Background
The global Cesarean section (C-section) rate nearly doubled between 2000 and 2015 when epidemiologists estimate that 21.1% of births occurred via C-section, with wide disparities in C-section rates ranging from 44.3% in Latin America and the Caribbean to only 4.1% in west and central Africa.Reference Boerma, Ronsmans and Melesse1 According to the Centers for Disease Control and Prevention, the rate of C-section in the US was 31.9% in 2016.2 While medically necessary C-sections can save the lives of mothers and infants, the World Health Organization has concluded, “at [the] population level, Cesarean section rates higher than 10% are not associated with reductions in maternal and newborn mortality rates.”3 The perceived overuse of C-section in many regions has led the International Federation of Gynecology and Obstetrics to label C-section use an “epidemic.”Reference Visser, Ayres de Campos and Barnea4
The global rise in C-section deliveries portends concern not only for women's health, but also for the health of the offspring who enter the world with this exposure.Reference Sandall, Tribe and Avery5 Data from prospective birth cohorts have shown that C-section delivery is associated with greater offspring weight gain and adiposity over the first year of life,Reference Mueller, Zhang, Hoyo, Østbye and Benjamin-Neelon6 as well as greater risk of becoming overweight or obese in childhoodReference Huh, Rifas-Shiman and Zer7 and adulthood.Reference Darmasseelane, Hyde, Santhakumaran, Gale and Modi8 The delivery mode-obesity link has been confirmed by 3 meta-analyses, with the most recent showing that C-section increases offspring obesity risk by 34%.Reference Li, Zhou, Liu, Kuhle, Tong and Woolcott9 In addition to obesity, C-section has been associated with offspring risk of asthma, allergies, and other immune-related disorders.Reference Renz-Polster, David and Buist10 While early findings on this topic were mixed,Reference Magnus, Håberg, Stigum, Almqvist, Cnattingius, Lichtenstein, Lundholm, Kero, Gissler, Grönlund, Salam, Margolis, McConnell, Huang, Chen, Zhao, Park, Kim, Choi, Juhn, Weaver, Katusic, Yunginger, Maitra, Sherriff and Strachan11 a more recent population-based study in Denmark, with more than 2 million children followed over a 35-year period, found C-section delivery was associated with increased risk of asthma, systemic connective tissue disorders, juvenile arthritis, inflammatory bowel disease, immune deficiencies, and leukemia.Reference Sevelsted, Stokholm, Bønnelykke and Bisgaard12 Moreover, in a Swedish cohort of over 1 million participants, C-section delivered children had a 21% higher risk of developing food allergies.Reference Mitselou, Hallberg and Stephansson13
We and other scientists have hypothesized that the observed associations of C-section delivery with off-spring health conditions are, at least in part, due to non-transmission of vaginal microbes from mother to newborn at birth.Reference Mueller, Bakacs, Combellick, Grigoryan and Dominguez-Bello14 Infants born vaginally harbor bacterial communities that resemble their mother's vaginal microbiota, while those born by C-section harbor communities similar to those found on the skin.Reference Dominguez-Bello, Costello and Contreras15 While randomization to delivery mode is not ethical, it is ethical and feasible to randomize C-section delivered neonates to be exposed vs. not exposed to their mother's vaginal microbiota, which they would have received had they not been C-section delivered. Such an experimental design affords the unique ability to test whether maternal vaginal microbiota indeed affects infant microbiota development and disease risk.
Vaginal Seeding
Vaginal seeding is a technique that uses gauze to transfer the maternal vaginal microbiota from the mother's vagina to a newborn delivered by C-section (see Figure 1). The theory behind this technique is that it will restore the newborn's microbiota to a state that more closely resembles that of a vaginally born baby and, therefore, possibly decrease the risk of C-section-associated diseases.Reference Iacobucci16
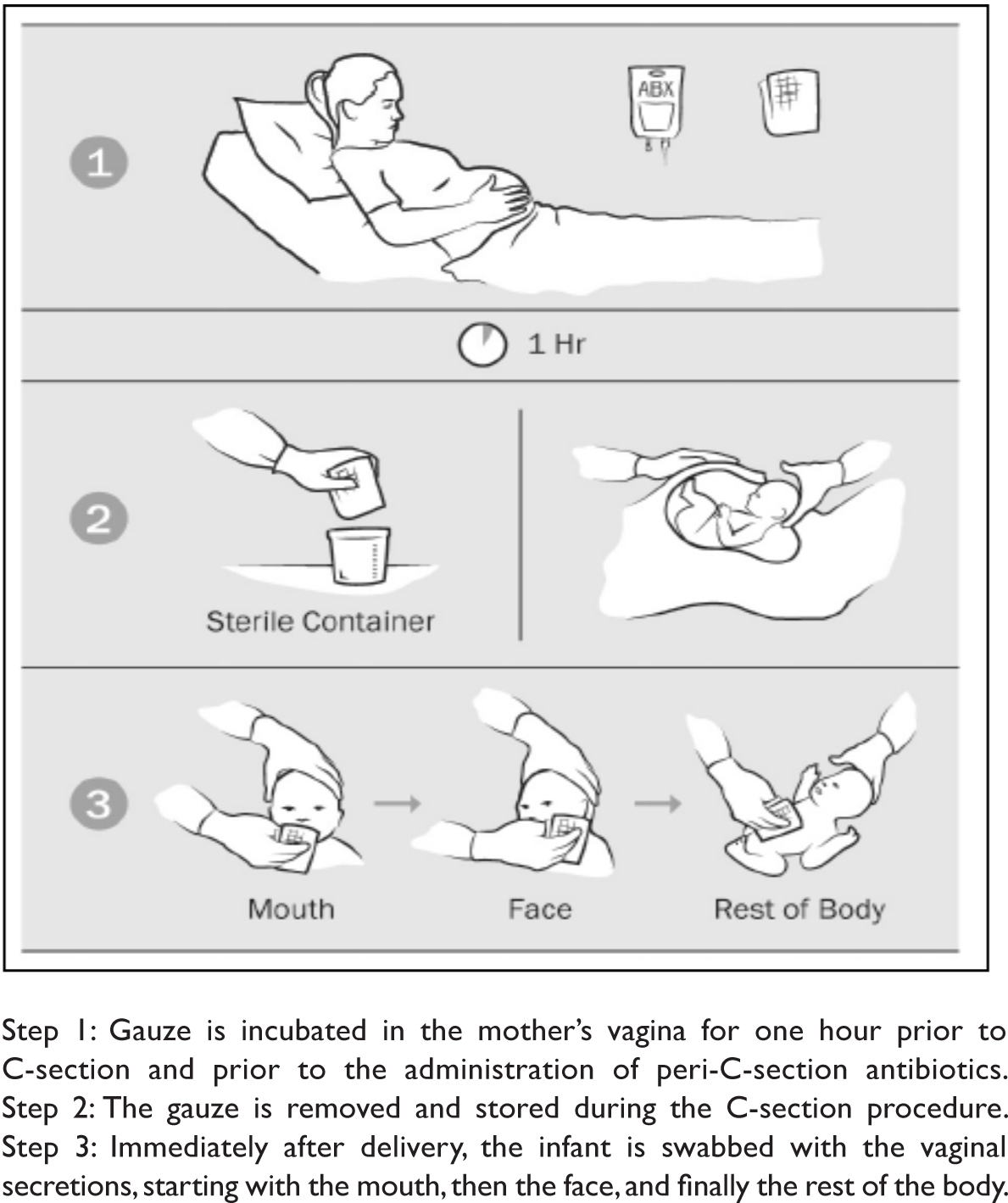
Figure 1. The Vaginal Seeding ProcessReference Dominguez-Bello, De Jesus-Laboy and Shen17
Dominguez-Bello and colleagues demonstrated in a non-randomized trial that vaginal seeding was associated with partial restoration of the microbiota of C-section born infants.18 However, it remains unknown if this improves health outcomes. It is also unknown if exposure to vaginal fluids may pose a minimal risk of transmission of vaginal pathogens to infants. There is one reported case in the literature of localized neonatal herpes simplex virus infection following vaginal seeding performed outside of a research protocol.Reference Huynh, Palasanthiran and McMullan19 While this single case report cannot prove whether the infection was attributable to vaginal seeding or another factor (e.g. kissing the baby), it does highlight the need for further research in a carefully monitored research setting.
Due to the scarcity of data on the benefits of vaginal seeding and concerns about its safety, clinicians have been advised against performing the procedure. In a 2016 editorial in the British Medical Journal, providers from several hospitals in the UK and Australia cautioned clinicians “not to perform vaginal seeding because we believe the small risk of harm cannot be justified without evidence of benefit.”Reference Cunnington, Sim, Deierl, Kroll, Brannigan and Darby20 Similarly, the American College of Obstetricians and Gynecologists (ACOG) does not recommend the use of vaginal seeding outside of a research protocol approved by a human subjects review board.21 ACOG's statement on vaginal seeding notwithstanding, there is anecdotal evidence to suggest that women scheduled to deliver babies via C-section are increasingly requesting that vaginal seeding be performed.Reference Haahr, Glavind and Axelsson22
Development of the Infant Microbiome and the Rationale for Vaginal Seeding
The typical microbiome development of a vaginally delivered newborn begins at birth when microorganisms from the maternal vagina, intestine, and skin colonize the baby. In general, the first colonizers are typically facultative anaerobes, after which strict anaerobes begin colonizing the gut over the first months, and eventually becoming adult-like around the age of 2 to 3 years.Reference Yassour, Vatanen, Siljander, Bokulich, Chung, Battaglia, Yatsunenko, Rey and Manary23 The microbial developmental trajectory of a C-section delivered infant is altered from that of a vaginally delivered infant. Vaginally delivered infants acquire bacterial communities resembling their own mother's vaginal microbiota, dominated by Lactobacillus, Prevotella, or Sneathia spp., whereas C-section infants harbor more bacterial communities typically found on the skin surface, e.g., Staphylococcus, Corynebacterium, and Propionibacterium spp.24 Colonization by several taxa common in the intestinal microbiota of vaginally delivered neonates (e.g., species of the genera Bacteroides, Bifidobacterium, and Lactobacillus) is often delayed or absent in C-section delivered neonates.25 The suspended development of these key bacteria can have important consequences for the infant, ranging from delayed immune system development to inability to fully metabolize oligosaccharides found in human breast milk.26
More than 20 studies, many longitudinal with repeated samples, have identified differences in the microbiota of vaginal versus C-section delivered infants.Reference Bäckhed, Roswall, Peng, Goedert, Hua, Yu, Shi, Chernikova, Koestler, Hoen, Kabeerdoss, Ferdous, Balamurugan, Sordillo, Zhou, McGeachie, Levin, Sitarik, Havstad, Martin, Makino, Cetinyurek Yavuz, Lee, Kim, Kang, Bosch, Levin, van Houten, Bokulich, Chung, Battaglia, Yassour, Vatanen, Siljander, Zeber-Lubecka, Kulecka, Ambrozkiewicz, Madan, Hoen, Lundgren, Chi, Xue, Lv, Mueller, Shin, Pizoni, Fouhy, Watkins, Hill, Liu, Qin, Song, Wampach, Heintz-Buschart, Fritz, Li, Wang, Wu, Tun, Bridgman, Chari, Shi, Guo, Chen, Wampach, Heintz-Buschart, Hogan, Hill, Lynch, Murphy, Gregory, LaPlante, Shan, Kumar and Gregas27 Yet, there is still some debate about how long the differences persist.Reference Chu, Ma, Prince, Antony, Seferovic and Aagaard28 One study found cross-sectional differences in 152 newborns at birth, but these differences were less apparent among the 60 infants who were not lost to follow-up at 6 weeks.29 Lack of observed association at 6 weeks in this study may have been due to the small sample size, bias introduced by differential loss to follow up, or differential distribution of factors that modify or mediate the influence of microbiome seeding. A larger and more recent prospective study on this topic found that delivery-mode differences in microbiome compositions persist out to at least 4 years of age.Reference Fouhy, Watkins and Hill30 Regardless of how long the impact on the microbiome remains, perturbation of the infant microbiome during a critical window of development may result in lasting health consequences.Reference Cox, Yamanishi and Sohn31
The rationale for efforts to restore the microbes that C-section born infants lack by facilitating this exposure through vaginal seeding is that infants will be colonized by the primordial human-evolved microbes that have colonized generations of babies at birth. If microbes are involved in the etiology of C-section-associated diseases, the hypothesis is that normalizing the first microbial exposure should normalize microbial development, facilitate programming of the immune system, and, ultimately, protect against C-section-associated diseases. Without randomized trials to test the hypothesis that vaginal seeding improves health in C-section babies, we will simply never know the answer to whether this increasingly popular technique has health benefits or risks.
Vaginal Seeding in Clinical Trials and Clinical Practice
Researchers, anxious to understand the impact of vaginal seeding on the long-term health of children, have already begun to conduct clinical trials to evaluate the safety and effectiveness of the procedure. Currently, there are at least two vaginal seeding trials underway in the US (NCT03298334, NCT03567707) and one in China (NCT03809390), per ClinicalTrials.gov.32 Several coauthors of this article (Dominguez-Bello, Hourigan, Mueller) are conducting a randomized controlled trial examining the impact of the vaginal microbiome on the health outcomes of babies born via C-section.33 The trial is taking place at Inova Health Care System in northern Virginia (“The Inova Study”). The second US trial is taking place at Mt. Sinai Hospital in New York.
The initial Inova Study of 50 mother-infant pairs, designed to better understand the study's recruitment/enrollment potential and to optimize study processes, is a beta version of a much larger planned and approved randomized controlled trial that will rigorously test whether vaginal seeding affects weight over the first 2 years after birth. In the original study protocol, the inclusion criteria included negative maternal testing for infections transmitted through vaginal and/or other body fluids performed as standard of care tests in early pregnancy,34 negative testing for Group B streptococcus at 35-37 weeks, and no symptoms of possible vaginal infection such genital lesions on delivery admission. As part of the approval process, FDA required the study team to modify the inclusion/exclusion criteria to include negative maternal testing for sexually transmitted diseases including gonorrhea, chlamydia, hepatitis B, hepatitis C, syphilis, and human immunodeficiency virus (HIV) again after 35 weeks gestation. The agency required the same modification for the clinical trial of vaginal seeding currently underway at Mt. Sinai Hospital in New York.35 Among the mother-neonate dyads included in the Inova study to date, no adverse events related to vaginal seeding have been reported.
The Inova Study has received considerable attention from pregnant women in the community, other states, and the media.Reference Stein36 The study team has also received requests for information from families wanting to perform vaginal seeding themselves, and, at this time, they are advising that “vaginal seeding should not be performed outside the context of an institutional review board-approved research protocol until adequate data regarding the safety and benefit of the process become available” as per the ACOG Committee Opinion.37
These requests may be fueled by podcasts and posts on the internet from a vocal community of supporters for a so-called “natural” approach to childbirth.Reference Wells, Harman and Wakeford38 As requests for vaginal seeding have increased, several doctors have posted pieces on online media sites and blogs. Many of these piecesReference Smith, Ruhl, Hayes and Mitra39 echo the ACOG recommendations but some take a decidedly more critical position (see, e.g., a 2016 Slate article titled: “Forget What You've Read. Swabbing Your Baby with Vaginal Juices is Pointless and Weird.”).Reference Austin40 An article on the blog “The Skeptical OB” titled “Warning: vaginal seeding doesn't work and may be harmful,” dismisses vaginal seeding as “a paradigm of much of what passes for ‘evidence’ in the world of natural childbirth.”Reference Tuteur41
ACOG has stated the need for studies that determine if vaginal seeding has benefits or risks for health.42 There is a pressing need for evidence-based guidance to clinicians and patients, and the compelling scientific evidence needs to be provided by randomized clinical trials that will show whether vaginal seeding has health benefits that outweigh any potential risks.
When a novel substance is being tested in human clinical trials, FDA has on many occasions issued an Industry Guidance document informing the research community as to how the substance will be regulated and whether or not an IND is required. FDA has not as yet issued public guidance on vaginal seeding; however, as they did in the early days with fecal microbiota transplantation (FMT), the agency is handling requests by investigators on a case-by-case basis.
Regulatory and Ethical Issues Associated with Vaginal Seeding
Regulation of Vaginal Seeding and the Vaginal Microbiome — Is it a Biologic or Drug (or Neither)?
An initial issue for any researcher conducting a clinical trial is whether submission of an Investigational New Drug Application (IND) to the US Food and Drug Administration (FDA) is required. While the practical requirement for an IND is to allow the testing of unapproved drugs and biologics in humans, the legal requirement for an IND is based on whether or not the treatment being tested is a biologic43 or a drug that has a connection to interstate commerce. The Federal Food, Drug, and Cosmetic Act defines the term drug, in part, as any article “intended for use in the diagnosis, cure, mitigation, treatment or prevention of disease in man or other animals” or a “substance (other than food) intended to affect the structure or any function of the body.”44 Thus, the definition is tied to the researcher's or manufacturer's intent. FDA's authority to require an IND stems from its authority under the Food, Drug, and Cosmetic Act to prevent the distribution of adulterated or misbranded products in interstate commerce. An unapproved drug is considered misbranded.
When a novel substance is being tested in human clinical trials, FDA has on many occasions issued an Industry Guidance document informing the research community as to how the substance will be regulated and whether or not an IND is required.45 FDA has not as yet issued public guidance on vaginal seeding; however, as they did in the early days with fecal micro-biota transplantation (FMT), the agency is handling requests by investigators on a case-by-case basis.
Both of the current US clinical trials on vaginal seeding are being conducted under an IND. In the case of the Inova Study, after the researchers submitted their study protocol to their institutional review board and the relevant data and safety monitoring board (DSMB),46 members of the DSMB advised the study team to investigate whether an IND would be required by the FDA. The investigator team contacted FDA in November 2017 and provided background information on the proposed study, including the study's inclusion and exclusion criteria that incorporate comprehensive screening for infectious diseases. The FDA advised that an IND would be required for the study. In January 2018, the study team submitted an application request for a pre-IND meeting in order to discuss with FDA why an IND application was required for the study. In its response declining the meeting request, the agency noted that the study's “plan to use maternal vaginal flora applied to infants in a study to determine possible impact on obesity in young children, utilizes administration of live organisms to affect structure/function of the body” and falls within IND requirements as per Section VI.B of FDA's 2013 “Guidance for Clinical Investigators, Sponsors and IRBs — Investigational New Drug Applications (INDs) — Determining Whether Human Research Studies Can Be Conducted Without an IND.”47 This decision by FDA appears to be based on the agency's decision that “maternal vaginal flora” meets the definition of a “drug/biologic” under the federal Food, Drug and Cosmetic Act. FDA's determination also presumes that FDA has jurisdiction over use of the substance/procedure.
Although FDA has claimed regulatory jurisdiction over FMT performed by a physician with donated stool from a patient's family or friends, some have argued it should be treated as the practice of medicine which is regulated by state medical boards and departments of health.
While FDA's authority under the Food, Drug, and Cosmetic Act is limited to drugs having a connection with “interstate commerce,” in passing the 1997 Food and Drug Administration Modernization Act (which amended the Food, Drug, and Cosmetic Act), Congress made clear that “the connection with interstate commerce required for [the agency's] jurisdiction … shall be presumed to exist.”48 Since then, FDA has interpreted its jurisdiction as being extremely broad and in virtually all cases the courts have upheld FDA's decisions.
In United States v. Kaplan (2016), a physician was found criminally liable under the Food, Drug, and Cosmetic Act after reusing single-use plastic needle guides during prostate biopsy exams.49 The physician/defendant appealed the lower court decision arguing that the Food, Drug, and Cosmetic Act did not apply. The 9th Circuit Court of Appeals' decision turned on its interpretation of Section 331(k) of the Food, Drug, and Cosmetic Act which prohibits any act “with respect to, a food, drug, [or] device … if the act is done while such article is held for sale … after shipment in interstate commerce and results in such article being adulterated or misbranded.”50 A drug is misbranded if it does not include the proper labeling. The court referenced a prior 9th Circuit case, U.S. v. Geborde,51 in which a disc jockey distributed a home-made designer drug called gamma hydroxy butyrate to several teenagers and one of the teens drank it and died. This was the only 9th Circuit case addressing the “held for sale” provision, and the court sided with the defendant. It distinguished Geborde, however, from the facts of Kaplan by pointing out that Geborde “was not a physician, made his own recreational drugs and distributed them free of charge,” and, thus, the transaction was of a “noncommercial nature.”52 The defendant in Kaplan argued that the needle guides were not “held for sale” because there was no sale. The 9th Circuit, however, rejected this argument, relying on a number of out-of-circuit cases which have held that “a physician's use of a device on a patient is covered by the statutory phrase ‘held for sale.’”53 The court ultimately sided with FDA concluding that Kaplan's medical practice was commercial in nature; he operated at a profit and what he charged patients for procedures also covered the products he used, therefore the needle guides were “held for sale.”
In US v. Regenerative Sciences (2014),54 the U.S. Court of Appeals, District of Columbia Circuit, sided with FDA concluding that cultured stem cells, originating from a patient's bone marrow and sent to an in-state lab for processing and then injected back into the same patient, constituted a drug when combined with an antibiotic prior to injection. The District Court indicated that, in claiming jurisdiction under the Commerce Clause, the FDA must establish that the substance at issue had been both (1) “held for sale” and, prior to such sale, (2) “ship[ped] in interstate commerce.”55 While the defendant agreed that the product was “held for sale,” it argued that the second requirement was not met because the entire process took place intrastate at the defendant's medical facilities. The Court relied on prior case law holding that “wholly intrastate manufacturers and sales of drugs” meet the interstate commerce requirement as long as “an ingredient used in the final product travelled in interstate commerce.”56 In the Regenerative Sciences case, the stem cells were combined with the antibiotic doxycycline before they were administered to the patient. Because the doxycycline was shipped across state lines and was a component of the substance given to the patient, that substance constituted a drug subject to FDA's regulatory authority.
Based on the decision in Regenerative Sciences, it is likely that a court would find that stool administered to patients from donated stool from a friend of family member meets the second prong of the jurisdictional test, i.e., the product must be shipped in interstate commerce, as the stool is combined with saline solution, which is shipped across state lines for sale. Based on Kaplan and cases from other circuits,57 it is likely that a court would find that the donor stool is “held for sale.” An argument can be made, however, that vaginal microbiota from a baby's mother is not being sold or distributed across state lines and therefore would arguably be the practice of medicine. In the case of vaginal seeding, the only material used in the vaginal seeding process, other than the mother's own vaginal secretions, is gauze. FDA might be able to argue that, because such gauze is sold in interstate commerce, the vaginal microbiota in combination with the gauze constitutes a drug or medical device/drug combination. FDA would further likely argue that the microbiota-soaked gauze would be considered “held for sale” as the hospital is charging the patient for the C-section and the gauze would be bundled into the hospital charge. If the facts were changed slightly, however, and the mother brought the gauze with her to the hospital, the gauze may no longer be considered “held for sale” and FDA may not have jurisdiction.
In addition to controversy about jurisdictional claims, just as the categorization by FDA of fecal matter as a drug/biologic has been controversial, the classification of vaginal secretions when administered to newborns from their mother shortly after birth is also likely to raise objections on the part of some researchers and possibly patients. There are several reasons for this. As others have noted, microbiota are not like “drugs” as traditionally understood. In the case of FMT, FDA is currently regulating the stool administered during the procedure as a biological / drug product. While FDA has not publicly addressed the regulation of vaginal seeding or vaginal micro-biota transplantation, the regulatory history of FMT may provide some insight as to how the agency may approach vaginal seeding or vaginal microbiota transplantation more broadly.
In the case of FMT, the FDA has changed its position regarding how it will regulate fecal microbiota over the past six years. After an initial determination in 2013 that fecal microbiota would be categorized as a “biological product” that would require an IND application, the agency reversed course in response to patient and provider opposition, opting to exercise enforcement discretion of the IND requirement for C. difficile infection that is non-responsive to traditional antibiotics. Then, in 2014 and 2016, the agency issued draft guidance that would require an IND application from stool banks that collect and distribute fecal material for FMT for C. difficile infections. While the agency has not yet finalized this guidance and has maintained its enforcement discretion posture for providers performing FMT on patients with recurrent C. difficile infections, the guidance gives some indication of FDA's current thinking on the matter of how it would prefer to regulate microbiota transplants.
Vaginal seeding, however, is distinct from FMT and vaginal microbiota transplantation in several ways. In the case of vaginal seeding, if a woman delivered her neonate vaginally, the neonate would naturally be exposed to her vaginal fluids and thusly the mother's vaginal microbiota. The C-section delivery constitutes a medical intervention that interrupts that process. Thus, there may be an argument that vaginal seeding is an attempt to replicate an exposure that occurs in the natural birthing process. While FDA may argue that it is being done to prevent disease or chronic conditions or tp affect the structure or function of the body, it seems very different from the typical drug as well as different from FMT and VMT.
In FMT the patient receives the fecal matter of a different individual. Outside of the context of the FMT, the patient would not otherwise be exposed to the other individual's fecal microbiota. Similarly, in a vaginal microbiome transplant, vaginal contents from one woman are transplanted into the vagina of another in an effort to alter the recipient's microbial composition (e.g., for treatment of bacterial vaginosis). Researchers and patients have made numerous arguments that fecal microbiota should not be classified as a drug or biologic and that the drug regulatory pathway is not appropriate for FMT. These arguments have included the difficulty of characterizing the active ingredients in FMT which is typically required of drugs, the cost of the drug approval process which may lead to an increase in unsafe “do-it-yourself ” FMTs, the inadequacy of the IND process to accommodate post approval changes to the manufacturing process, and the availability of off-label prescribing that may discourage research into the use of FMT for other indications.
Characterization, which refers to the use of external techniques to “characterize” a substance to ensure that the substance is what it claims to be, has been considered a particularly challenging stumbling block in treating fecal microbiota as a drug/biologic. The issue of characterization is particularly important for microbiota transplantation because, unlike most other regulated products, microbiome products include a microbial community and other elements that differ from person to person and within the same person at different times. This makes characterization extremely challenging.
Maternal vaginal secretions are a heterogeneous substance, composed primarily of bacteria and water, but also containing viral and fungal organisms, metabolic products of these organisms, salts, dead cells, and mucus from the cervix and vaginal tract. Lactobacillus spp. typically dominate the secretions; however, members of the genera Atopobium, Corynebacterium, Anaerococcus, Peptoniphilus, Prevotella, Gardnerella, Sneathia, Eggerthella, Mobiluncus, and Finegoldia are also less commonly found in vaginal secretions.
Similar to the fecal microbiome, the vaginal micro-biome differs between women and within the same woman over time. While typically less diverse than the fecal microbiome, the vaginal microbiome is dynamic and changes in response to multiple factors, such as pregnancy, hormonal fluctuations, intercourse, and environmental exposures.Reference MacIntyre, Chandiramani and Lee58 Defining the exact contents of donor vaginal seeding material is a major obstacle due to this variation. In addition, the intent of vaginal seeding is to restore exposure of an infant to its own mother's vaginal microbiota, and the vaginal seeding material may be very specific for each mother-infant dyad.
FDA has largely dealt with the “lot-to-lot” variability challenge in the context of stool-based products through the Good Manufacturing Practices framework for drugs and live biotherapeutic products. Whether this makes sense in the context of vaginal seeding done between mother and infant is not at all clear, as it would require major scaling up of the product to justify the cost of implementing Good Manufacturing Practices. Rather than defining what the exact contents of the transferred substance are, it may be more than sufficient to characterize the major bacteria species and what is not contained in the secretions (like pathogens) through a variety of clinical and laboratory tests.
In addition to the identity of the biological product, a critical part of the Chemistry, Manufacturing and Control information requirements for new drugs/biological products is to establish their safety, potency or strength, quality, and purity. Determining dose, potency, and purity are major hurdles for stool used for FMT and may also be for the vaginal secretions used in vaginal seeding.
Because of the obstacles to approving stool or fecal microbiota as a drug, some have argued that other regulatory pathways may be more appropriate for FMT, such as those used for blood, human cells and tissues, or cord blood. These alternative regulatory pathways may also be more appropriate for vaginal microbiota associated with vaginal seeding. These pathways are more flexible and allow for more rapid updating of screening protocols for donors and the product to be “transplanted.”
Another concern about the drug regulatory pathway in the context of FMT has been the do-it-yourself phenomenon. This could also happen in the context of vaginal seeding. Mothers could place, or request that their physician or nurse place, a gauze pad or a swab in their vagina prior to their C-section procedure and then after the baby is born apply the gauze to their baby themselves without any physician intervention or the need for any product other than what they have produced from their own bodies. This makes it even less likely that a court would consider the “product” “held for sale.” Mothers could even perform vaginal seeding in their own home after discharge from the hospital (although the vaginal microbiome postpartum may not be similar to the vaginal microbiome just before birth). Such do-it-yourself practice might be safer in the context of vaginal seeding than in FMT if the mother asks her physician if there is any likelihood of an immediate risk to the baby if she exposes the baby to her vaginal fluids. However, trials would be needed to test such an approach if vaginal seeding in a controlled context first proves to be efficacious.
The most controversial regulatory issue in the context of FMT has been whether stool from stool banks should be regulated as drugs. This raises the question of whether vaginal microbiota for vaginal seeding might ever be banked. While the current research on vaginal seeding involves vaginal microbiota from the baby's mother, it is possible that researchers may find that certain mothers have vaginal microbiota that are more beneficial to health than others. This could ultimately lead to banking vaginal secretions and making them available for sale. It is also possible that researchers will identify particular microbial strains that offer infants born via C-section the protection of the mother's vaginal microbiota and will attempt to develop a probiotic based on vaginal microbiota for administration to these newborns. In both of these cases there is a stronger argument for regulating vaginal seeding as a drug than in the context of applying a mother's own vaginal microbiota directly to her newborn.
Because of the obstacles to approving stool or fecal microbiota as a drug, some have argued that other regulatory pathways may be more appropriate for FMT, such as those used for blood, human cells and tissues, or cord blood.Reference Sachs and Edelstein59 These alternative regulatory pathways may also be more appropriate for vaginal microbiota associated with vaginal seeding. These pathways are more flexible and allow for more rapid updating of screening protocols for donors and the product to be “transplanted.”Reference Khoruts, Hoffmann and Palumbo60
Ethical Challenges Associated with Vaginal Seeding
In addition to the myriad legal and regulatory questions, vaginal seeding also implicates a number of ethical concerns with regard to clinical practice and research. The concept of informed consent and the regulations that require its use are intended to provide patients and research subjects with full information about a treatment or procedure, including its risks and benefits, so they can make educated and autonomous decisions about how to proceed. In the case of vaginal seeding, there is preliminary evidence that the practice may result in a C-section delivered infant microbiome that more closely resembles that of vaginally-delivered infants.61 To date, however, the scientific evidence of the benefits is very limited and little is known about the potential short- or long-term risks of the procedure on the infant.
vulnerable populations in the research setting
Human subjects research regulation has long recognized pregnant women and children as vulnerable populations. In the case of pregnant women, there have often been blanket exclusions from research because of this vulnerability resulting in their underrepresentation in studies and limited data on the effects of various medications.Reference van der Zande, van der Graaf and Oudijk62 The inability of neonates to provide informed consent or assent to participate in research warrants increased scrutiny of studies involving this population. The Nuremberg Code63 expressly prohibited research involving any participants lacking legal capacity to provide voluntary consent. Although research guidelines were subsequently issued with less restrictive requirements that permitted research with children,64 there is a distinct regulatory framework for research with children that requires research studies involving greater than minimal risk to either provide direct benefit to the individual subject or provide critical information about the participant's disease or condition.65
Due to the nature of studies on vaginal seeding, research can only be conducted in the vulnerable populations of pregnant women and children. The current Inova clinical trial is considered minimal risk, and safeguards are in place to minimize the possibility of coercion and to ensure that risks are fully disclosed during the informed consent process. Trained study personnel meet with pregnant mothers who are eligible. They explain the goals and study procedures, possible risks and benefits and provide time and privacy for adequate decision making. The participant is then asked to provide informed consent for herself and her baby. One of the concerns here goes to whether or not the vaginal seeding permanently changes the child's microbiome. In part, that may be the goal; however, there are arguably reasons to scrutinize such changes more carefully than those that are transitory. This argument is similar to those expressed by opponents of allowing parents to consent to germline therapy on their children, which creates genetic changes in the child that are carried onto future generations. However, at this stage of research we do not know whether any changes to the newborn's microbiota as a result of vaginal seeding will be permanent and, if so, whether they would be carried on to one's offspring.
ethical issues in the clinical setting
Despite the lack of evidence, the studies that have suggested that vaginal seeding may be beneficial in restoring the microbiome of C-section delivered infants have generated keen interest and widespread coverage in the media, including the 2014 documentary “The Miracle of Microbirth” about the practice.66 At times, this has resulted in pitting patient wishes to have the procedure against the lack of clear evidence of safety and effectiveness of the procedure, and the potential risks associated with the practice of vaginal seeding outside of a controlled research setting (in which women are rigorously screened with pathogen testing) including transmission of infectious diseases such as gonorrhea, chlamydia, herpes simplex virus infection, and Group B streptococcal infection.
Given the ACOG committee opinion and BMJ article in the same vein, patient requests that vaginal seeding be performed on their C-section delivered infants present an ethical challenge to physicians. On the one hand, there is clearly a consensus among clinicians that the practice should not be performed outside of a research setting. On the other, there is not strong evidence that the potential risks outweigh possible, if unproven, benefits. The Inova Health System randomized controlled clinical trial “Vaginal microbiome seeding and health outcomes in Cesarean-delivered neonates” explores the impact of vaginal seeding on postnatal weight gain, among other metabolic and immunoinflammatory outcomes, associated with substantial lifelong morbidity. Empirical evidence from rigorously designed, randomized controlled trials is needed to inform any argument about whether potential benefits outweigh potential risks.
Some may argue that patients who seek out vaginal seeding are similar to patients who seek alternative, nontraditional remedies such as herbs or acupuncture. With the current emphasis on “patient-centered care” in medicine and an increasingly competitive healthcare marketplace, how should physicians ethically navigate a patient request for vaginal seeding in a manner that preserves patient autonomy and trust in the provider-patient relationship? The manner in which a provider addresses a patient request for vaginal seeding will have important implications for trust. As others have noted, patients requesting nontraditional treatments in a medical context are also seeking the scientific imprimatur.Reference Glazer67 Physicians refusing to perform the vaginal seeding procedure need to explain the risks of infection transference associated with the procedure and encourage patients to avoid doing it at home given those potential risks. While patient autonomy in decision making about care should be respected, the clinician also has a professional obligation to provide care that serves the patient's best interest and minimizes harm.
Conclusion
The transfer of the maternal vaginal microbiota through vaginal seeding to infants born by C-section holds promise for reducing C-section-associated diseases, but current and future studies are needed to determine the benefits and risks of vaginal seeding and will influence the regulation of this nascent technique. Until then, a regulatory framework that promotes safety, but also allows flexibility and promotes research, is needed. Regulators should consider pathways outside the traditional drug/biologic pathways such as those for blood and human tissues and cells as well as modified IND requirements within the drug/biologic pathways to accommodate the unique features of vaginal microbiota used in the vaginal seeding process.



