INTRODUCTION
Sponges are important components of low intertidal and subtidal communities from tropical to polar environments (Bell, Reference Bell2008a). They provide diverse ecosystem services, such as the uptake of nitrogen and carbon dioxide by their symbionts, the production of biologically active metabolites, and an efficient filtering capacity (Reiswig, Reference Reiswig1971; Hay, Reference Hay1996; Pile et al., Reference Pile, Patterson and Witman1996; Díaz & Ward, Reference Díaz and Ward1997). They also perform several ecological roles including the provision of shelter or food for other organisms or as agents of biological disturbance due to the seasonal growth and regression of sponge tissues that create free space for other inferior competitive organisms (Wulff, Reference Wulff2006; Abdo, Reference Abdo2007; Bell, Reference Bell2008a).
Many sponges show spatial and temporal variation in their abundance pattern that may be related to abiotic factors such as depth, sedimentation, nutrient concentration, solar radiation, salinity, substrate type and inclination (Palacios, Reference Palacios1978; Jokiel, Reference Jokiel1980; Wilkinson & Evans, Reference Wilkinson and Evans1989; Bell & Barnes, Reference Bell and Barnes2000; Maldonado et al., Reference Maldonado, Giraud and Carmona2008) as well as biotic factors including competition, predation and food availability (Trussell et al., Reference Trussell, Lesser, Patterson and Genovese2006; Wulff, Reference Wulff2006, Reference Wulff2012). Due to the increasing nutrients load in coastal systems, algae and sponges have increased their abundance (Valiela et al., Reference Valiela, Mc Clelland, Hauxwell, Behr, Hersh and Foreman1997; Bell, Reference Bell2008b) and, consequently, the study of their interaction has grown. While some studies suggest positive interactions between macroalgae and sponges (e.g. Palumbi, Reference Palumbi1985; Ávila et al., Reference Ávila, Blancas-Gallangos, Riosmena-Rodríguez and Paul-Chávez2010), others have reported negative interactions such as reduction of the sponge lateral growth due to the contact with algae or due to a shading effect (Freeman & Thacker, Reference Freeman and Thacker2011; González-Rivero et al., Reference González-Rivero, Ferrari, Schönberg and Mumby2012).
Less attention has been paid to the factors affecting growth and abundance of sponges in intertidal and other relatively harsh habitats. Desiccation is one of the most important abiotic factors affecting species distribution and community structure in intertidal habitats (e.g. Bertness et al., Reference Bertness, Crain, Silliman, Bazterrica, Reyna, Hidalgo and Farina2006; Daleo et al., Reference Daleo, Escapa, Alberti and Iribarne2006). In stressful environments, positive interactions may play a significant role in structuring communities, as a consequence of the ability of some species to ameliorate the stress factors of these habitats (Bertness & Callaway, Reference Bertness and Callaway1994; Bertness et al., Reference Bertness, Leonard, Levine, Schmidt and Ingraham1999; Bruno et al., Reference Bruno, Stachowicz and Bertness2003). For example, in rocky intertidals of the SE Pacific coast, some macroalgae improve water retention under their canopies during low tide, showing positive associations with other macroalgae in the upper intertidal (Molina-Montenegro et al., Reference Molina-Montenegro, Muñoz, Badano, Morales, Fuentes and Cavieres2005). Understanding which variables affect coastal community composition and abundances and the relationships of their most conspicuous components, will allow us to predict how these communities will evolve under the increasing anthropogenic impact of coastal systems (e.g. high nutrient loads, pollution, species removal) and to provide specific information for implementing conservation measures. Thus, the general aims of this study were to assess the biotic and abiotic variables that affect the distribution of the fast-growing massive sponge Hymeniacidon cf. perlevis Montagu 1814, and to assess if harsh conditions influence the relationship between Hymeniacidon and the fast-growing macroalgae Ulva lactuca Linnaeus 1753. Specifically, we evaluated the abundance pattern of Hymeniacidon and Ulva. We also assessed the relationship between Ulva and some physical variables on the abundance of Hymeniacidon at both intertidal and shallow subtidal. In this context, we predicted (1) a positive correlation between the cover of Hymeniacidon and Ulva in the intertidal but negative correlation between them in the relatively more benign subtidal; (2) strong correlations between the intertidal Hymeniacidon cover and some physical variables but weak or no correlations between the subtidal Hymeniacidon cover and physical variables.
MATERIALS AND METHODS
Study site
The study was conducted at San Antonio Bay, northern Patagonia (40°46′S 64°54′W), an 80 km2 body of marine water affected by a semidiurnal macrotidal regime (up to 9 m tidal amplitude, see Daleo et al., Reference Daleo, Escapa, Alberti and Iribarne2006; Martinetto et al., Reference Martinetto, Daleo, Escapa, Alberti, Isacch, Fanjul, Botto, Piriz, Ponce, Casas and Iribarne2010, Reference Martinetto, Teichberg, Valiela, Montemayor and Iribarne2011). The extensive intertidal is mainly composed of mud, sand and pebble flats (Figure 1) and, given the geomorphology of the bay, the low intertidal remains exposed up to 5 h every low tide (MG personal observation). The bay is a hypersaline system, due to the low average precipitation (250 mm year−1). The mean annual atmospheric temperature is 15.1°C, with extreme temperatures occurring in July (austral winter) and February (austral summer, −7.7 and 41.4°C, respectively). The humidity is 57% and the annual average wind speed is 18 km h−1, reaching an average of 25 km h−1 in spring-summer (Lucas et al., Reference Lucas, Guerrero, Mianzan, Acha and Lasta2005; Genchi et al., Reference Genchi, Carbone, Piccolo and Perillo2010; González et al., Reference González, Narvarte and Verona2010). San Antonio Bay is also subject to high anthropogenic N loading (Teichberg et al., Reference Teichberg, Fox, Olsen, Valiela, Martinetto, Iribarne, Muto, Petti, Corbisier, Soto-Jiménez, Páez-Osuna, Castro, Freitas, Zitelli, Cardinaletti and Tagliapietra2010) since the main freshwater input is nitrogen-rich groundwater introduced via the septic system from the nearby city of San Antonio Oeste, which affects an inner channel that runs beside the city (see Martinetto et al., Reference Martinetto, Daleo, Escapa, Alberti, Isacch, Fanjul, Botto, Piriz, Ponce, Casas and Iribarne2010, Reference Martinetto, Teichberg, Valiela, Montemayor and Iribarne2011). In this bay, Hymeniacidon cf. perlevis, with a cushion-shaped morphology, short papillae, small digitations emerging from its surface, and orange to yellow colour, is a common and abundant sponge that occurs in the intertidal and subtidal. It is able to feed on a wide variety of particulate sources, including microbes such as Escherichia coli (Fu et al., Reference Fu, Sun, Zhang and Zhang2006; Maldonado et al., Reference Maldonado, Cao, Cao, Song, Qu and Zhang2012). Ulva lactuca is the most conspicuous macroalga of this system, which in San Antonio Bay can reach a growth rate up to 20–25% day−1 (see Martinetto et al., Reference Martinetto, Teichberg, Valiela, Montemayor and Iribarne2011). Previous observations show that both Hymeniacidon and Ulva inhabit the intertidal and the entire subtidal of the shallow inner channel that runs beside the city (that reaches a depth of 0.5 m).

Fig. 1. Intertidal zone of San Antonio Bay, northern Patagonia (top). The sponge Hymeniacidon cf. perlevis and the green alga Ulva lactuca occur both in the intertidal and the shallow subtidal (bottom).
Relationship between Hymeniacidon and Ulva
To describe the seasonal variation in the abundance of Hymeniacidon and Ulva (per cent cover), we performed seasonal samplings from February 2012 to December 2013, at two heights: the intertidal, an extensive zone with low slope where the variation in exposure time was minimal between sampling areas; and the subtidal, with 0.5 m depth that is the maximum depth during low tide. The number of samplings varied according to weather conditions. In the intertidal, we performed a total of 29 samplings along the 2-year period (2012: 3 samplings in summer, 3 in autumn, 5 in winter and 4 in spring; 2013: 4 in summer, 3 in autumn, 2 in winter and 4 in spring). Meanwhile at the subtidal we performed a total of 27 samplings along the period (2012: 2 samplings in summer, 3 in autumn, 5 in winter and 4 in spring; 2013: 4 in summer, 3 in autumn, 2 in winter and 4 in spring). At each height, 10 quadrats (0.25 m2) were randomly placed on the substrate along a transect parallel to the waterline, where Hymeniacidon and Ulva were both present, and photographed with a Cannon® Powershot D20 (75 cm from the bottom approximately). For each quadrat, two photographs were obtained (one of Ulva canopy and another of the substrate after removing the Ulva canopy). Digital photographs were used to measure the per cent cover of Ulva and Hymeniacidon with the ImageJ software (Rasband, Reference Rasband1997–2014; http://rsb.info.nih.gov/ij/download.html) by manual drawing of the surface area. In addition, to assess Hymeniacidon abundance outside Ulva canopy, 10 quadrats were placed and photographed at each tidal height, in sites where Ulva was absent.
Physical variables
Atmospheric and seawater temperatures (°C), wind speed (km h−1), rainfall (mm), solar ultraviolet radiation (UV, 400–280 nm, expressed as kJ m−2 day−1) and photosynthetically active radiation (PAR,700–400 nm, expressed as kJ m−2 day−1) were used as physical variables to correlate with the cover of Hymeniacidon in sites without Ulva. Physical variables were measured every 3 h and then averaged for 1 week. Data for the 2-year study period were obtained from the weather stations of the National Meteorological Service (http://www.smn.gov.ar/) and the Photobiological Station of Playa Unión (http://www.efpu.org.ar/). Seawater temperature was measured every 90 min with three dataloggers (Thermo Button) permanently submerged in different points of the inner channel of San Antonio Bay.
To examine differences in desiccation we placed water-saturated artificial sponges (3 cm side squared pieces of plastic foam) inside and outside the Ulva canopy (N = 20 per treatment) in the intertidal during the low tide (sensu Bertness et al., Reference Bertness, Leonard, Levine, Schmidt and Ingraham1999). Artificial sponges were collected just before the incoming tide and desiccation was calculated as the difference in initial and final water masses divided by the time the sponges remained deployed (g min−1). Inside the canopy, artificial sponges were placed below a plastic frame (a small ring of 5 cm in diameter with 3 legs of 4 cm height) to avoid water transfer from Ulva to the artificial sponges. These frames were assumed not to affect the temperature surrounding the artificial sponges. The same experiment was replicated twice for each season in 2012.
Data analyses
Per cent cover data of Hymeniacidon obtained for each tidal height was grouped by season for further analyses. Differences in mean per cent cover of Hymeniacidon were tested with a factorial two-way ANOVA (Zar, Reference Zar1999), with season and presence of Ulva as main factors. Cover data were fourth root transformed to meet the assumptions of parametric statistics. Post hoc comparisons were performed using LSD test. To analyse the relationships between covers of Hymeniacidon and Ulva, and between Hymeniacidon cover and the physical variables, Spearman correlation tests (r s) were used (non-parametric tests were used since assumptions were not met for Ulva cover after data transformation). Mean desiccation rates were compared with a two-way ANOVA (Zar, Reference Zar1999), with seasons and presence of Ulva as main factors. LSD test was used for post hoc comparisons.
RESULTS
Relationship between Hymeniacidon and Ulva
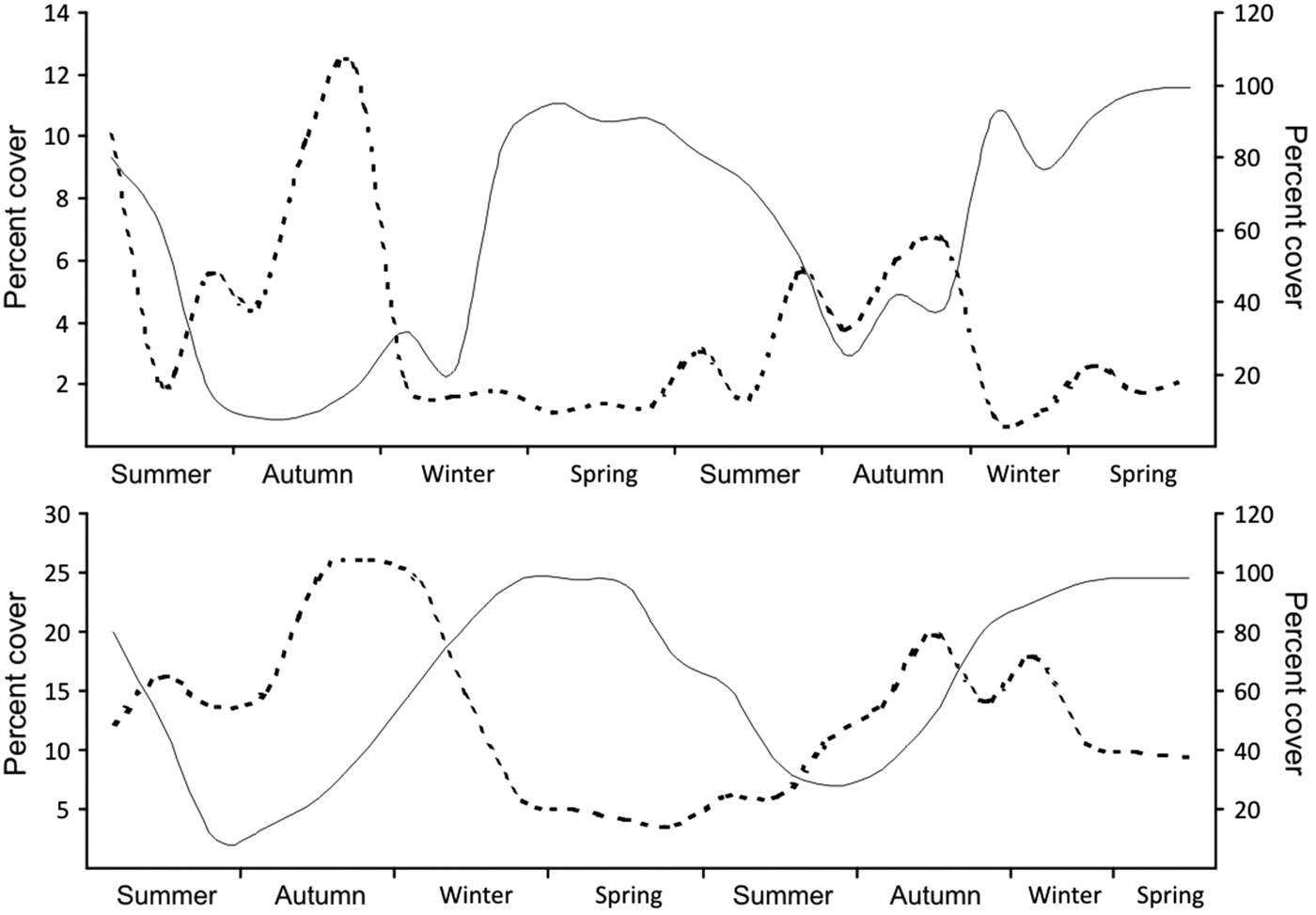
Hymeniacidon cf. perlevis and Ulva lactuca showed seasonal variation at both tidal heights over the 2-year period (Figure 2). During cover peaks, Ulva always had higher cover than Hymeniacidon. Cover of Hymeniacidon differed between tidal heights with maximum cover approximately double in the subtidal that in the intertidal (t = −17.86, df = 558, P < 0.01).

Fig. 2. Per cent cover of Hymeniacidon cf. perlevis (dashed line, left axis) and U. lactuca (solid line, right axis) during 2 year in the intertidal (top panel) and the subtidal (bottom panel). Note the different scale of y-axes.
Cover patterns of Hymeniacidon and Ulva were opposite. For example at the intertidal (Figure 2 top panel), while Hymeniacidon reached the highest cover in autumn (201 ± 113 and 103 ± 75 cm2 in 2012 and 2013, respectively), Ulva presented the lowest cover (274 ± 165 and 885 ± 374 cm2, respectively), then Hymeniacidon decreased up to spring when Ulva peaked (28 ± 36 and 37 ± 39 cm2 and 2301 ± 156 and 2320 ± 348 cm2, for Hymeniacidon and Ulva, respectively). Similarly, at the subtidal (Figure 2 bottom panel) Hymeniacidon cover peaked in autumn and persisted during the winter (550 ± 366 and 218 ± 206 cm2 and 395 ± 320 and 132 ± 133 cm2, for both in 2012 and 2013, respectively) when Ulva cover was scarce (395 ± 245 and 939 ± 403 cm2, respectively), and reached the lowest cover when Ulva cover peaked, in spring (72 ± 65 and 89 ± 95 cm2 and 2424 ± 73 and 2410 ± 115 cm2, for Hymeniacidon and Ulva, respectively).
In sites without Ulva, Hymeniacidon cover peaked in autumn at the intertidal (223 ± 131 cm2 in 2012 and 135 ± 97 cm2 in 2013) and in autumn and winter at the subtidal (445 ± 245 and 370 ± 320 cm2 and 524 ± 352 and 399 ± 238 cm2, for the both seasons in 2012 and 2013, respectively). The lowest cover was observed in spring both in the intertidal (36 ± 31 and 42 ± 47 cm2 for both years, respectively) and the subtidal (115 ± 103 and 254 ± 195 cm2, respectively).
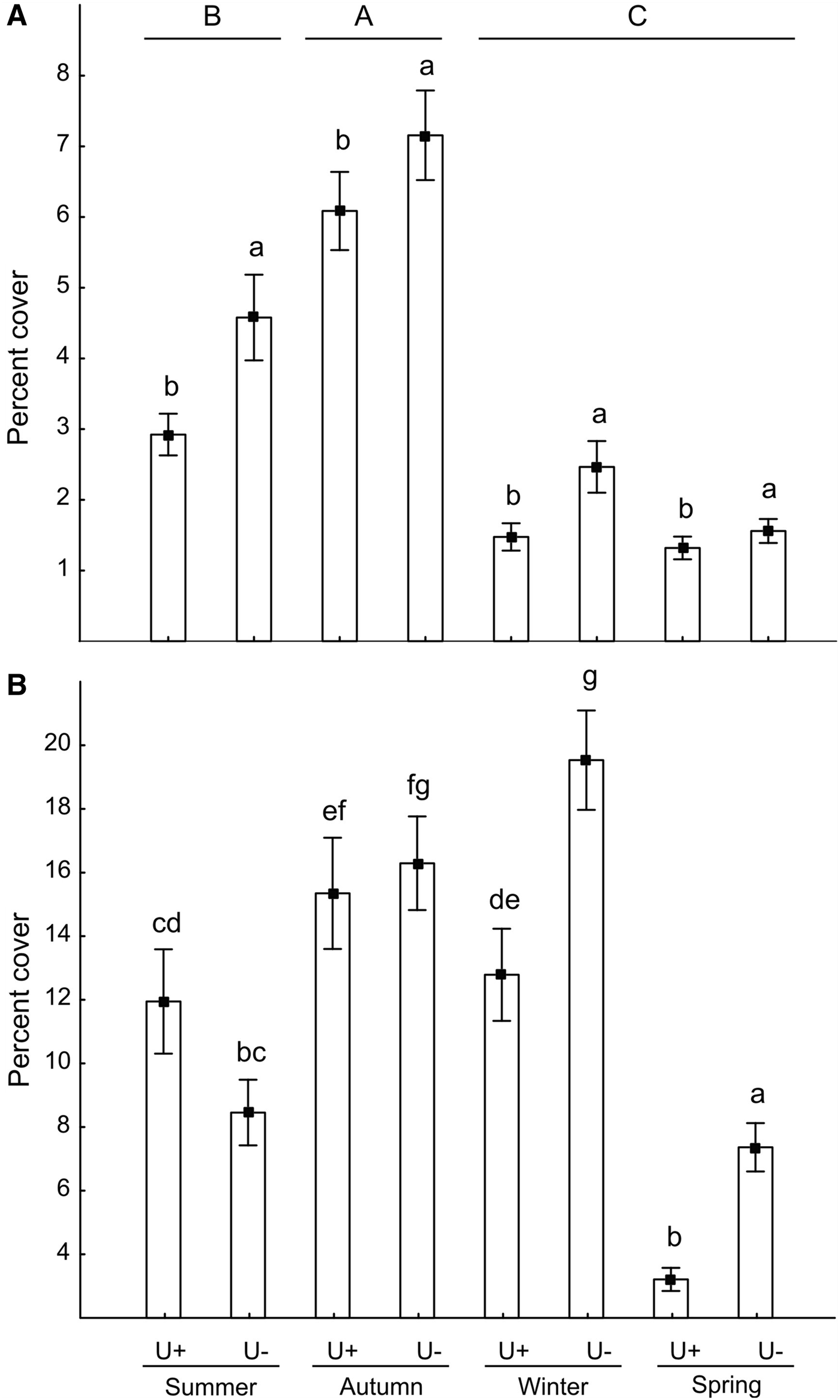
At the intertidal, Hymeniacidon cover differed between seasons and sites. Hymeniacidon cover was the highest in autumn (F 3, 572 = 81.9, P < 0.01, LSD test) and in sites without Ulva (F 1, 572 = 16.9, P < 0.01, LSD test; Figure 3A). At the subtidal, an interaction between seasons and sites was found (F 3, 572 = 1.2, P < 0.01, Figure 3B). Hymeniacidon cover was lower in sites with Ulva in winter and spring, and did not differ between sites for the remaining seasons (LSD test). The Spearman (r s) coefficient showed a relatively strong and negative correlation between Ulva and Hymeniacidon in sites with Ulva at both tidal heights (r s = −0.66, P < 0.01 and r s = −0.78, P < 0.01, respectively).

Fig. 3. Per cent cover of Hymeniacidon cf. perlevis (mean ± SE) with and without U. lactuca (U+ and U−, respectively) in the intertidal (A) and the subtidal (B). Different lower case and upper case letters indicate differences between treatments and among seasons. Note the different scale of y-axes.
Relationships between Hymeniacidon and physical variables
Striking seasonal variations were found in the physical variables over the study period (Figure 4). UV and PAR radiations showed a seasonal pattern, peaking in spring-summer (from 994 to 1162 kJ m−2 day−1 for UV and from 6748 to 9105 kJ m−2 day−1 for PAR, Figure 4A). Atmospheric and seawater temperatures showed a similar pattern, varying over seasons, with higher values in midsummer (February, up to 38.5 and 28°C for atmospheric and seawater temperatures, respectively) and lower in winter (August, up to 5°C, Figure 4B). Rainfalls were higher in summer and lower in spring (32 and 4 mm, respectively; Figure 4C). Summer and spring were the windiest seasons over the whole study period with peaks over 63 km h−1. Overall, all seasons showed relatively strong winds (Figure 4D).

Fig. 4. UV and PAR daily dose (A, solid and dashed line respectively), air and water temperature (B, solid and dashed line respectively), precipitation (C) and wind speed (D) during 2 years in the study area.
In the intertidal, Hymeniacidon cover was not correlated with any of the physical variables considered (UV: r s = −0.31; PAR: r s = −0.33; atmospheric temperature: r s = 0.06; seawater temperature: r s = 0.05; rainfall: r s = 0.11; wind: r s = −0.13, P > 0.05 for all). At the subtidal, Hymeniacidon cover showed negative correlations with UV radiation (r s = −0.832, P < 0.05), PAR radiation (r s = −0.831, P < 0.05) and water temperature (r s = −0.68, P < 0.05).
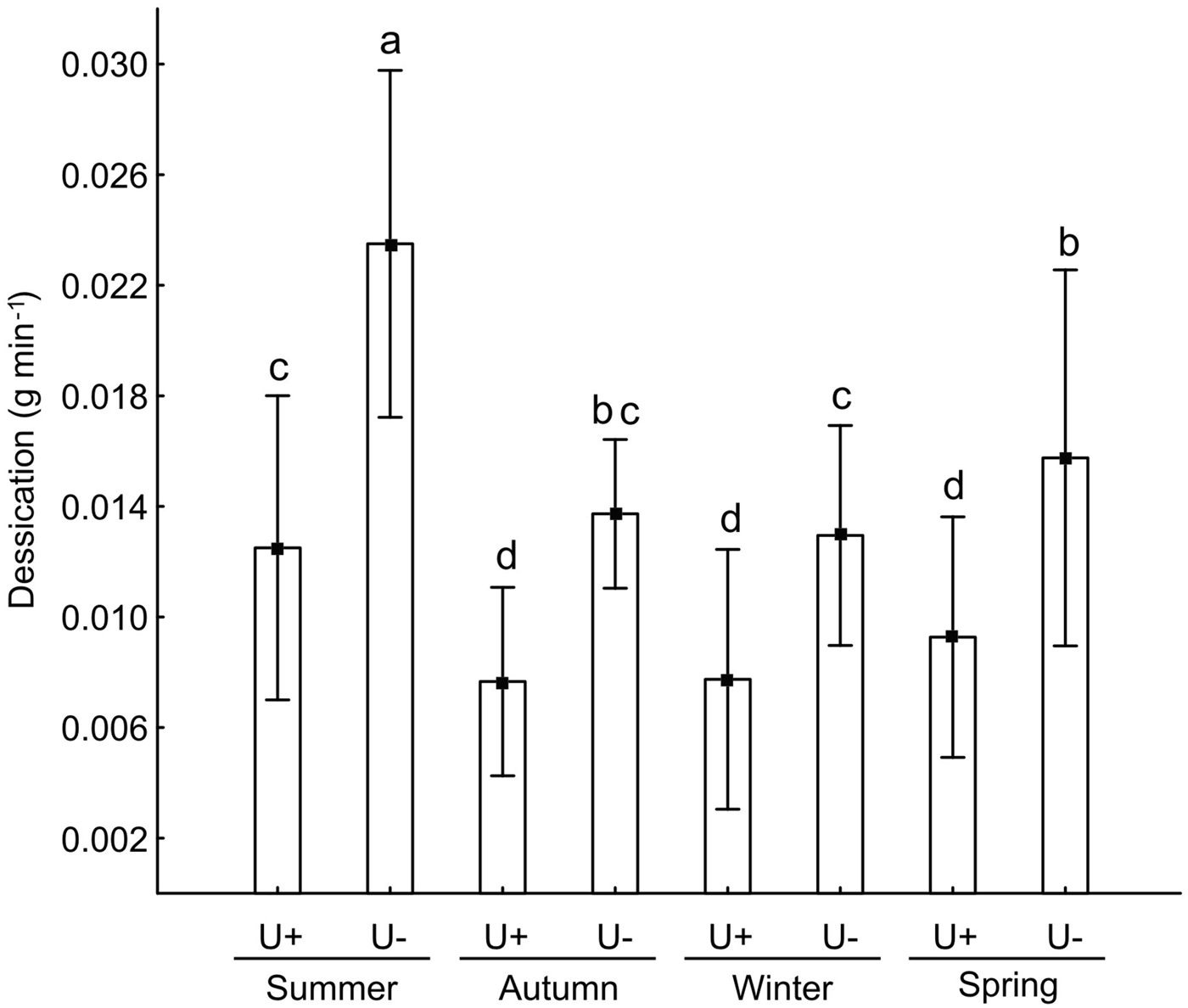
An interaction between factors was found for desiccation in the intertidal (F 3,232 = 4.2, P < 0.05). The highest desiccation was observed in U− in summer, followed by U− in spring, autumn and winter. Desiccation at U− in winter was similar to that at U+ in summer, and then U+ in spring, autumn and winter (Figure 5).

Fig. 5. Desiccation (mean ± SE) with and without U. lactuca (U+ and U−, respectively). Different lower case letters indicate differences between treatments and among seasons.
DISCUSSION
This study suggests that the abundance pattern of Hymeniacidon may be driven by biotic relationships at the intertidal while a more complex interaction between variables (combining biotic and abiotic stressors) may influence its abundance pattern at the subtidal. Negative relationships between Hymeniacidon and Ulva were observed at both tidal heights. Environmental conditions (e.g. desiccation) are harsher outside the Ulva canopy at the intertidal, but only subtidal sponges seem to be affected by the physical variables considered in this study. Thus, seawater temperature as well as UV and PAR solar radiation was negatively correlated with subtidal Hymeniacidon abundance.
Both positive and negative relationships between sponges and macroalgae have already been reported for different habitats (Palumbi, Reference Palumbi1985; Bell, Reference Bell2002; Ávila et al., Reference Ávila, Blancas-Gallangos, Riosmena-Rodríguez and Paul-Chávez2010; Cárdenas et al., Reference Cárdenas, Davy and Bell2012). Our results suggest a negative relationship between Hymeniacidon and Ulva, since Hymeniacidon was more abundant in sites and seasons when Ulva was scarce. Some authors suggest that sponge assemblages are more influenced by substrate inclination than by algae abundance and that the negative correlation between them is due to their different spatial distribution (algae dominate horizontal substrates while sponges dominate more vertical ones; Preciado & Maldonado, Reference Preciado and Maldonado2005). Though we do not measure substrate inclination, our study area is an intertidal flat with a homogenous and smooth slope, without crevices or vertical walls. In subtidal systems a negative relationship between sponge community and turf-forming algae was suggested since sponge community decrease and turf-forming algae increase when the canopy of a foundation species, a canopy-forming alga, was removed (Cárdenas et al., Reference Cárdenas, Davy and Bell2015). Also, the lateral growth rate of clionaids decrease when they are in contact with macroalgae (Lopez-Victoria et al., Reference Lopez-Victoria, Zea and Weil2006; González-Rivero et al., Reference González-Rivero, Ferrari, Schönberg and Mumby2012) and the physiology of the sponge Aplysina cauliformis is affected in contact with a green macroalga, though without affecting its biomass (Easson et al., Reference Easson, Slattery, Baker and Gochfeld2014).
The stress-gradient hypothesis predicts that community structuring processes are context dependent of the environmental conditions (either biotic or abiotic). Thus, the importance of negative biotic effects is higher under benign environmental conditions, while positive facilitative effects become more important as environmental stress increases (Bertness & Callaway, Reference Bertness and Callaway1994). Under such benign conditions, the dominance of foundation species may result in competitive exclusion of other organisms that overlap in terms of resources requirements (Paine, Reference Paine1966; Power et al., Reference Power, Tilman, Estes, Menge, Bond, Mills, Castilla, Lubchenco and Paine1996), whereas in stressful environments foundation species are expected to play a critical role in structuring communities (Silliman et al., Reference Silliman, Bertness, Altieri, Griffin, Bazterrica, Hidalgo, Crain and Reyna2011). Patagonian shores present higher desiccation rates than other previously studied intertidals and this is known to be the main factor structuring the shore communities (Bertness et al., Reference Bertness, Crain, Silliman, Bazterrica, Reyna, Hidalgo and Farina2006; Silliman et al., Reference Silliman, Bertness, Altieri, Griffin, Bazterrica, Hidalgo, Crain and Reyna2011). We expected to find a positive correlation between Hymeniacidon and Ulva in a harsher site (low intertidal) and a negative correlation in a more benign site (shallow subtidal). However, we observed negative associations at both heights, suggesting that competition may play an important role at both heights. Hence, three alternative explanations arise: (1) we considered both tidal heights as contrasting environments of the stress gradient at San Antonio Bay, since while the subtidal was permanently submerged; the intertidal was subjected to air exposure during 10 h per day (considering both low tides). During emersion intertidal sponges are subjected to loss of oxygen and food supply, increased salinity and exposure to solar radiation and, eventually, loss of cellular water upon draining and drying of the internal cavity and aquiferous system (Rützler, Reference Rützler1995). However, these two environments would not be contrasting enough for Ulva and Hymeniacidon. (2) Predation and reproduction seem to have large effects on sponges’ abundance (Stone, Reference Stone1970; Tanaka, Reference Tanaka2002; Wulff, Reference Wulff2006; Bell, Reference Bell2008a; Gaino et al., Reference Gaino, Cardone and Correiro2010). However, during the first year of sampling no recruitment was observed on settlement plates deployed in the subtidal (MG, unpublished data). On the other hand, predation on Hymeniacidon perlevis has not been reported yet (Bell, Reference Bell2008a) and no evident predator of Hymeniacidon could be identified at San Antonio Bay. Nevertheless, these biotic processes, not considered in this study, may be playing an important role in determining Hymeniacidon abundance pattern, overwhelming the effect of the physical variables. (3) Both species Hymeniacidon and Ulva may have different optimal environmental requirements. In San Antonio Bay Ulva abundance is strongly driven by seasonal environmental factors, such as the availability of nutrients and temperature (Martinetto et al., Reference Martinetto, Teichberg, Valiela, Montemayor and Iribarne2011). On the other hand, studies about the environmental requirements of Hymeniacidon are scarce, but growth of Hymeniacidon cover occur between 17–20°C on controlled temperature aquarium (Stone, Reference Stone1970). Here only subtidal Hymeniacidon cover was correlated with seawater temperature and radiation, and while Ulva showed similar abundances at both heights, Hymeniacidon doubled the abundance at the subtidal. The pattern observed for Ulva suggests that none of the heights are limiting environments for its growth, agreeing with previous studies which demonstrate that Ulva has a great capacity to thrive in the harsh intertidal, maintaining or even enhancing photosynthesis during air exposure and high levels of desiccation, and showing a fast adaptation of growth under different light conditions (Henley et al., Reference Henley, Lindley, Levavasseur, Osmond and Ramus1992; Altamirano et al., Reference Altamirano, Flores-Moya and Figueroa2000; Gao et al., Reference Gao, Shen, Wang, Niu, Lin and Pan2011). Even though Hymeniacidon is an intertidal sponge that seems to resist high desiccation rates, the observed pattern of Hymeniacidon suggests that the subtidal would be a more suitable environment. This may be explained by the supply of food particles which is continuous in the subtidal and restricted to high tide in the intertidal (see also Reiswig, Reference Reiswig1971; Steindler et al., Reference Steindler, Beer and Ilan2002). The latter may explain the opposite abundance pattern showed by Ulva and Hymeniacidon, the seasonal variation of abundances in both species and the differences in abundance between heights. However, none of these three explanations satisfactorily elucidate why Hymeniacidon is more abundant in sites where Ulva is absent. Macroalgae can exert several negative effects on survivorship of sponges by releasing allelochemical compounds (González-Rivero et al., Reference González-Rivero, Ferrari, Schönberg and Mumby2012), shading (Freeman & Thacker, Reference Freeman and Thacker2011), and/or the enhancement of sedimentation (Maldonado et al., Reference Maldonado, Giraud and Carmona2008). None of these negative chemical or physical effects have been reported on Hymeniacidon, however it seems unlikely that Ulva may affect Hymeniacidon by enhancing sedimentation since, as already observed (Stone, Reference Stone1970), in San Antonio Bay, Hymeniacidon is able to grow on muddy gravel and soft mud. However, to understand the mechanisms underlying such interactions, disentangling the physical from chemical effects of Ulva on Hymeniacidon, manipulative experiments are needed.
Under a scenario of climate change, with increasing human activities, nutrient loads and rising water temperatures, it is essential to enhance our knowledge of the factors controlling intertidal and shallow subtidal communities in stressful environments to predict how natural communities will perform. However, further studies are needed to determine the role of other potential stressors, such as food availability and reproduction events on the abundance pattern of Hymeniacidon.
ACKNOWLEDGEMENTS
We thank María Cecilia Salas, Roxana Soler, Sabrina Sepúlveda and Guillermo Svendsen for help and support during samplings. We also thank Dr E. Hajdu (Universidade Federal do Rio de Janeiro) for the sponge identification and Dr W. Helbling (Estación de Fotobiología Playa Unión/CONICET) for providing radiation data and further related advice. This article benefited from the critical reading, comments and useful suggestions of three anonymous reviewers.
FINANCIAL SUPPORT
MG was funded by a CONICET doctoral scholarship. This study forms part of the PhD thesis of MG and was partially supported by the Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT, Grant PICT CONAE-CONICET No. 04/2010 to MN).