INTRODUCTION
Numerous studies in vertebrates have found evidence that inbreeding compromises the immune system (reviewed by Keller and Waller, Reference Keller and Waller2002) giving rise to the hypothesis that inbreeding reduces an individual's ability to resist parasites and pathogens. In contrast to the findings in vertebrates, most studies on insects have failed to find support for this hypothesis. For example, Gerloff et al. (Reference Gerloff, Ottmer and Schmid-Hempel2003) did not find a significant effect of inbreeding (brother–sister mating) on innate immune defence (measured as the encapsulation response against nylon monofilament) in the bumble-bee, Bombus terrestris. In the termite, Zootermopisis angusticollis, Calleri et al. (Reference Calleri, McGrail, Reid, Rosengaus, Vargo and Traniello2006) found that inbreeding decreased neither the encapsulation response against nylon monofilament nor the resistance against a bacterial or a fungal disease (Calleri et al. Reference Calleri, McGrail, Reid, Rosengaus, Vargo and Traniello2006). Similarly, studies using inbred lines in the flour beetle, Tribolium castaneum, (Stevens et al. Reference Stevens, Yan and Pray1997) and in the sand cricket, Gryllus firmus, (Rantala and Roff, Reference Rantala and Roff2006) failed to find a significant effect of inbreeding on immunity. However, in the autumnal moth, Epirrita autumnata, Rantala and Roff (Reference Rantala and Roff2007) did find that the effect of inbreeding on the immune response differed between the sexes: in females, inbreeding significantly reduced the encapsulation response against nylon monofilament, whereas it did not have a significant effect on the male immune response. Likewise, in Drosophila melanogaster, Spielman et al. (Reference Spielman, Brook, Briscoe and Frankham2004) found that both inbreeding and loss of genetic diversity reduced the resistance of larvae to bacterial diseases. Thus, more studies testing the effect of inbreeding on insect immunity are needed before any generalization can be made, if indeed one exists.
Insect immunity is characterized by the inducible expression of a large array of antimicrobial peptides and by the constitutive melanization–encapsulation response. Encapsulation is an immune response through which insects defend themselves against endoparasitoid wasps and flies, mites, fungi, nematodes, bacteria and viruses (Washburn et al. Reference Washburn, Kirkpatrick and Vokman1996; reviewed by Gupta, Reference Gupta2001). During the encapsulation process, specialized haemocytes recognize invading particles as non-self and cause other haemocytes to aggregate and encapsulate the particle. A cascade of reactions involving the tyrosinephenyloxidase pathway causes the melanization of the capsule and the death of the invading particle (Fisher, Reference Fisher1963). Phenoloxidase (PO) is a key enzyme in the synthesis of melanin and the ability to produce melanin is an important aspect of the immune response (Gillespie et al. Reference Gillespie, Kanost and Trenczek1997). In insects, the easiest and the most commonly used method to assay the strength of the encapsulation response is to measure the cellular/melanization encapsulation response against a novel and standardized antigen such as a nylon monofilament (e.g. Köning and Schmid-Hempel, Reference Köning and Schmid-Hempel1995; Rantala et al. Reference Rantala, Koskimäki, Taskinen, Tynkkynen and Suhonen2000; Kapari et al. Reference Kapari, Haukioja, Rantala and Ruuhola2006; Córdoba-Aguilar et al. Reference Córdoba-Aguilar, Contreras-Garduño, Peralta-Vázquez, Luna- González, Campa-Córdova and Ascencio2006; Contreras-Garduño et al. Reference Contreras-Garduño, Córdoba-Aguilar, Lanz-Mendoza and Cordero Rivera2009). It has been shown that the ability to encapsulate abiotic material is strongly related to the ability to encapsulate a parasite (Paskewitz and Riehle, Reference Paskewitz and Riehle1994; Gorman et al. Reference Gorman, Cornel, Collins and Paskewitz1996). In the autumnal moth, Epirrita autumnata, Rantala and Roff (Reference Rantala and Roff2007) found that the ability to encapsulate nylon monofilament is associated with an ability to resist an entomopathogenic fungal disease, Beauveria bassiana, suggesting that this widely used method to measure the strength of immune defence in insects is also a biologically relevant method.
The mealworm beetle Tenebrio molitor L. (Coleoptera, Tenebrionidae) is a cosmopolitan pest of stored grains. Previous studies of this species have found that the immune defence is condition-dependent, being downregulated by nutritional stress (Rantala et al. Reference Rantala, Kortet and Vainikka2003b; Siva-Jothy and Thompson, Reference Siva-Jothy and Thompson2002) and juvenile hormone treatment (Rantala et al. Reference Rantala, Kortet, Kotiaho, Vainikka and Suhonen2003a, Rolff and Siva-Jothy, Reference Rolff and Siva-Jothy2002). The aim of this study was to test whether inbreeding in T. molitor reduces body condition and has an effect on the immune response as measured by the encapsulation response (potential immune response) and resistance against the entomopathogenic fungus B. bassiana (realized immune responses). Beauveria bassiana grows commonly in soils throughout the world (Mietkiewski and Tkaczuk, Reference Mietkiewski and Tkaczuk1998) and acts as a parasite on many kinds of insects and arthropods, including our model species, Tenebrio molitor (e.g. Dromph, Reference Dromph2003). Thus, it is a natural pathogen in this system and one that T. molitor is likely to encounter.
MATERIALS AND METHODS
Insects
The beetles used in the experiment were taken from a laboratory stock population originating from a large outbred colony from Imazon Sweden and maintained at the University of Turku for 2 generations by the authors at 28°C under a 14:10 h light/dark photo-period and fed with wheatbran and fresh apple. The grandparental generation was derived as pupae from a large bulk laboratory stock (maintained at 500–1000 individuals). Shortly after emergence the beetles were placed in individual plastic film roll canisters with an excess of apple. Sexes were physically isolated to ensure virginity. Upon eclosion to adults, male and female pairs were set up and the offspring (parental generation) were reared in full sib-families in small plastic boxes (0·5 litre) and fed with wheatbran and fresh apple ab libitum.
Crossing design
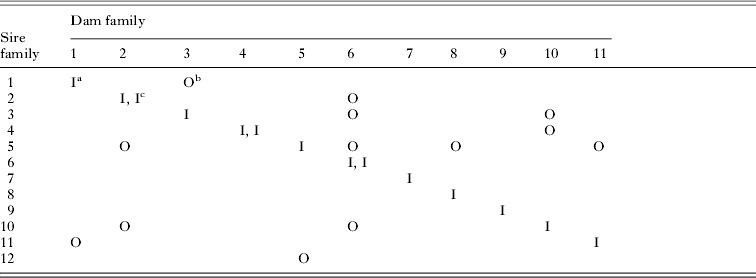
After eclosion of the parental generation we arranged matings to create inbred and outbred families. Inbred families were created by brother-sister mating, and outbred families by mating between randomly chosen, unrelated individuals (Table 1). Females were allowed to lay eggs for 3 days after which females were removed from the vial. From each family we randomly chose 40 small larvae that were split between 4 (0·25 litre) cages (10 per cage). Some families had less than 40 larvae and for those families we reduced the number of cages. We checked cages daily to find newly eclosed individuals. After eclosion, sexes were physically isolated to ensure virginity. Beetles were included in the experiments when aged between 9 and 14 days post-eclosion. The developmental time of an individual was measured as the time between the establishment of the cage (egg-laying) and eclosion. Before the immune assays, we measured the wet body mass of each beetle to the nearest 0·1 mg.
Table 1. Matrix showing the sets of crosses used, where the numbers represent different oubred families

a Inbred family formed by brother-sister mating of a sire and dam from outbred family designated as 1.
b Outbred family formed by mating of a sire from outbred family 1 with a dam from outbred family 2.
c Two inbred families created.
Encapsulation rate assay
The beetles were chilled on ice for 20 min to anaesthetize them. Following the chilling we inserted a 2 mm long piece of nylon monofilament (diameter 0·18 mm and knotted at one end) through a puncture in the pleural membrane between the second and third sternite. The knot allowed us to non-destructively remove the monofilament. The beetle's immune system was given 3 h to react to this object while the insects were kept individually in film roll canisters at 28±1°C. Previous studies have found that a time period of 3 h produces the largest differences between individuals (e.g. Rantala et al. Reference Rantala, Jokinen, Kortet, Vainikka and Suhonen2002). After 3 h the implant was removed and frozen for later examination. The removed monofilament was photographed under a light microscope from 3 different angles using a digital video. These pictures were analysed using an image analysis program (Image Pro, Media Cybernetics Inc., Carlsbad, CA, USA). The degree of encapsulation was measured as the mean grey value of reflecting light from 2 pictures of the implants. The scale was calibrated to indicate that the darkest grey reflected the highest encapsulation rate (total black). It has previously been shown that the repeatability of the measurement is very high (repeatability, R=0·997; Rantala et al. Reference Rantala, Jokinen, Kortet, Vainikka and Suhonen2002).
Fungal assay
The day after the encapsulation assays, we tested the resistance against the entomopathogenic fungus B. bassiana (strain Mycotrol). The animals used in the fungal assay were the same individuals as those used in the encapsulation rate assay. Half of the individuals from each family were infected with the fungi and half were used as controls. Fungal spores were suspended in distilled water (for more detailed methods see Valtonen et al. Reference Valtonen, Viitaniemi and Rantala2010) and the beetles were exposed to the fungus by dipping them in spore solution for 5 s. The dose was determined by giving a separate group of larvae originating from stock population 5 increasing doses of conodians (20 males/dose) and selecting the dose that came closest to killing 50% of the animals (1·42×106 spores/ml). Controls were dipped in distilled water without fungal spores. After treatment the beetles were individually incubated in a hermetic box at the constant room temperature (21±1°C: because of logistical limitations this temperature differed from that of the encapsulation experiment) and fed with fresh apple ad libitum. Mortality was checked daily and the cause of death (sporulation) was verified. If beetles survived more than 2 weeks (14 days) they were recorded as having survived.
Statistical procedures
Because the data consisted of matings between different families and the overall resulting set of mating combinations was highly unbalanced (Table 1), we adopted a mixed model analysis of variance approach. Specifically, the data were analysed using the ‘animal’ model (Kruuk, Reference Kruuk2004) as implemented by the asreml© program. Mating type (inbred or outbred) and sex were entered as fixed factors and individual as a random factor. Because survival data were coded as 0,1 a binomial error term was used for these analyses. No interactions were significant in any of the analyses (P>0·5) and are not included in the results presented below. Significant inbreeding depression indicates the presence of directional dominance variance but its magnitude cannot be estimated from the present data. Heritability estimates in the present case are potentially inflated by dominance variance and thus significant heritabilities, while indicative of genetic variation, may be overestimates of narrow sense heritabilities.
RESULTS
Effects of inbreeding on weight, development time and survival
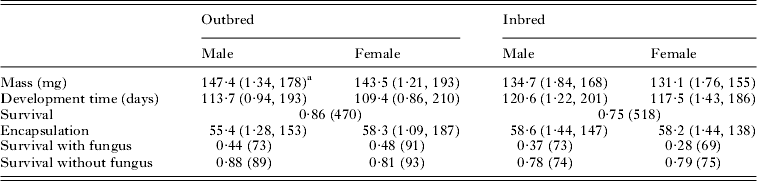
Visual inspection of the summary statistics (Table 2) suggests that males were larger than females and both sexes suffered a reduction in mass when inbred. This was confirmed by the statistical analysis which showed highly significant effects due to mating type (inbred/outbred) (F 1,28·3=18·91, P<0·001) and sex (F 1,674·3=15·04, P<0·001). Development time was significantly longer in inbred individuals than outbred individuals (F 1,25·7=8·90, P=0·006) and shorter in males than in females (F 1,766·1=13·75, P<0·001). Many larvae died before their sex could be established: therefore, the analysis of the effect of inbreeding on pre-adult survival was done using mating type only. The survival probability of inbred individuals was significantly less than that of outbred individuals (F 1,45·3=6·54, P=0·014, Table 2).
Table 2. Basic statistics (average values) nested by mating type (inbred or outbred) and sex
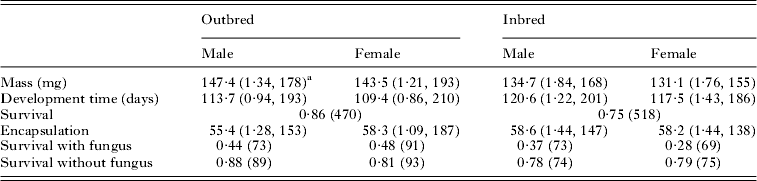
a s.e., sample size. Only sample size is given for proportions.
Effects of inbreeding on the immune response
There was no significant effect of inbreeding or sex on the encapsulation response (F 1,26·8=0·19, P=0·667 for inbreeding and F 1,605·2=1·61, P=0·205 for sex). Survival following fungal treatment is potentially confounded by the prior treatment measuring the encapsulation response: therefore, we included the encapsulation response in the statistical model. The presence or absence of inoculation with the fungus was taken as a fixed effect. Because the animal model failed to converge using a binomial error term, we used a normal error term. There was no significant effect due to pedigree (χ 12=1·038, P>0·25: log-likelihood ratio test). There was also no significant effect due to the encapsulation response (F 1,605= 0·52, P=0·473) or sex (F 1,606·6=0·52, P=0·473) but both infection and inbreeding reduced survival (F 1,597·9=144·63, P<0·001 and F 1,111·5=4·69, P= 0·032, respectively; Table 2).
In summary, the encapsulation response was not significantly correlated with mating type (inbred vs outbred) nor with survival following fungal treatment but survival following fungal treatment was significantly reduced in inbred individuals.
Heritability estimates
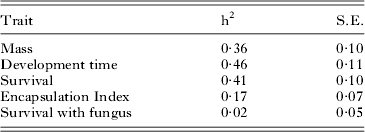
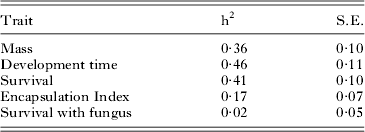
Because the encapsulation response showed no significant relationship with mating type or sex, we dropped these variables from the estimation of heritability. To estimate the heritability of survival following fungal treatment we were necessarily restricted to only those individuals that were treated, which greatly reduced the sample size and statistical power to detect an effect. Mating type was entered as a fixed effect and a binomial error term used: in this case the model converged although, as suspected from the previous analysis, the heritability estimate was not significantly different from zero. In contrast, all other heritabilities were significant and moderate to large in value (Table 3).
Table 3. Heritability estimates obtained using the ‘animal’ model
DISCUSSION
In this study we found that one generation of inbreeding did not affect encapsulation response in T. molitor, despite significant inbreeding depression in other life-history traits. This is a somewhat unexpected result because the encapsulation response has been found to be condition-dependent in T. molitor (Siva-Jothy and Thompson, Reference Siva-Jothy and Thompson2002; Rantala et al. Reference Rantala, Kortet and Vainikka2003b) and also in many other insect species (e.g. Koskimäki et al. Reference Koskimäki, Rantala, Suhonen, Taskinen and Tynkkynen2004; Contreras-Garduño et al. Reference Contreras-Garduño, Lanz-Mendoza and Córdoba-Aguilar2007; Yang et al. Reference Yang, Ruuhola and Rantala2007). Our finding is consistent with many previous studies on insects that have found that inbreeding did not have a significant effect on potential immune responses (Gerloff et al. Reference Gerloff, Ottmer and Schmid-Hempel2003; Calleri et al. Reference Calleri, McGrail, Reid, Rosengaus, Vargo and Traniello2006; Rantala and Roff, Reference Rantala and Roff2006; but see Rantala and Roff, Reference Rantala and Roff2007). Although inbreeding did not affect encapsulation response (innate immunity) it did reduce an individual's ability to resist the entomopathogenic fungi, suggesting that realized immune responses are more sensitive to inbreeding than innate immunity in T. molitor. On the other hand, maximal immunity is not always optimal (e.g. Schmid-Hempel, Reference Schmid-Hempel2003). Instead optimal investment on immunity may depend on trade-offs between the costs and benefits of immunity to other life-history components such as growth, reproduction or somatic maintenance and inbreeding might have an effect on the cost and benefits of immune defence. Thus the optimal level of investment on potential immune responses and different components of immunity in insects might differ between inbred and outbred individuals.
Other studies have also found that ‘potential’ and ‘realized’ immunity are not necessarily associated. For example, Schwarzenbach and Ward (2006) selected the yellow dung fly, Scathophaga stercoraria for increased phenoloxidase (PO) activity, which is an important component of the encapsulation response. They found no difference in resistance to entomopathogenic fungi between high PO and control selection lines (Schwarzenbach and Ward, Reference Schwarzenbach and Ward2007). Wilson et al. (Reference Wilson, Cotter, Reeson and Pell2001) showed that PO activity was associated with resistance to B. bassiana (the same fungus used in our study) in Spodoptera littoralis but not in S. exampta larvae. Schwartz and Koella (Reference Schwartz and Koella2002) showed in Anopheles gambiae that bead encapsulation does not reflect a mosquito's susceptibility to malaria parasites in the field. Similarly, variation in PO activity did not predict resistance to parasites in the cricket, Gryllus texensis (Adamo, Reference Adamo2004) or in the water flea, Daphnia magna, populations (Mucklow et al. Reference Mucklow, Vizoso, Jensen, Refardt and Ebert2004).
Studies on insects have shown that activation of the immune system is costly and can reduce survival (e.g. Moret and Schmid-Hempel, Reference Moret and Schmid-Hempel2000; Armitage et al. Reference Armitage, Thompson, Rolff and Siva-Jothy2003; but see Suhonen et al. Reference Suhonen, Honkavaara and Rantala2010). Thus the cost of encapsulation rather than the fungal treatment might be hypothesized to be the causative factor that reduced survival of the inbred beetles relative to the outbred ones (or at least exacerbated the effect). However, a previous study with the beetles originating from same population found that activation of the immune system by the nylon monofilament did not reduce survival (Vainikka et al. Reference Vainikka, Rantala, Seppälä and Suhonen2007). On the other hand, previous studies on T. molitor have found that activation of the immune system by LPS leads to a long-lasting antimicrobial response, which provided a survival benefit when the larvae were exposed to fungal infection, a phenomenon known as ‘immune-priming’ (see Moret and Siva-Jothy, Reference Moret and Siva-Jothy2003). Likewise, previous activation of the immune system by nylon monofilament increased the encapsulation response against nylon monofilament following immune measurements (Kivleniece et al. Reference Kivleniece, Krams, Krama, Daukste and Rantala2010). Thus, it is plausible that ‘immune-priming’ may have protected the beetles somewhat from the fungal challenge. Under this scenario, the difference in survival between the inbred and the outbred beetles underestimated what would have happened in the absence of immune-priming.
We found a strong family effect on the encapsulation response, suggesting genetic variation (and/or maternal effects) on the encapsulation response, which is consistent with studies in other species (e.g. Cotter and Wilson, Reference Cotter and Wilson2002; Cotter et al. Reference Cotter, Kruuk and Wilson2004; Schwarzenbach et al. Reference Schwarzenbach, Hosken and Ward2005; van Ooik et al. Reference van Ooik, Rantala and Saloniemi2007). However, there was no statistically significant family effect on disease resistance, which, given that there was a significant effect of inbreeding, we suggest that this may be due to low statistical power. Previous studies in T. molitor suggest that females prefer the pheromones of males with a strong encapsulation response (Rantala et al. Reference Rantala, Jokinen, Kortet, Vainikka and Suhonen2002). Thus, in the light of this study it seems that females might enhance the encapsulation of their offspring by preferring males with a strong encapsulation response.
Surprisingly, in this study we found no association between resistance against the fungal disease and the encapsulation response against the nylon monofilament. Previous studies in insects have found that the ability to encapsulate abiotic material is strongly related to the ability to encapsulate a parasite (Paskewitz and Riehle, Reference Paskewitz and Riehle1994; Gorman et al. Reference Gorman, Cornel, Collins and Paskewitz1996). Likewise, in the autumnal moth, Epirrita autumnata, we found that the encapsulation response against nylon monofilament is associated with resistance against B. bassiana (Rantala and Roff, Reference Rantala and Roff2007). It is important to note that the immunological methods and the pathogen strain used with E. autumnata were exactly the same as those used in the present experiment. Thus, there seem to be substantial differences in association between the potential and the realized immune responses between species. Because we measured the encapsulation response of an individual against nylon monofilament before we tested their resistance against the fungi, it is possible that the method used may have had an effect on the association between the encapsulation response and the resistance against the fungi. It is possible that individuals that had strong immune responses against the nylon monofilament might have had relatively less resources left to resist fungal infection than those individuals whose immune system responded less towards nylon monofilament, which might mask an association between resistance against nylon monofilament and resistance against the fungal diseases. On the other hand, if there is a different limiting substance for the encapsulation response in E. autumnata than T. molitor, we could expect to find a different association between these parameters. For example, if tyrosine is a limiting factor then an individual that had already successfully encapsulated an implant might not have sufficient tyrosine left to synthesize melanin in the encapsulation response towards the fungi, although they would have more phenoloxidase (PO) enzyme and haemocytes available than those individuals that had not been able to encapsulate the implant. It is also possible that encapsulation simply does not reflect resistance against B. bassiana in insects, and the association between the encapsulation response and the survival following fungal treatment in E. autumnata is caused by some unknown factor, like body condition, that may have an influence on both traits. Mallon et al. (Reference Mallon, Loosli and Schmid-Hempel2003) found in Bombus terrestris that bumblebee colonies that were more resistant against gut trypanosomal parasites had a lower encapsulation response against the nylon monofilament. These results suggest a trade-off in this species between specific and non-specific components of the immune system at the colony level (Mallon et al. Reference Mallon, Loosli and Schmid-Hempel2003). Although, they did not measure the association between the encapsulation response and resistance against parasites at an individual level, it seems that an association between innate immunity and specific immunity is more complicated than previously thought and there might be large differences between taxa.
Studies on vertebrates have often found that females have superior immunity compared to the males (Zuk and McKean, Reference Zuk and McKean1996; Møller et al. Reference Møller, Sorci and Erritzøe1998). The ultimate mechanism for sex differences in immune function has been suggested to be differential selection on the sexes favouring different investment levels in immune defence (Zuk, Reference Zuk1990; Zuk and McKean, Reference Zuk and McKean1996; Rolff, Reference Rolff2002) and the proximate mechanism is suggested to be the detrimental side effects of testosterone on immunity (Folstad and Karter, Reference Folstad and Karter1992; Zuk and McKean, Reference Zuk and McKean1996). Thus, the disparity in how sexes allocate resources is thought to result in sexual dimorphism in immune defence, even among taxa lacking testosterone (Zuk and Stoehr, Reference Zuk and Stoehr2002). However, studies on invertebrates have found no consistent pattern in sex differences in immunity. Although in some species females have been found to have superior immunity (e.g. Gray, Reference Gray1998; Kurtz et al. Reference Kurtz, Wiesner, Götz and Sauer2000; Adamo et al. Reference Adamo, Jensen and Younger2001;Vainio et al. Reference Vainio, Hakkarainen, Rantala and Sorvari2004; Schwarzenbach et al. Reference Schwarzenbach, Hosken and Ward2005; Gershman, Reference Gershman2008), there are many studies that have not found any sex differences in immunity (e.g. Gillespie and Khachatourians, Reference Gillespie and Khachatourians1992; da Silva et al. Reference da Silva, Dunphy and Rau2000; Yourth et al. Reference Yourth, Forbes and Smith2001, Reference Yourth, Forbes and Smith2002a, Reference Yourth, Forbes and Smithb; Rantala and Roff, Reference Rantala and Roff2005; Lindsey and Altizer, Reference Lindsey and Altizer2009) and sometimes males have been found to possess stronger immunity (Siva-Jothy and Thompson, Reference Siva-Jothy and Thompson2002; Fedorka et al. Reference Fedorka, Zuk and Mousseau2004; Zuk et al. Reference Zuk, Simmons, Rotenberry and Stoehr2004; Rantala et al. Reference Rantala, Roff and Rantala2007; Kelly and Jennions, Reference Kelly and Jennions2009). In concordance with experimental studies, a meta-analysis by Sheridan et al. (Reference Sheridan, Poulin, Ward and Zuk2000) did not detect sex differences in parasite infections found among arthropod hosts. In the present study we did not find any sex differences in immunity in T. molitor. In contrast to previous studies (Rantala and Roff, Reference Rantala and Roff2007) we did not find any sex differences in the effect of inbreeding on immunity and other life-history traits. Thus, the sex differences in genetic architecture in immunity seem to differ between species, which could explain the inconsistency between studies testing sex differences on immunity in insects. Of particular significance, we found that inbreeding reduced the resistance against the entomopathogenic fungi, but it did not have any effect on the encapsulation response, suggesting fundamental differences in the amount of directional dominance in these components of immune defence. Inbreeding reduced survival following fungal treatment, which is a direct measurement of the strength of immunity, but the effect of inbreeding was not visible when we measured the strength of immune defence indirectly by using the nylon monofilament method. Thus, our study highlights the importance of measuring several different parameters of immunity and pathogens and parasites when testing the effect of inbreeding on immune defence.
ACKNOWLEDGEMENTS
Special thanks to Ulla Anttila for assistance in the laboratory. This work was supported by the Academy of Finland to M.J.R.