including CD55 and Sca-1 are included others, such as CD44, Na+K+ATPase and β1-integrin, which lack GPI anchors are excluded (Mordue et al. 1999). During the formation of the PV, a number of molecules are secreted from the rhoptry organelles. The newly formed PV and the PVM are extensively modified by secreted parasite proteins (reviewed by Cesbron-Delauw, 1994). Dense granule proteins are released approximately 10 min after invasion and are targeted to the intravacuolar network (IVN), the PVM or to the lumen of the PV (reviewed by Cesbron-Delauw, 1994).
Consistent with the involvement of micronemes in the attachment of the parasite to the host cell, these proteins are similar to molecules with known adhesive properties. However, with the notable exceptions of cyclophilin and NTPase isoenzymes I and II (Asai et al. 1995), the functions of the vast majority of proteins secreted from the dense granules (GRA1–8) have remained elusive. Similarly, of the proteins secreted from rhoptries (ROP1–9), the only protein that has a clear function is ROP2, which has been demonstrated to possess a non-cleavable mitochondrial-like targeting sequence, which is likely to contribute to the mitochondrial-PVM association (Sinai & Joiner, 2001).
Based on secondary structure predictions and the localization of dense granule proteins to the PV and PVM, a number of potential functions have been postulated. For example, several GRA proteins, including GRA5, GRA6, GRA7 and GRA8 have predicted transmembrane regions and are inserted in the PVM and thus may be involved in the PVM-organelle association (Sinai & Joiner, 2001) or facilitate transport of molecules into or out of the PV. GRA1 is soluble whereas GRA2, GRA4 and GRA6 are targeted to the IVN, which extends from the parasite surface.
The dense granule protein GRA3 is known to strongly associate with the PVM, but the exact nature of this association has been enigmatic (Ossorio, Dubremetz & Joiner, 1994). It was suggested that GRA3 associates stably with the PVM via a hydrophobic interaction, but no transmembrane domain or post-translational modification has yet been described. Herein we describe the convergence of two studies that were initiated independently and solve the enigma of the mechanistic basis of GRA3 association with the PVM and explore what was thought to be the existence of a family of GRA3-related proteins. The results obtained show that GRA3, as previously published, is in fact an artificial chimera, comprising the N-terminus and C-terminus of two different proteins. One of these is the authentic GRA3 and the other that we have termed Tg65 is of yet unknown function. The process by which these molecules were identified, their characterization and the potential role of GRA3 in the host–parasite relationship are described.
Sequence data were obtained from the T. gondii and N. caninum expressed sequence tag (EST) projects http://ParaDB.cis.upenn.edu/toxo1/blast.html (Ajioka et al. 1991) and http://paradb.cis.upenn.edu/cgi-bin/est/neo/blast.html, from the T. gondii genome project ToxoDB: The Toxoplasma Genome Resource (http://www.toxodb.org) (Kissinger et al. 2003) and from The Sanger Center (http://www.sanger.ac.uk/Projects). Homology predictions were performed using the BLAST tool at http://www.ncbi.nlm.nih.gov. Post-translational modification and topology predictions were analysed with the aid of a variety of prediction programs: PSORTII (http://psort.ims.u-tokyo.ac.jp) (Nakai & Horton, 1999) to predict protein sorting signals; TMHMM 2.0 (http://www.cbs.dtu.dk/services/TMHMM-2.0) (Krogh et al. 2001) to predict transmembrane helices; and SignalP 1.1 (http://www.cbs.dtu.dk/services/SignalP) (Nielsen et al. 1997) to identify potential signal peptide cleavage sites.
T. gondii tachyzoites RH strain were grown in the peritoneum of BALB/c mice as previously described (9). N. caninum (NC1 strain) tachyzoites were grown in the human fibroblast cell line (MRC-5) in DMEM medium (Invitrogen Ltd, Paisley, UK) containing 5% FCS, 1% penicillin/streptomycin, 1% glutamine.
The Beverley strain of T. gondii was used in the preparation of bradyzoites. Brains from ND4 mice infected 17–21 weeks previously were harvested and homogenized in 2 ml of sterile 0·1 M PBS by 6 passages through a 21-gauge needle. Approximately 10–20 cysts were used to infect C57BL/6 mice by the oral route. Cysts were harvested and purified from the brains of C57BL/6 mice after 4 weeks of infection as previously described by Denton et al. (1996). Purified cysts were stored at −70 °C until required.
RNA isolation was performed following a protocol based on a single-step acid guanidinium thiocyanate-phenol-chloroform for RNA isolation, developed by Chomczynski & Sacchi (1987).
Complementary DNA (cDNA) was produced from total RNA using Moloney murine leukaemia virus reverse transcriptase (Invitrogen), as previously described by Lyons et al. (2001).
Genomic DNA was extracted from 109 tachyzoites by a routine protocol described previously by Johnson et al. (1986). The DNA pellet was resuspended in 0·5 ml of TE (10 mM Tris–HCl/1 mM EDTA, pH 8·0) buffer and stored at 4 °C until used in PCR.
All PCR amplification reactions contained 0·5 U of cloned Pfu DNA polymerase (Stratagene Europe, Amsterdam, The Netherlands), 2·5 μl of 10X Pfu DNA polymerase buffer [(10 mM KCl, 10 mM (NH4)2SO4, 20 mM Tris–HCl (pH 8·75), 2 mM MgSO4, 0·1% Triton X-100, 1 mg/ml BSA)], 0·5 mM dNTPs (Promega) and 25 pmol of forward and reverse primer in a 25 μl volume. Template was cDNA derived from T. gondii or N. caninum, or from T. gondii gDNA. Reactions consisted of an initial denaturation at 94 °C for 3 min followed by 35 cycles of denaturation at 94 °C for 30 sec, annealing for 45 sec from 52 °C to 60 °C and extension at 72 °C for 1 min. A final extension was carried out at 72 °C for 10 min.
A portion of Tg65 was amplified from cDNA by using forward primer 5′ GGA TCC ATT CGG CAC GAG CTC GTC CGA 3′ and reverse primer 5′ GGT ACC TGC GGC TTC CGA TGA CGG CAT 3′. BamHI and KpnI sites were engineered into either the forward or the reverse primers respectively (underlined). Both products were blunt-end ligated into pUC18 using the Sureclone Ligation (Amersham Biosciences, Little Chalfont, UK) and subcloned by sticky-end ligation into the BamHI and KpnI sites of the N-terminal HisTag expression vector pQE-30 (Qiaexpression system, Qiagen, Crawley, UK). Plasmids were purified from cultures using the Qiagen miniprep kit (Qiagen) according to the manufacturer's instruction. Automated sequencing was performed on all cloned products (MWG Biotech Ltd, Germany) and sequences were assembled using Sequencher™ (Gene Codes, USA).
Protein was purified from inclusion bodies under denaturing conditions (according to Qiaexpression system instruction) and purity of eluted protein was determined by SDS–PAGE.
Using the pMAL expression system (New England Biolabs, USA), GRA3-Maltose binding protein (MBP) fusion proteins were produced. A GRA3 fragment was amplified by PCR using Type I RH genomic DNA as template, digested with Xmn I and Sal I, purified using Qia-quick spin columns (Qiagen, USA) and ligated into the pMAL-p2 cloning vector. The following primers were used: forward 5′-GAG GGA AGG ATT TCA GAA TTC ATG GAC CGT ACC ATA TGT-3′ and reverse 5′-CAG GTC GAC GGT TTG TTT CTT GGA GGC-3′. Bacteria expressing the fusion proteins were induced for 2 h at 37 °C with IPTG.
The pVAX1 plasmid (Invitrogen) was modified by ligation of the GATEWAY adaptation cassette (reading frame c) into EcoRV cut pVAX1. The correct orientation of the cassette was confirmed by restriction enzyme digests, which made use of the EcoRI site in the MCS of pVAX1 and the asymmetric position of the EcoRI site within the GATEWAY adaptation cassette.
Adapted PCR primers were designed to insert GRA3 into the Gateway compatible pVAX1. These were made by adding 5′ attB sites (in bold) to the forward (5′-GGG GAC AAG TTT GTA CAA AAA AGC AGG CTT GGA CCG TAC CAT ATG TCC C-3′) and reverse (5′-GGG GAC CAC TTT GTA CAA GAA AGC TGG GTT TAA AAG GCG TCC GTA TAG GG-3′) oligonucleotides. Purified GRA3 DNA was recombined into pDONR.207 (Invitrogen) by incubating 300 ng of plasmid with 500 ng of PCR-amplified GRA3 and 4 μl of BP clonase for 1 h at 25 °C. l μl of the BP reaction was transformed into DH5α E. coli (Cohen, Chang & Hsu, 1972) and colonies were selected by using gentamicin (12·5 μg/ml). Cloned inserts were isolated and recombined into the GATEWAY adapted pVAX1 by incubating 300 ng of pDONR.207 and 300 ng of pVAX1 with 4 μ1 of LR clonase for 1 h at 25 °C. The pVAX1 vector was subsequently transformed into DH5α E. coli and colonies were selected by using kanamycin (12·5 μg/ml) and incubated overnight at 37 °C.
Sixty μg of the recombinant HisTag fusion protein was emulsified in Freund's Complete Adjuvant (Sigma) (1[ratio ]1) by repeated passage through a double-hubbed emulsification needle. This was administered subcutaneously (s/c) into female BALB/c mice. On day 14, a secondary immunization composed of 60 μg fusion protein emulsified in Freund's Incomplete Adjuvant (Sigma, USA) (1[ratio ]1) was administered s/c. For DNA vaccination, 5 female BALB/c mice were inoculated by intramuscular injection with 100 μg pVAX (GRA3) (divided dose of 50 μg in each thigh muscle). Mice were boosted with a further 100 μg pVAX (GRA3) at day 14 as previously described by Nielsen et al. (1999). Mice were bled by cardiac puncture and the plasma portion obtained by centrifugation.
The monoclonal antibodies TG17-43 and 2H11 that recognize GRA1 and GRA3 respectively were used in Western blot studies at a 1/1000 dilution (Achbarou et al. 1991).
The ESA was obtained from T. gondii tachyzoites (RH strain) as described by Neudeck et al. (2002). Briefly, 1×108 tachyzoites were incubated for 3 h in PBS (pH 7·2) and 1% FCS (Harlan) at 37 °C. After centrifugation for 15 min at 400 g the supernatant fraction was saved and, after further centrifugation at 1200 g for 15 min, the protein concentration of the supernatant containing the ESA was quantified (Bradford, 1976).
T. gondii tachyzoites (RH strain) were adjusted to 2×109 per ml in PBS and an equal volume of SDS–PAGE sample buffer (0·6 M Tris, pH 6·8, 2% SDS, 20% glycerol, 10% 2-mercaptoethanol) was added to give a final concentration of 109/ml. The solution was boiled for 5 min prior to separation by SDS–PAGE in 10%–12% polyacrylamide gels. The gels were electrophoresed at a constant 150 V for 1–1·5 h in a 1X Tris–glycine buffer (0·02 M Tris base, 0·192 M glycine, 1% SDS solution).
Proteins were electrophoretically transferred from SDS–polyacrylamide gels, to nitrocellulose membranes using Novex Xcell II electrophoresis apparatus for 1 h at 30 V in Tris–glycine buffer containing 20% methanol (Invitrogen).
Nitrocellulose membranes were blocked overnight at room temperature with BLOTTO (5% dried milk powder, 0·1% Tween 20). Primary antibody was added to BLOTTO (1[ratio ]500 dilution) and incubated at room temperature for 1 h followed by 3×15 min washes in 1X TPBS (0·1% Tween 20, 1X PBS). Anti-mouse Ig horseradish peroxidase (HRP) conjugate was diluted 1[ratio ]5000 in BLOTTO, added to the membranes and incubated at room temperature for 1 h followed by a further 3×15 min washes. Detection was by chemiluminescence (Pierce, USA).
Long-distance genome walking PCR, or adaptation of asymmetric PCR, was used in order to isolate the unknown 5′ genomic sequence of Tg65 adjacent to the known sequence. The method used was as described by Min & Powell (1997). Initially, the known sequence of Tg65 was extended by asymmetric PCR from 50 ng of gDNA using 50 pmols of a gene-specific reverse primer GW1 (5′-CTCATCGCCTCAGGAAGTGCTCTAC-3′) in a 50 μl reaction containing, 2·5 U of Expand High Fidelity Taq polymerase, 5 μl of 10X Expand High Fidelity Taq polymerase with MgCl2 (Roche Molecular Biochemicals, Lewes, UK) and 0·2 mM of each dNTP (Promega). Conditions for PCR were 35 cycles of denaturation at 96 °C for 10 sec, annealing at 64 °C for 10 sec, and extension at 68 °C for 4 min. The single-stranded gDNA (ssgDNA) product was purified using the PCR purification kit (Qiagen) and eluted in 30 μl of 10 mM Tris–HCl, pH 8·3 and adjusted to a volume of 10 μl by centrifugal evaporation. The ssgDNA was incubated at 95 °C for 5 min and immediately chilled on ice for 2–3 min.
A dC-tail was added to the unknown 5′end with the DNA tailing kit (Roche Molecular Biochemicals). Four μl of 5X tailing buffer (1 M potassium cacodylate, 125 mM Tris–HCl, 1·25 mg/ml BSA, pH 6·6), 3 μl of 5 mM CoCl2, 1 μl of 100 pmol dCTP and 50 U of terminal deoxyribonucleotidyl transferase were added to 4 μl of the purified ssgDNA and incubated at 37 °C for 20 min followed by an incubation at 70 °C for 10 min.
Five μl of the tailing reaction was used in a nested PCR containing the reagents used in the initial extension step. Primers used were the gene-specific reverse primer GW2 (5′-GGG GCT CTC CAA ATC TCA GGA-3′), designed upstream from GW1 and an oligodG-anchor primer (5′-CGA GGA ATT CGG GGG GGG GGG G-3′). Cycling conditions were 40 cycles of denaturation at 96 °C for 30 sec, annealing at 65 °C for 15 sec and extension at 68 °C for 5 min. A final extension was at 65 °C for 5 min. The product was run on a 1% agarose gel, purified and cloned into pDRIVE (Qiagen) for subsequent sequence analysis.
The GFP expression plasmid ptubP30-GFP/sag-CAT (Striepen et al. 1998) was used to develop 3 constructs: GRA3 (SP)-GFP, comprising the predicted signal sequence of GRA3 with a C-terminal GFP extension; GRA3 (M)-GFP, comprising the predicted mature GRA3 with a C-terminal GFP extension and GRA3 (E)-GFP, comprising the entire GRA3 protein with a C-terminal GFP extension. Each region of GRA3 was amplified using pfu polymerase with annealing at 56 °C. Primers for the signal peptide region were, ForGFP (5′-CCC GGA AGA TCT AAA ATG GAC CGT ACC ATA TGT CCC-3′) and sigrevGFP (5′-CTA GTT CCT AGG GCA AGG CCT CCC AAG GCA GCA GC-3′). Primers for the mature peptide region were, matpepGFPfor (5′-CCC GGA AGA TC TAA AGC GGA TCA GCC TGA AAA TCA T-3′) and longGFPR (5′-CAA CGA TTC CTA GGG GTT TGT TTC TTG GAG GCT TTG-3′). The region of DNA encoding the complete GRA3 gene was amplified using primers ForGFP and longGFPR. A BglI site was engineered in the forward primers (underlined) and an AvrII site in the reverse (underlined). Then 300 ng of product were ligated into 150 ng of PtubP30-GFP/sag-CAT that had been prepared by digestion with BglI and AvrII using T4 ligase (Promega).
T. gondii tachyzoites from the RH strain were grown in the human foreskin fibroblast cell line (HFF) in Dulbecco's modified Eagles medium (DMEM) (Invitrogen) containing 10% foetal calf serum (FCS; Harlan Sera-Lab, UK) at pH 7·2, 125 μg penicillin/streptomycin (Sigma), 2 mM glutamine (Biowittaker, USA) at 37 °C under 5% CO2. Tachyzoites were resuspended in Cytomix (KCl 120 mM, CaCl2 0·15 mM, KH2PO4–K2HPO4 10 mM, HEPES–KOH, 25 mM, EDTA 2 mM, MgCl2 5 mM) containing ATP (2 mM) and GSH (5 mM) to a final concentration of 3·3×107 parasites/ml. Then 300 μl of the parasite suspension were electroporated with 50 μg of the plasmid construct in a 0·2 cm gap cuvette with a Gene Pulser II (Bio-Rad, USA) at 25 uFD, 1·5–1·6 kV and 2×105 parasites were added to each well of a 24-well plate containing an HFF monolayer on a cover-slip and incubated at 37 °C, 5% CO2 for either 24, 48 or 72 h.
Cover-slips were washed 3 times in sterile 1X PBS and incubated with 5 μg/ml 4′,6′-diamidino-2-phenylindole hydrochloride (DAPI) (Sigma) in PBS for 30 min. The cover-slips were then rinsed for a further 3 times with PBS prior to examination using an Axiovert inverted fluorescence microscope (Zeiss, Germany) or an Axioplan microscope (Zeiss) equipped with a cooled CCD camera (MicroMAX, USA). All pictures were taken with the 63X oil immersion objective. Image capture, deconvolution and processing of the images were performed with Slidebook 10.0 and Openlab 3.0 softwares (Improvision Ltd, USA). Optical sections were taken through the depth of the cell and the software used to deconvolve these images and construct 3-D volume views. Images were exported as TIFF files and processed using Adobe Photoshop 6.0 (Adobe, USA).
A Vero cell monolayer that had been infected for 24 h with T. gondii tachyzoites was resuspended using 5 mM EDTA in PBS and the infected cells were pelleted and then fixed for 15 min with 2% paraformaldehyde and 0·1% glutaraldehyde in 0·2 M sodium phosphate buffer, pH 7·4. The pellet was dehydrated in ethanol and embedded in LR White (London Resin Co). Thin sections were collected on carbon-coated grids and saturated for 30 min on 2·5% non-fat dry milk and 0·1% Tween 20 in PBS (PBS-milk-Tween). The grids were then floated successively for 1 h each on Mab T6 2H11 (ascitic fluid) diluted 1/100, rabbit anti mouse serum diluted 1/400, followed by 10 nm Protein A gold, all diluted in PBS-milk-Tween, with PBS washes between each step. The grids were then stained with uranyl acetate lead citrate and observed with a Jeol C120 electron microscope operated at 80 KV.
For the production of N- and C-terminal his-tagged recombinant GRA3, several sets of primers were designed based on the published GRA3 sequence (U13771), but no product could be amplified using either cDNA (derived from bradyzoites or tachyzoites) or genomic DNA. DNA from 3 GRA3 expressed sequence tag (EST) clones derived from an Me49 in vivo library (TgESTzz-33d01.r1; -26e04.r1; -41b02.s1) was next used as template to test out each primer individually for its ability to PCR amplify GRA3 when paired with either the T3 or T7 primer present in the EST cloning vector. Using this approach, the N-terminal sense primers paired with T3 amplified an expected ~1 kb transcript whereas the C-terminal antisense primers paired with either T3 or T7 failed to amplify (data not shown). Sequence analysis of the 1 kb transcript identified a 669 nucleotide open reading frame (ORF). BLAST analysis against the Toxoplasma EST database (Ajioka et al. 1998) identified the contig ctoxqual_533 (an assembly of 33 EST sequences) indicating that this ORF was likely to be expressed.
BLAST analysis of the published GRA3 sequence (U13771) against the Toxoplasma EST database identified 2 contigs, the ctoxqual_533 described above, which shared an identical N-terminus with the first 598 nucleotides of the published GRA3 sequence (U13771), and a EST ctoxqual_786 (an assembly of 14 EST sequences) that shared an identical C-terminus to published GRA3. Alignment of published GRA3, ctoxqual_533, _786 and the DNA sequence derived from the Me49 bradyzoite GRA3 ESTs above showed that, where the identity between published GRA3 and ctoxqual_533 stopped, the identity between GRA3 and ctoxqual_786 began and at this junction there was an EcoRI site. A separate independently initiated study that explored the relationship of these contigs by a similar PCR approach reached identical conclusions. The most parsimonious explanation for these results is that the published GRA3 sequence (U13771) is a chimera that arose by chance during the construction of the expression cDNA library used to clone GRA3. In effect, the published GRA3 sequence is an artificial tail-to-tail fusion between 2 distinct proteins.
DNA from the EST pN82944, which encodes a portion of the protein encompassed by the contig ctoxqual_786, shares an identical C-terminus to the published GRA3 and was expressed and purified in order to raise antibodies for localization and immunochemical studies. Antibodies raised against this peptide recognized a protein of approximately 65 kDa in Toxoplasma lysate antigen (TLA) (Fig. 1A). To obtain the full-length coding sequence of this 65 kDa antigen, hereafter referred to as Tg65, successive rounds of 5′RACE and genomic walking experiments were performed. Although this was useful in obtaining most of the unknown 5′ region of the GRA3 gene (see below), it failed to obtain the entire ORF. With the release of the ToxoDB genome database (http://www.toxodb.org), a large DNA fragment encompassed within contig TGG_784 was identified that included the genomic locus for Tg65. In order to obtain the full coding sequence, TGG_784 was analysed for potential Kozak sequences (5′-A/GCCATGG-3′) (Kozak, 1983) upstream from the known sequence. A potential site was identified at 4480 bp and a set of primers (forward 5′-GAC GCC CTTAACAGTAAGTCTC-3′ and reverse 5′-GATGGTCGCTCTCTCCCAGTC-3′) was designed to amplify the region between the area upstream from this potential Kozak-like sequence (5′-GTCATGG-3′) and a portion of the known sequence. Sequencing of this fragment, confirmed this to be the initiator codon as an upstream stop codon was present in the cDNA. The Tg65 genomic locus spans 3590 nucleotides, is comprised of 7 introns (Genbank Accession number AY223805 (Fig. 1B) and is spliced to yield a 1772 nucleotide ORF (Genbank Accession number AY223804).

Fig. 1. (A) The polyclonal antiserum was raised against the unique sequence of pN82944 and reacted with the homologous recombinant protein (Control) recognizing a band of 17 kDa and a presumed dimer of 34 kDa. This antiserum recognized a 65 kDa protein in Toxoplasma lysate antigen (TLA). (B) The genomic sequence spans 4783 bp and the 1817 bp coding sequence is composed of 8 exons (white) and 7 introns (black) the sizes of which are annotated above and below, respectively. The ATG start codon is found in exon 1 at 121 bp and the TAA stop codon is situated in exon 7 at 3573 bp.
The entire sequence of the predicted protein contained in the ctoxqual_533 sequence was readily amplified from genomic DNA by PCR (forward primer; 5′-TAGATCATCCGAACGCTCACG-3′ and reverse primer; 5′- AAAAACAACAGTACACTCAGCCC-3′). It contains an ORF of 669 nucleotides (Fig. 2A) that is translated as a protein of 264 amino acids in length with a predicted molecular weight of approximately 24 kDa (Genbank Accession number AANO3693). This sequence has a predicted von Heijne signal peptide (von Heijne, 1986), a C-terminal transmembrane domain consistent with membrane localization and a ‘KKXX’ ER retrieval motif at its extreme C-terminus. To establish that this sequence is in fact GRA3, the protein was expressed as a maltose binding protein (MBP) fusion in E. coli and tested for monoclonal antibody (MAb) 2H11 reactivity. 2H11 has previously been shown to react with a 24 kDa dense granule protein that is empirically defined as GRA3. This experiment was necessary since close examination of the published GRA3 sequence identified within its 3′ UTR another significant 638 nucleotide ORF in the −1 frame. Moreover, this other ORF had similarity with the contig, ctoxqual_3365 (comprising an assembly of 2 ESTs) present in the Toxoplasma EST database and possessed 38% sequence similarity with a mammalian PITSLRE protein kinase β gene, hence, as we had not yet obtained the entire sequence of Tg65 it was formally possible that this ORF was GRA3.
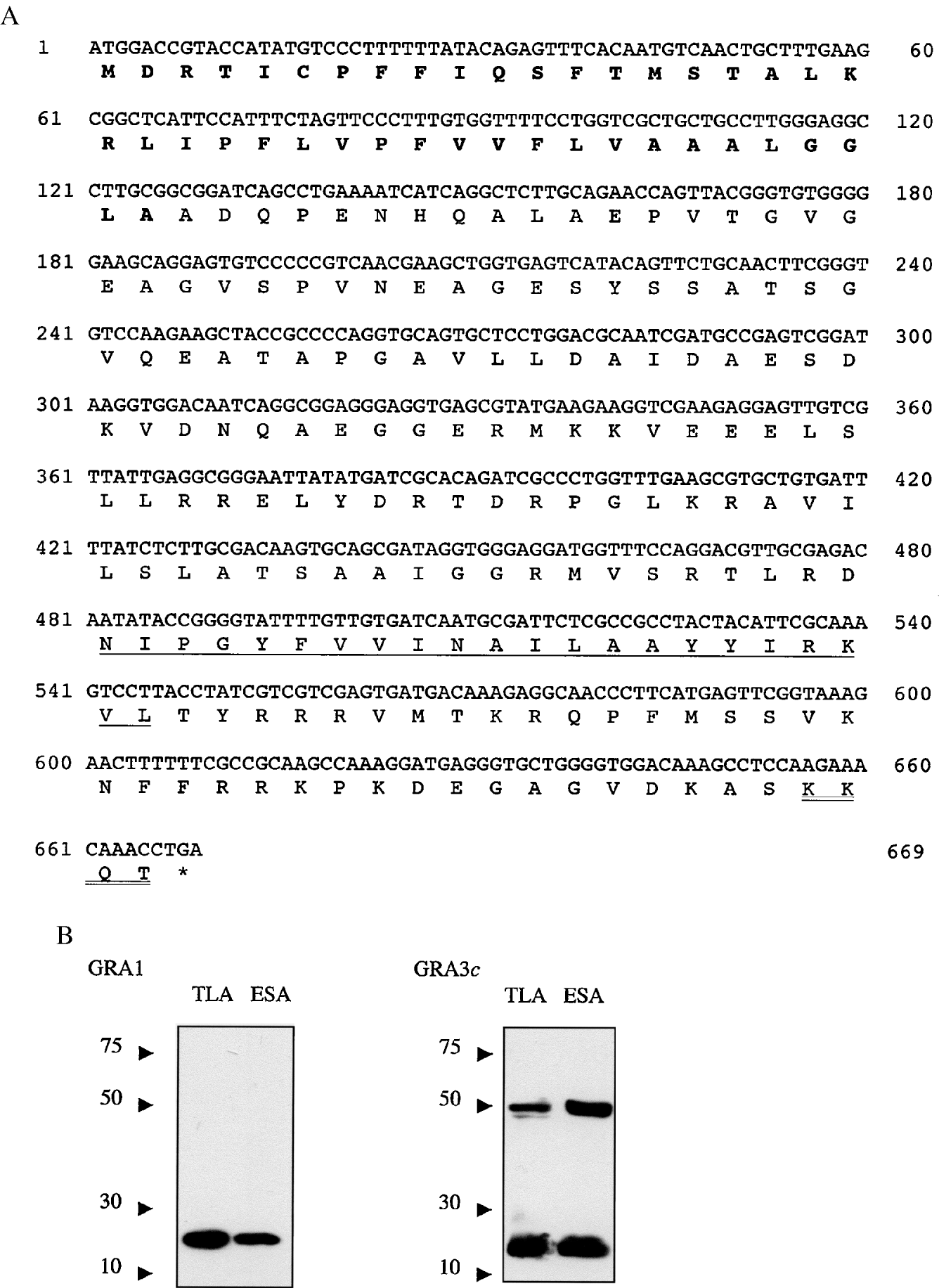
Fig. 2. (A) The ORF of GRA3 has a predicted molecular weight of 24 kDa. It contains a predicted cleavable signal peptide (BOLD) and a transmembrane region in the mature peptide (underlined) At the C-terminal there is a KKXX retrieval/retention motif (double underlined). (B) Antibodies to GRA3 recognize a protein of the predicted 24 kDa molecular weight and a probable dimer of approximately 48 kDa in both TLA and excretory–secretory antigen (ESA). In control lanes, monoclonal antibody TG17–43 which recognizes GRA1 reacts with a protein of the predicted molecular weight (23 kDa) in both TLA and ESA.
The ability of 2H11 to react specifically with the recombinant MBP-GRA3 protein, established that the 669 nucleotide ORF is in fact the authentic dense granule protein GRA3 (Fig. 3). Furthermore, antibodies raised to the corrected GRA3, obtained by DNA vaccination of mice, recognized a protein of approximately 24 kDa and a probable dimeric form of 48 kDa in TLA (Fig. 2B). A similar pattern of recognition was observed in T. gondii ESA confirming that this antigen is secreted by tachyzoites.

Fig. 3. GRA3-MBP fusion protein is recognized by 2H11 monoclonal antibody. Lysates from E. coli containing the plasmid pMAL-p2 encoding the GRA3-MBP fusion protein were subjected to Western blotting with the polyclonal antibody anti-MBP (left panel) and with the monoclonal antibody 2H11 (right panel). No reactivity was evident in non-induced cultures (U), but a protein of the predicted GRA3-MBP fusion was recognized following induction with IPTG (I).
Having shown that GRA3 is a secretory protein recognized by the MAb 2H11, which in immunoEM studies has been shown to label the IVN and the PVM (Achbarou et al. 1991; Fig. 5, this paper), we next tested whether the predicted signal sequence of GRA3 can direct GRA3 into the secretory pathway and target the protein to the dense granules. For this, 3 constructs all with a C-terminal GFP extension were made using the GFP expression plasmid ptubP30-GFP/sag-CAT (Striepen et al. 1998), namely, GRA3 (M)-GFP, comprised of the predicted mature GRA3, GRA3 (SP)-GFP, comprised of the predicted signal sequence of GRA3 and GRA3 (E)-GFP, comprised of the entire GRA3 protein. Transfection of tachyzoites with these constructs resulted in viable fluorescent parasites after 48 h. GFP localized to the cytoplasm of GRA3 (M)-GFP transfected parasites as expected (Fig. 4, Panel A). In GRA3 (SP)-GFP parasites, GFP localization in both extracellular (Fig. 4, Panel B) and intracellular parasites (data not shown) was discrete and granular within the parasite, consistent with dense granule staining (27). GFP was also present in the PV of transfected parasites (data not shown). A similar pattern of GFP localization was evident in GRA3 (E)-GFP transfected parasites (Fig. 4, Panel C). Taken together, these findings indicate that the signal peptide is both sufficient and necessary to direct GRA3 secretion via the dense granules into the PV.

Fig. 4. The signal sequence of GRA3 is necessary and sufficient to target GRA3 into the secretory pathway and into the PV as determined by GFP localization by fluorescence microscopy. In extracellular parasites the mature GRA3 protein (lacking the signal peptide) fused with GFP is retained within the cytoplasm of the parasite (Panel A). The signal peptide fused with GFP is targeted to discrete areas, consistent with the location of dense granules within the parasite (Panel B). In intracellular parasites, it is evident in a similar location within the parasite but is also secreted into the parasitophorous vacuole (Panel C). [Morphology was visualized by differential interference contrast microscopy (DIC). GFP location was visualized independently or in conjunction with DAPI by fluorescent microscopy (GFP and GFP/DAPI). Merged DIC and fluorescent images are labeled DIC/GFP and DIC/GFP/DAPI.]

Fig. 5. Immuno-electron microscopy of GRA3/GRA3 in Toxoplasma gondii infected Vero cells. Mab 2H11 reacts with dense granules, IVN and PVM (A). The anti-GRA3 label covers all the PVM surface, including the areas covered on the cytoplasmic face by host cell ER (B, er). (DG: dense granules; HC: host cell; M: mitochondria; N: nucleus; PVM: parasitophorous vacuole membrane; R: rhoptries; T: tachyzoite.)
A putative protein with homology to T. gondii GRA3 was found in the N. caninum EST project. Primers were designed to flank the putative coding region and PCR was performed from cDNA with annealing temperature of 56 °C. The sequence of NcGRA3 is highly similar to T. gondii GRA3 with many areas of identity (Genbank Accession number AY157874). In common with the T. gondii protein, a von Heijne signal sequence, a transmembrane region and a C-terminal ‘KKXX’ ER retrieval signal are evident in NcGRA3.
Herein we provide the corrected sequence of T. gondii dense granule protein GRA3 and describe the recharacterization of this protein. GRA3 is a 223 amino acid protein with a predicted molecular weight of 24 kDa. It has a predicted N-terminal signal sequence and a C-terminal transmembrane domain. Furthermore it has a C-terminus ER retrieval motif that may function in PV-ER organelle recruitment and/or association. A N. caninum homologue identified in the course of these studies is predicted to share all these features. The previously reported sequence for GRA3 is an artificial chimera comprising the N-terminus of GRA3 and the C-terminus of a 65 kDa protein that we have designated Tg65. Tg65 has none of the features normally associated with dense granule proteins such as a von Heijne signal sequence or a transmembrane region and is predicted to be a cytosolic protein.
Our finding that the monoclonal antibody 2H11 recognizes the corrected GRA3 is important as it validates many of the previous studies that used this antibody as a marker for dense granules (Achbarou et al. 1991). However, other features apparent on the corrected sequence prompt reinterpretation of the findings and conclusions from these previous studies (Bermudes et al. 1994). For example, immunolocalization studies using 2H11 demonstrated that GRA3 associates with the PVM and PVM extensions, which protrude into the cytoplasm of the host cell (Bermudes et al. 1994). This ability of GRA3 to associate with the membrane of the parasitophorous vacuole has been difficult to explain, since the previously published sequence did not have a transmembrane domain. Furthermore, the sequence lacks any motifs suggestive of myristoylation, isoprenylation or glycophosphatidyl inositol (GPI) addition. One explanation for the stable membrane association of GRA3 that has been put forth is that soluble GRA3 following exocytosis from dense granules undergoes a conformational change in association with oligomerization and insertion into the PVM. Other suggestions for the membrane association included the possibility that this protein may have a high affinity interaction with another PVM protein (Bermudes et al. 1994). The prediction of a transmembrane region in the corrected sequence, however, illustrates that no unusual or elaborate explanation for the localization and behaviour of this protein is necessary.
The presence of at least 1 transmembrane region is now a common feature of GRA proteins known to target the PVM after they are secreted from the dense granules including GRA5, GRA6 and GRA7. Furthermore, this observation is consistent for the homologous N. caninum proteins NcGRA6, NcGRA7 and NcGRA3 (identified as described herein). As GRA3 shares the same N-terminus originally reported for GRA3, the prediction of a signal sequence reported by Bermudes et al. (1994) remains unchanged. Consistent with this prediction we demonstrate that GRA3 is secreted from the tachyzoite stage when incubated in vitro. Through the use of GFP-fusion reporter constructs we demonstrate that the signal sequence efficiently delivers GFP to the PV. A fusion of the predicted mature protein to GFP remains in the cytoplasm of T. gondii. This demonstrates that the signal sequence is necessary and sufficient to facilitate secretion of protein into the PV.
The T. gondii PVM forms intimate associations with host cell organelles including the mitochondria and endoplasmic reticulum (Jones & Hirsch, 1972; Sinai et al. 1997). This association is likely to be mediated by parasite-derived proteins displayed on the PVM. Notably, ROP2 possesses a non-cleavable mitochondrial-like targeting sequence that may account for the mitochondrial-PVM association (Sinai & Joiner, 2001). It has been postulated that GRA3 plays a role in PVM-organelle association. In keeping with this, using the monoclonal antibody 2H11, GRA3 has been found to co-fractionate with elements of host cell endoplasmic reticulum (Sinai et al. 1997). The previously published GRA3 sequence gave no clues as to how this association could be formed. However, the T. gondii GRA3 sequence and the N. caninum homologue each have a KKXX C-terminal ER retrieval motif, which is known to retrieve soluble proteins back to the ER. This motif is not present on any of the other known GRA proteins from T. gondii or N. caninum. It is thus formally possible that this motif is responsible for the association of this molecule with the endoplasmic reticulum of host cells as evident from previous empirical studies (Sinai et al. 1997). ImmunoEM studies (using 2H11) reported herein, confirm that GRA3 is targeted to the PVM and IVN. 2H11 staining is apparent throughout the PVM including where it apposes the host cell ER and mitochondria. This places GRA3 in the correct location, but in itself does not provide proof of the role of this molecule in the ER association with the PVM. The impact of sequestration of the host cell ER to the perimeter of the PV remains to be determined for both host and parasite. Determination of the topology of GRA3 and the construction and characterization of GRA3 deficient parasites will be necessary to elucidate the potential role of GRA3 in this process.
The function of Tg65 also identified during the course of these studies is not obvious through similarity searches or predictive algorithms. However, it would appear to be an important apicomplexan protein as highly homologous hypothetical proteins are evident in Plasmodium falciparum (Accession number AAN35752) and in P. yeolii (Accession number EAA21589). Furthermore, BLAST analysis of the ongoing Eimeria tenella and Theileria annulata genome projects (http://www.sanger.ac.uk/DataSearch/) identifies putative homologues.
T. gondii is an obligate intracellular pathogen that resides inside host cells inside a specialized non-fusogenic vacuole (PV), delimited by the PVM, decorated by host cell mitochondria and endoplasmic reticulum. Identification of the molecular components underpinning the unique nature of this intracellular niche will greatly facilitate the understanding of the biology of vacuolar pathogens and interactions of these pathogens with the host cell (Sinai et al. 1997). Parasite proteins contained in dense granules and secreted into the vacuole after cell invasion contribute to the modification of the PVM. Our studies describe the characterization of a T. gondii GRA protein that is likely to have such role, perhaps by facilitating PVM-organelle association. Future studies with the correct sequence can now determine the role of this protein in host–parasite interactions.
Preliminary genomic and cDNA sequence data was accessed via http://ToxoDB.org and http://www.tigr.org/tdb/t_gondii/. Genomic data were provided by The Institute for Genomic Research (supported by the NIH grant no. AI05093). EST sequences were generated by Washington University (NIH grant no. 1R01AI045806-01A1). F.L.H. was supported by a University of Strathclyde Studentship. Additional support was from the Scottish Hospital Endowments Research Trust, NIH, USA RO1 AI-43228, AI-27530 and AI-21423, and the Wellcome Trust. Electron microscopy was performed at the CCME, Université de Montpellier 2.
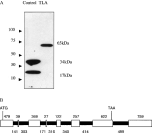
Fig. 1. (A) The polyclonal antiserum was raised against the unique sequence of pN82944 and reacted with the homologous recombinant protein (Control) recognizing a band of 17 kDa and a presumed dimer of 34 kDa. This antiserum recognized a 65 kDa protein in Toxoplasma lysate antigen (TLA). (B) The genomic sequence spans 4783 bp and the 1817 bp coding sequence is composed of 8 exons (white) and 7 introns (black) the sizes of which are annotated above and below, respectively. The ATG start codon is found in exon 1 at 121 bp and the TAA stop codon is situated in exon 7 at 3573 bp.

Fig. 2. (A) The ORF of GRA3 has a predicted molecular weight of 24 kDa. It contains a predicted cleavable signal peptide (BOLD) and a transmembrane region in the mature peptide (underlined) At the C-terminal there is a KKXX retrieval/retention motif (double underlined). (B) Antibodies to GRA3 recognize a protein of the predicted 24 kDa molecular weight and a probable dimer of approximately 48 kDa in both TLA and excretory–secretory antigen (ESA). In control lanes, monoclonal antibody TG17–43 which recognizes GRA1 reacts with a protein of the predicted molecular weight (23 kDa) in both TLA and ESA.
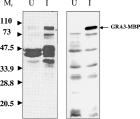
Fig. 3. GRA3-MBP fusion protein is recognized by 2H11 monoclonal antibody. Lysates from E. coli containing the plasmid pMAL-p2 encoding the GRA3-MBP fusion protein were subjected to Western blotting with the polyclonal antibody anti-MBP (left panel) and with the monoclonal antibody 2H11 (right panel). No reactivity was evident in non-induced cultures (U), but a protein of the predicted GRA3-MBP fusion was recognized following induction with IPTG (I).

Fig. 4. The signal sequence of GRA3 is necessary and sufficient to target GRA3 into the secretory pathway and into the PV as determined by GFP localization by fluorescence microscopy. In extracellular parasites the mature GRA3 protein (lacking the signal peptide) fused with GFP is retained within the cytoplasm of the parasite (Panel A). The signal peptide fused with GFP is targeted to discrete areas, consistent with the location of dense granules within the parasite (Panel B). In intracellular parasites, it is evident in a similar location within the parasite but is also secreted into the parasitophorous vacuole (Panel C). [Morphology was visualized by differential interference contrast microscopy (DIC). GFP location was visualized independently or in conjunction with DAPI by fluorescent microscopy (GFP and GFP/DAPI). Merged DIC and fluorescent images are labeled DIC/GFP and DIC/GFP/DAPI.]

Fig. 5. Immuno-electron microscopy of GRA3/GRA3 in Toxoplasma gondii infected Vero cells. Mab 2H11 reacts with dense granules, IVN and PVM (A). The anti-GRA3 label covers all the PVM surface, including the areas covered on the cytoplasmic face by host cell ER (B, er). (DG: dense granules; HC: host cell; M: mitochondria; N: nucleus; PVM: parasitophorous vacuole membrane; R: rhoptries; T: tachyzoite.)