Introduction
Surgical resection, if applicable, is the first choice of treatment for pancreatic cancer. However, only about 20–25% of all pancreatic cancer patients are resectable. Reference Willett, Czito and Bendell1,Reference Krishnan, Rana and Janjan2 Standard management of unresectable pancreatic cancer has not been established. It has been reported that the median survival time (MST) with best supportive care is less than 6 months in patients with unresectable locally advanced or metastatic pancreatic cancer. Reference Tsavaris, Tentas and Tzivras3,Reference Shibamoto, Nishimura and Abe4 Chemotherapy, radiotherapy and chemoradiotherapy may be conceivable options of treatment for unresectable pancreatic cancer. However, the superiority of chemoradiotherapy over chemotherapy alone remains controversial; two studies suggested the superiority of chemoradiation over chemotherapy alone, Reference Loehrer, Feng and Cardenes5,6 whereas others did not. Reference Chauffert, Mornex and Bonnetain7–Reference Hammel, Hugel and van Laethem10 A reason why chemoradiotherapy has not been proven to be definitely superior to chemotherapy alone may be the use of relatively low doses of radiation therapy. Since the pancreas is surrounded by radiosensitive gastrointesitinal tracts, tolerable doses of 50–54 Gy or less have been employed in previous studies. Reference Blakaj, Stein and Khan11,Reference Jaoude, Kouzy and Nguyen12 In view of the relative radioresistance of pancreatic cancer, higher doses may be desirable to definitely improve local control. Reference Shibamoto, Kubota and Kishii13,Reference Shibamoto, Manabe and Baba14
Recently-established intensity-modulated radiation therapy (IMRT) enables delivery of higher doses (≥55 Gy) to the pancreatic tumour while administering tolerable doses (<50 Gy) to the gastrointestinal tract included in the target volume. This can be achieved especially using the simultaneous integrate boost (SIB) technique. To our knowledge, however, IMRT has not yet been employed in prospective studies comparing chemoradiotherapy with chemotherapy. To properly evaluate the role of radiotherapy in the treatment of unresectable pancreatic cancer, it seems desirable to use IMRT in a prospective study.
We have been using IMRT with helical tomotherapy for the treatment of pancreatic cancers in combination with systemic chemotherapy. Using the SIB technique, doses of 55–60 Gy with a daily dose of 2–2·5 Gy have been focussed onto tumour regions, while the dose to the gastrointestinal tract was kept to be lower than 50 Gy. The standard dose to the tumour was 60 Gy delivered preferably in 25 fractions. Our policy was to use concurrent chemotherapy and high-dose IMRT (CCIMRT) even in patients with distant metastasis; metastatic tumours were included in the treatment field whenever possible. The purpose of the present study was to evaluate the outcome of CCIMRT. For comparison, patients treated with chemotherapy or radiotherapy alone were also analysed.
Materials and Methods
Patient characteristics
The subjects of this study were patients histologically or clinically diagnosed with pancreatic cancer who received CCIMRT using helical tomotherapy at our hospital between May 2008 and December 2019. For reference, patients who received chemotherapy or IMRT alone during the same period were also analysed. Other eligibility criteria were: (1) no previous treatment; (2) primary case and (3) unresectable tumour. Generally, unresectable pancreatic cancer was defined as a tumour having distant metastasis to other organs and/or a tumour having invasion to or contact with the major arteries, portal vein and superior mesenteric vein, also taking patient age into consideration. A total of 46 patients were treated: 17 by CCIMRT, 16 by chemotherapy and 13 by IMRT. This study was approved by the institutional review board. Informed consent was obtained from all patients.
Chemotherapy
For CCRT, the following regimens were employed: intravenous (iv) gemcitabine (GEM, 1000 mg/m2, day 1 and 8) + oral S-1 (60 mg/m2, day 1–14) repeated every 3 weeks; oral S-1 (80 mg/m2, day 1–14) repeated every 3 weeks; and iv GEM (1000 mg/m2, day 1, 8 and 15) plus nab-paclitaxel (nab-PTX, 125 mg/m2, day 1, 8 and 15) repeated every 4 weeks (GnP). For chemotherapy alone, the following regimens were employed: GnP; iv oxaliplatin (85 mg/m2, day 1) + levofolinate (200 mg/m2, day 1) + irinotecan (180 mg/m2, day 1) + 5-fluorourcil (400 mg/m2 single shot and 2400 mg/m2 over 24 hours, day 1) repeated every other week (FOLFIRINOX) and GEM monotherapy (1000 mg/m2, day 1, 8 and 15) repeated every 4 weeks. Dose reduction by 20% and elongation of the chemotherapy interval by 1–2 weeks were considered in cases of severe toxicity and/or high age.
Radiation therapy
IMRT was delivered with the helical mode of TomoTherapy Hi-Art or HDA system (Accuray Inc., Sunnyville, CA, USA). Patients were fixed with the BodyFix system (Medical Intelligence, Schwabmuenchen, Germany) and scanned for treatment planning with a 4- or 16-row multidetector computed tomography (CT) with a slice thickness of 2·5 mm under shallow breathing and breath holding during the inspiratory and expiratory phases. Subsequent planning was made with the TomoTherapy planning station. Contouring was performed on non-contrast CT images with fusion or with reference to contrast-enhanced CT and PET-CT images according to the previous study. Reference Shibamoto, Naruse and Fukuma15 The gross tumour volume (GTV) was the primary pancreatic tumour, lymph node metastasis, and whenever possible, liver metastasis. The GTVs during the three phases were superimposed to create GTV-all. The clinical target volume (CTV) was the GTV-all plus 5-mm margins and also included appropriate ranges of the para-aortic lymph node regions over the plexus region. The planning target volume 1 (PTV1) was the CTV plus anteroposterior and lateral 5-mm margins and craniocaudal 10-mm margins. PTV2 was GTV-all plus 3–5-mm margins in all directions. The SIB technique was used to deliver different doses to PTV1 and PTV2.
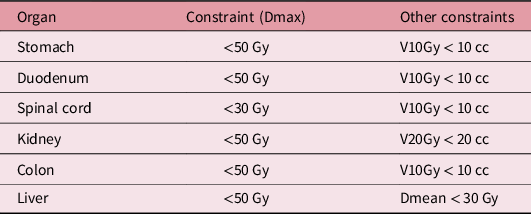
Typically, the prescribed doses to 50% of PTV 1 and PTV2 were 50 and 60 Gy, respectively, in 25 fractions. Depending on the size of PTV, 24 or 30 fractions were also used, and 55 Gy in 25 fractions was also used in the CCIMRT and IMRT-alone groups. Planning dose constraints for organs at risk are shown in Table 1. The maximum dose to the gastrointestinal tract was set to be below 50 Gy.
Table 1. Dose constrains for organs at risk
Follow-up evaluation
All patients were followed at 1- to 3-month intervals with physical examination and CT. Upon this analysis, special attention was paid to the locoregional status. A 20% or greater increase in the tumour diameter or appearance of a new regional lesion was regarded as locoregional failure. Acute gastrointestinal toxicity, including nausea and vomiting, diarrhea and abdominal pain, and chronic gastrointestinal toxicity were evaluated according to the Common Terminology Criteria for Adverse Events Version 4.0.
Statistical analysis
The Chi-square or Mann–Whitney U test was used to examine differences in patient characteristics and gastrointestinal toxicities among the CCIMRT, chemotherapy-alone, and IMRT-alone groups. Overall survival (OS) was defined as the period from the start of treatment to the date of death or last follow-up. The Kaplan–Meier method was used to estimate OS and locoregional progression-free survival (LRPFS). The log-rank test was used for comparisons of OS and LRPFS. All statistical tests were two-sided. Statistical significance was defined with a p-value < 0·05. All statistical analyses were performed using Mac Statistical Analysis Version 3.0 (ESUMI Co., Ltd., Tokyo, Japan).
Results
Patient and tumour characteristics
Table 2 shows characteristics of the patients, treatments and tumours in the 3 groups. In the CCIMRT group, the schedule of 60 Gy delivered in 25 fractions was employed in 11 patients (65%). The schedule of 55 Gy in 25 fractions was used in four patients (24%) in the CCIMRT group and six (46%) in the IMRT-alone group. The tumour marker data were lacking in two patients.
Table 2. Patient, tumour and treatment characteristics

GEM, gemcitabine; GnP, gemcitabine + nab-paclitaxel; FOLFIRINOX, oxaliplatin + levofolinate + irinotecan + 5-fluorouracil.
Treatment outcome
All but one patient were followed until death, and the remaining one patient was alive at 14·5 months at the time of this analysis. Figure 1 shows OS curves for the three groups. The median survival time (MST) was 15 months in the CCIMRT group and 9 months in the chemotherapy and IMRT groups (p = 0·12). The 1-year OS rate was 69% in the CCIMRT group, 27% in the chemotherapy group and 38% in the IMRT group. The 2-year OS rate was 20, 0 and 12% in the three groups, respectively. In nine patients of the CCIMRT group who received GEM-containing chemotherapy, the MST was 16·5 months, and the 1- and 2-year OS rates were 78 and 16%, respectively. In six non-metastatic patients of the CCIMRT group who received GEM-containing chemotherapy, the MST was 20 months, and the 1- and 2-year OS rates were 83 and 30%, respectively.

Figure 1. Overall survival curves for the CCIMRT group (solid line, n = 17), chemotherapy group (dotted line, n = 16) and IMRT group (broken line, n = 13). p = 0·12.
Figure 2 shows OS curves according to the presence/absence of distant metastasis at diagnosis for the three groups. In patients without distant metastasis, the MST was 20 months in the CCIMRT group, 13·5 months in the chemotherapy group, and 12 months in the IMRT group (p = 0·38). The 1-year OS rate was 73, 53 and 60%, respectively, and the 2-year OS rate was 37, 0 and 20%, respectively. In patients with distant metastasis at diagnosis, the MST was 15 months in the CCIMRT group, 8·5 months in the chemotherapy group, and 4·5 months in the IMRT group (p = 0·43). The 1-year OS rate was 60, 18 and 25%, respectively.

Figure 2. Overall survival curves for the CCIMRT group (solid line), chemotherapy group (dotted line) and IMRT group (broken line). Left panel: patients without distant metastasis at diagnosis (n = 12, 5 and 5, respectively; p = 0·38). Right panel: patients with distant metastasis (n = 5, 11 and 8, respectively; p = 0·43).
Figure 3 shows LRPFS curves for the three groups. The median time to locoregional progression was 17 months in the CCIMRT group, 5·5 months in the chemotherapy group and 10·5 months in the IMRT groups (p = 0·012). The 1-year LRPFS rate was 73, 0 and 40% in the three groups, respectively.

Figure 3. Locoregional progression-free survival curves for the CCIMRT group (solid line, n = 17), chemotherapy group (dotted line, n = 16), and IMRT group (broken line, n = 13). p = 0·012.
Table 3 shows the results of univariate analysis of potential prognostic factors in all 46 patients. The presence of distant metastasis at diagnosis, pretreatment high CA19-9 level (above median) and pretreatment high CEA level (above normal range) were associated with worse OS and LRPFS.
Table 3. Univariate analysis of all 46 patients

Acute Grade 2 gastrointestinal toxicity (nausea, diarrhea) was observed in 12% (2/17) of the patients in the CCIMRT group, 19% (3/16) in the chemotherapy group and 7·7% (1/13) in the IMRT group. Acute Grade 3 diarrhea was observed in 6·3% (1/16) in the chemotherapy group, and late Grade 3 gastrointestinal bleeding was observed in 6·3% (1/16) in the chemotherapy group. No other Grade 3 or higher toxicity was observed. The difference in the Grade ≥ 2 acute gastrointestinal toxicity among the three groups was not significant (p = 0·38).
Discussion
Pancreatic cancer is not a radiosensitive tumour, and conventional radiation therapy with doses of 50–54 Gy has not been so useful to improve the prognosis of the patients. However, delivering higher doses may be beneficial in a proportion of patients who do not develop metastasis at an early period. A method to deliver a high dose is intraoperative radiation therapy. Reference Shibamoto, Manabe and Baba14,Reference Shibamoto, Manabe and Ohshio16,Reference Karasawa, Sunamura and Okamoto17 By delivering a single high dose (25–30 Gy) in addition to external beam radiotherapy, better local control was reported, and also cure has been reported in some patients. Reference Karasawa, Sunamura and Okamoto17,Reference Shibamoto, Sugie and Ito18 However, intraoperative radiotherapy is now not widely used because of the limitations in facilities using this modality. The use of stereotactic body radiotherapy (SBRT) may be an alternative approach. By using SBRT in combination with GEM chemotherapy, median survival times of 12 months or longer are reported. Reference Gurka, Collins and Slack19,Reference Herman, Chang and Goodman20 However, the margins need to be narrow to protect the gastrointestinal tract, and so marginal recurrence may be a concern when SBRT is used alone. Instead, IMRT may be a more feasible modality to reasonably deliver high radiation doses. There have been reports on the use of IMRT for pancreatic cancer, but in most studies, the administered doses were similar to those used in conventional radiotherapy or were only modestly increased. Reference Wang, Ren and Ma21–Reference Huguet, Hajj and Winston25 Concurrent IMRT and chemotherapy has been reported feasible, Reference Huguet, Hajj and Winston25 but IMRT with a high dose, as defined by the biological effective dose with an α/β ratio of 10 Gy (BED10) > 70 Gy, Reference Jaoude, Kouzy and Nguyen12 has been scarcely reported. Reference Loehrer, Feng and Cardenes5,Reference Klaassen, MacIntyre and Catton8–Reference Hammel, Hugel and van Laethem10 Our present study has shown the feasibility of concurrent high-dose IMRT and chemotherapy with acceptable toxicities.
This study mainly used 60 Gy in 25 fractions to PTV2; the BED10 was 74·4 Gy. The dose to PTV1 was mainly 50 Gy, and the dose to the gastrointestinal tract was kept at less than 50 Gy in 25 fractions using the SIB technique. Still higher doses may be delivered when the treatment volume is limited to the primary tumour, Reference Nakamura, Hiraoka and Itasaka22,Reference De Felice, Benevento and Bulzonetti26 but doing this may allow early regional recurrence. Therefore, we think our method and doses of IMRT with the SIB technique are reasonable to combine with concurrent chemotherapy.
Our study is retrospective and each treatment was mostly allocated at the discretion of attending physicians. Therefore, the results of the CCIMRT, chemotherapy, and IMRT groups cannot be compared; however, LRPFS in the CCIMRT group seemed to be favourable, suggesting the benefit of high radiation doses in combination with chemotherapy on locoregional control. The outcomes of the CCIMRT group compare favorably with those of prospective studies of chemoradiation therapy. Reference Loehrer, Feng and Cardenes5–Reference Hazel, Thirlwell and Huggins9 From the results of this study, prospective randomised studies of CCIMRT versus chemotherapy alone seem to be warranted.
In this study, patients with distant metastases were also treated with radiation therapy. Metastatic tumours were included in the treatment volume whenever possible. Using helical tomotherapy, such a treatment is readily afforded. Takaoka et al. Reference Takaoka, Shibamoto and Murai27 treated chemorefractory multiple liver metastases with helical tomotherapy and obtained prolongation of OS. In future studies, it seems that Stage IV patients with oligometastatic tumours can be regarded as candidates for IMRT. CCIMRT covering the primary tumour and oligometastatic lesions should be considered.
There are many limitations in this study. In addition to the retrospective study design and small patient number, selection criteria for treatment choice were not defined. More than half of the patients had metastatic disease. The three groups of patients had great heterogeneity even within the group of CCIMRT. Although our policy of delivering high doses in combination with chemotherapy was uniform, the dose fractionation schedules slightly varied with patients. So, prospective studies with more patients are needed to definitely show that our approach is promising. Although chemotherapy regimens were various, it was suggested that a GEM-based regimen could be combined with high-dose IMRT, and our preliminary results appear promising. In further studies, we plan to continue this treatment with a fixed schedule of 60 Gy in 25 fractions to the tumour and a GEM-based chemotherapy regimen.
Conclusion
This study demonstrated the feasibility of concurrent high-dose IMRT and chemotherapy. The preliminary results seem encouraging. Future studies incorporating high-dose IMRT into treatment protocols for unresectable as well as oligometastatic pancreatic caners seem to be warranted.
Acknowledgement
The authors are grateful to Kimihide Nakamura M.D. PhD. and Katsunori Matsushita M.D. PhD. for providing clinical data. We also thank Mr. Norifumi Kato and Mr. Dai Sasaki for treating the patients.
Financial Disclosure
None.
Financial Support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of Interest
None.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation Hokuto Hospital and with the Helsinki Declaration of 1975, as revised in 2008, and has been approved by the institutional committees Hokuto Hospital. Informed consent was obtained and chart reviews were performed after approval by the ethics committee of Hokuto Hospital.









