Introduction
Velo-cardio-facial syndrome (VCFS) is a genetic disorder associated with microdeletions at chromosome 22q11 (Shprintzen et al. Reference Shprintzen, Goldberg, Lewin, Sidoti, Berkman, Argamaso and Young1978), typical facial morphology, cardiovascular anomalies, velopharyngeal insufficiencies, mild to borderline learning disabilities, cognitive deficits and a high prevalence of psychiatric disorders including ADHD (Papolos et al. Reference Papolos, Faedda, Veit, Goldberg, Morrow, Kucherlapati and Shprintzen1996) and schizophrenia-like psychosis (Murphy et al. Reference Murphy, Jones and Owen1999). This high prevalence of psychiatric disorders is likely to be caused by haploinsufficiency of one or more genes on 22q11.
One of the candidate genes located at 22q11 is the gene for catechol-O-methyltransferase (COMT), an enzyme that degrades dopamine. Particularly in the prefrontal cortex (PFC) degradation of dopamine is largely dependent on activity of COMT, whereas in other brain regions degradation of dopamine is mainly by monoamino-oxidase (MAO) and the dopamine transporter (DAT) (Chen et al. Reference Chen, Lipska, Halim, Ma, Matsumoto, Melhem, Kolachana, Hyde, Herman, Apud, Egan, Kleinman and Weinberger2004). A common mutation in the COMT gene, leading to an amino acid substitution [Valine (Val) to Methionine (Met)] results in decreased enzymatic activity with the Met/Met variant of COMT showing 40% less enzymatic activity than the Val/Val variant (Lachman et al. Reference Lachman, Papolos, Saito, Yu, Szumlanski and Weinshilboum1996; Chen et al. Reference Chen, Lipska, Halim, Ma, Matsumoto, Melhem, Kolachana, Hyde, Herman, Apud, Egan, Kleinman and Weinberger2004).
The COMT Val 158Met polymorphism has been reported to: (1) affect midbrain tyrosine hydroxylase levels and dopamine synthesis (Akil et al. Reference Akil, Kolachana, Rothmond, Hyde, Weinberger and Kleinman2003); (2) modulate dopaminergic interactions between the PFC and the midbrain (Meyer-Lindenberg et al. Reference Meyer-Lindenberg, Kohn, Kolachana, Kippenhan, McInerney-Leo, Nussbaum, Weinberger and Berman2005; Smolka et al. Reference Smolka, Schumann, Wrase, Grusser, Flor, Mann, Braus, Goldman, Buchel and Heinz2005); and (3) affect cognitive functions dependent on PFC, including working memory and attention (Egan et al. Reference Egan, Goldberg, Kolachana, Callicott, Mazzanti, Straub, Goldman and Weinberger2001; Malhotra et al. Reference Malhotra, Kestler, Mazzanti, Bates, Goldberg and Goldman2002; Meyer-Lindenberg et al. Reference Meyer-Lindenberg, Kohn, Kolachana, Kippenhan, McInerney-Leo, Nussbaum, Weinberger and Berman2005). People with VCFS carry only one copy of the COMT allele on their intact chromosome. It has been suggested that dopaminergic neurotransmission in VCFS is compromised, thereby increasing susceptibility for psychosis (Dunham et al. Reference Dunham, Collins, Wadey and Scambler1992). Therefore studying the COMT Val 158Met polymorphism in VCFS may increase our understanding of brain development and predisposition for psychosis in this population.
An increasing number of studies have investigated the effects of the COMT Val 158Met polymorphism on cognitive function in people with VCFS. Some have reported that in VCFS children the low-activity Met allele is associated with better performance on prefrontal-dependent cognitive tasks (Bearden et al. Reference Bearden, Jawad, Lynch, Sokol, Kanes, McDonald-McGinn, Saitta, Harris, Moss, Wang, Zackai, Emanuel and Simon2004; Shashi et al. Reference Shashi, Keshavan, Howard, Berry, Basehore, Lewandowski and Kwapil2006). However Glaser et al. (Reference Glaser, Debbane, Hinard, Morris, Dahoun, Antonarakis and Eliez2006) reported no difference on tasks of executive function between Met-hemizygous and Val-hemizygous groups in a combined children/adult VCFS population. In addition the COMT Val 158Met polymorphism may affect overall cognitive ability. For example, some studies have reported that the Met allele is associated with a decline in verbal IQ (Gothelf et al. Reference Gothelf, Eliez, Thompson, Hinard, Penniman, Feinstein, Kwon, Jin, Jo, Antonarakis, Morris and Reiss2005), and poorer performance on a task of language expression and spatial working memory (Baker et al. Reference Baker, Baldeweg, Sivagnanasundaram, Scambler and Skuse2005); whereas others found the Met allele to be associated with higher verbal IQ (Shashi et al. Reference Shashi, Keshavan, Howard, Berry, Basehore, Lewandowski and Kwapil2006). Thus the effect of COMT Val 158Met polymorphism and cognitive function in people with VCFS is not easily understood, and the discrepancy in results of the reported studies may have arisen because of differences in dopaminergic neurotransmission due to developmental changes during adolescence, since both children, adolescents, and young adults were included in the study populations (Wahlstrom et al. Reference Wahlstrom, White, Hooper, Vrshek-Schallhorn, Oetting, Brott and Luciana2007). The only study to date on COMT Val 158Met polymorphism in adults with VCFS showed that those with the Met allele displayed more severe excitement symptoms, and worse performance on theory of mind, Trails B, olfactory identification, communication and social functioning compared with those with the Val allele (Bassett et al. Reference Bassett, Caluseriu, Weksberg, Young and Chow2007). However, thus far there is no evidence that the COMT Val 158Met polymorphism affects the prevalence of schizophrenia in adults with VCFS (Bassett et al. Reference Bassett, Caluseriu, Weksberg, Young and Chow2007; Murphy et al. Reference Murphy, Jones and Owen1999).
The effects of the COMT Val 158Met polymorphism on brain anatomy in people with VCFS have been less frequently reported, but Gothelf et al. (Reference Gothelf, Eliez, Thompson, Hinard, Penniman, Feinstein, Kwon, Jin, Jo, Antonarakis, Morris and Reiss2005) found the Met allele was associated with a smaller frontal lobe volume. Others, however (Kates et al. Reference Kates, Antshel, AbdulSabur, Colgan, Funke, Fremont, Higgins, Kucherlapati and Shprintzen2006), did not find an effect of the COMT Val 158Met polymorphism alone, but did observe a gender-moderated effect of COMT genotype on both frontal lobe anatomy but not on frontal-lobe-dependent cognitive tasks.
Thus to date there have been relatively few studies on the effect of COMT Val 158Met polymorphism in people with VCFS, and those which are available focused on children and adolescents. Hence, it is unclear how the COMT Val 158Met polymorphism affects brain anatomy and cognitive function in adults with VCFS – and especially in adults. It has been suggested that PFC dopamine levels peak during adolescence and decline thereafter (Andersen et al. Reference Andersen, Dumont and Teicher1997), and that COMT activity reaches its optimal levels in early adulthood in the PFC, which might explain the discrepancies in studies on COMT Val 158Met polymorphism in children, adolescents and young adults with VCFS (Tunbridge et al. Reference Tunbridge, Harrison and Weinberger2006). We previously reported on brain anatomy and cognitive function in an adult VCFS population (van Amelsvoort et al. 2004 a, b). We have retrospectively obtained COMT genotype data from this sample. Here, we explore the relationship between COMT Val 158Met polymorphism and (1) brain morphometry and (2) measures of intelligence, attention, working memory, visuospatial function and memory in adults with VCFS. Because COMT is also expressed in abundance in non-frontal brain regions (Tunbridge et al. Reference Tunbridge, Harrison and Weinberger2006), we tested the hypothesis that in adults with VCFS, genetic variation in COMT activity is associated with anatomical differences in frontal brain regions but also in other regions including cerebellum, regions which have been implicated in VCFS in previous studies. We further hypothesized that, in agreement with Bassett et al. (Reference Bassett, Caluseriu, Weksberg, Young and Chow2007), in adults with VCFS those who were Val-hemizygous would perform better on frontal cognitive tests.
Method
Subjects
The study was approved by the local Ethics Committee and after complete description of the study to the subjects, written informed consent was obtained from themselves and/or their carers. All subjects were screened for medical conditions affecting brain functioning using a semi-structured clinical interview and routine clinical blood tests. To establish a DSM-IV (APA, 1994) diagnosis a semi-structured psychiatric interview was performed [Schedule for Clinical Assessment in Neuropsychiatry (SCAN); Wing et al. Reference Wing, Babor, Brugha, Burke, Cooper, Giel, Jablenski, Regier and Sartorius1990] as described elsewhere (Murphy et al. Reference Murphy, Jones and Owen1999). We included 26 subjects with clinical features of VCFS and an established 22q11 deletion who have participated in a previously reported study on brain anatomy and/or cognitive function in VCFS (Van Amelsvoort et al. Reference Van Amelsvoort, Daly, Henry, Robertson, Ng, Owen, Murphy and Murphy2004a, Reference Van Amelsvoort, Daly, Robertson, Suckling, Ng, Critchley, Owen, Henry, Murphy and Murphyb). Twelve subjects had psychosis, five val-hemizygotes and seven met-hemizygotes, were clinically stable and taking antipsychotic medication. No other psychiatric disorders were present in the 26 subjects with VCFS.
Genetics
Blood samples were collected from 26 participants, and DNA was extracted. Fluorescence in situ hybridization (FISH) was used to detect a 22q11 deletion (Oncor Inc., Gaithersburg, MD). The COMT Val 158Met polymorphism was genotyped using the SNaPshot technique of single base extension (Applied Biosystems, Foster City, CA, USA) according to manufacturer's instructions. The initial PCR reaction was performed using a Touchdown-PCR-protocol, with the following primers: forward: 5′-ACTGTGGCTACTCAGCTGTG-3′ and reverse: 5′-CCTTTTTCCAGGTCTGACAA-3′. The allele at the SNP position was determined by use of a 30 bp extension primer (5′-ATCACCCAGCGGATGGTGGATTTCGCTGGC-3′). All alleles were resolved on an ABI 3100 sequencer (Applied Biosystems, Foster City, CA, USA).
Magnetic resonance imaging acquisition and measurements
Magnetic resonance imaging (MRI) of the brain was performed on a 1.5-T MRI system (Signa; General Electric Co, Milwaukee, WI, USA) at the Maudsley Hospital, London, UK. A coronal volumetric spoiled gradient acquisition in the steady state dataset covering the whole head was acquired (repetition time, 13.8 ms; echo time, 2.8 ms; 124 sections; 1.5-mm section thickness). This dataset was used to perform manual tracing of brain volumes, using Measure software (Barta et al. Reference Barta, Dhingra, Royall and Schwartz1997). Volumetric analysis by manual tracing was performed for total intracranial volume, caudate, putamen, hippocampus, amygdala, frontal, occipito-parietal and temporal lobes, cerebellum and ventricular and peripheral cerebrospinal fluid (CSF) by means of region of interest boundaries as previously described (Murphy et al. Reference Murphy, DeCarli, Schapiro, Rapoport and Horwitz1992, Reference Murphy, DeCarli, Daly, Haxby, Allen, White, McIntosh, Powell, Horwitz and Rapoport1993a, Reference Murphy, DeCarli, Schapiro, Rapoport and Horwitzb; Van Amelsvoort et al. Reference Van Amelsvoort, Daly, Robertson, Suckling, Ng, Critchley, Owen, Henry, Murphy and Murphy2001, Reference Van Amelsvoort, Daly, Henry, Robertson, Ng, Owen, Murphy and Murphy2004a). The volume of each region was calculated by multiplying the summed pixel cross-sectional areas by section thickness. Intra-rater and inter-rater reliabilities (range, 0.90–0.99) were determined by intra-class correlation computation for all brain regions traced by two operators and were highly significant (F>4.0 and p<0.002).
In addition, for determination of grey and white matter densities we carried out voxel based morphometry on a second dataset that we acquired in the same individuals: a whole brain near axial dual-echo fast spin-echo (FSE) dataset aligned with the anterior commissure (AC)–posterior commissure (PC) plane [relaxation time (TR)=4000 ms, effective echo time (TE)=20 and 85 ms, 3 mm slice thickness]. This dataset was used to examine between-group differences in grey and white matter volume using a previously published methodology (Sigmundsson et al. Reference Sigmundsson, Suckling, Maier, Williams, Bullmore, Greenwood, Fukuda, Ron and Toone2001; McAlonan et al. Reference McAlonan, Daly, Kumari, Critchley, Van Amelsvoort, Suckling, Simmons, Sigmundsson, Greenwood, Russell, Schmitz, Happe, Howlin and Murphy2002; Van Amelsvoort et al. Reference Van Amelsvoort, Daly, Henry, Robertson, Ng, Owen, Murphy and Murphy2004a). Voxels representing extracerebral tissue were automatically set to zero (Suckling et al. Reference Suckling, Brammer, Lingford-Hughes and Bullmore1999a) and the probability of each intracerebral voxel belonging to grey matter, white matter, CSF, or dura/vasculature tissue classes was then estimated by a modified fuzzy clustering algorithm (Suckling et al. Reference Suckling, Sigmundsson, Greenwood and Bullmore1999b). On the basis of prior results, we equated these probabilities to the proportional volumes of each tissue class in the often heterogeneous volume of tissue represented by each voxel (Bullmore et al. Reference Bullmore, Brammer, Rouleau, Everitt, Simmons, Sharma, Frangou, Murray and Dunn1995). Thus, for example, if the probability of grey matter class membership was 0.8 for a given voxel, it was assumed that 80% of the tissue represented by that voxel was grey matter. Because the voxel size was predetermined (2.2 mm3), we then estimated the volume in millilitres of grey matter, white matter and CSF in each voxel. Summing these voxel tissue class volumes over all intracerebral voxels yielded global tissue class volumes. To allow estimation of between-group differences at each intracerebral voxel (spatial extent statistics), the short echo (proton-density-weighted) FSE images were co-registered using an affine transformation (Press et al. Reference Press, Taukolsy, Vetterling and Flannery1992; Brammer et al. Reference Brammer, Bullmore, Simmons, Williams, Grasby, Howard, Woodruff and Rabe-Hesketh1997) with a template image in the coordinate system of standard space as defined by Talairach & Tournoux (Reference Talairach and Tournoux1988). This individually estimated transformation was then applied to each of that subject's grey and white tissue probability maps.
Neuropsychological testing
Overall intellectual functioning, memory, visuospatial and perceptual ability, executive functioning, and attention were measured. Details of the tests employed have been described elsewhere (Van Amelsvoort et al. Reference Van Amelsvoort, Henry, Morris, Owen, Linszen, Murphy and Murphy2004b) but the battery was chosen on suitability for a learning disabled population and addressing measures of overall IQ, attention, memory, executive function and visuospatial function. The test battery included: a short version of the Weschler Adult Intelligence Scale – Revised (WAIS-R; Wechsler, Reference Wechsler1981) consisting of five subtests (Vocabulary, Comprehension, Similarities, Block Design and Object Assembly); the Doors and People Test of Visual and Verbal Recall and Recognition (Baddeley et al. Reference Baddeley, Emslie and Nimmo-Smith1994); two subtests from the Wechsler Memory Scale – Revised (WMS-R; Wechsler, Reference Wechsler1987): the Logical Memory (immediate and delayed recall of two short stories) and Paired Associates (immediate and delayed recall of matched pairs) subtests; the Visual Space and Object Perception Battery (VOSP; Warrington & James, Reference Warrington and James1991); Computerised Tower of London Task (3-D CTL Test) (Shallice, Reference Shallice1982; Morris et al. Reference Morris, Downes, Sahakian, Evenden, Heald and Robbins1988, Reference Morris, Downes, Robbins, Gilhooly, Keane, Logie and Erdos1990); Computerised Executive Golf Task (Morris et al. Reference Morris, Downes, Sahakian, Evenden, Heald and Robbins1988; Baker et al. Reference Baker, Rogers, Owen, Frith, Dolan, Frackowiak and Robbins1996); Controlled Oral Word Association Test (Benton & Hamsher, Reference Benton and Hamsher1976); Weigl test (Goldstein & Scheerer, Reference Goldstein and Scheerer1941); and the Continuous Performance Test (Conner, Reference Conner1995).
Statistics
The study population was divided into two groups according to COMT genotype: val-hemizygous and met-hemizygous VCFS subjects. Differences in age, and IQ between the two groups were compared using a t test; differences in gender and psychiatric diagnosis using a χ2 test.
Manually traced brain volumes, computerized tissue class volumes, and cognitive variables
Data were analysed with SPSS 12.0 for Windows (SPSS Inc., Chicago, IL). COMT effects on total and regional brain volumes, total tissue class volumes and cognitive variables were examined using a univariate analysis of covariance (ANCOVA) using COMT genotype, and gender as fixed factors and age and total intracranial volume (in case of MRI data) as covariates. We subsequently employed Bonferroni corrections for multiple testing using p<0.0045 for the manually traced volumes, p<0.017 for the tissue class volumes, and p<0.0011 for the cognitive variables as levels of statistical significance (two-tailed).
Analysis of MRI data using computerized voxel-wise analysis
Between-group differences in grey and white matter were localized by fitting an appropriate GLM at each intracerebral voxel. Inference was via a permutation distribution of spatial extent statistics with significance levels set to control for multiple comparisons by having less than one estimated false positive region (cluster) across the image (p<0.001). In brief, the processing proceeded as follows. Maps of the standardized GLM model coefficient of interest (group) at each voxel were thresholded such that only voxels with probability <0.05 were retained. The sum of voxelwise statistics for each three-dimensional suprathreshold cluster was the test statistic, the sign indicating a relative excess or deficit in local tissue density. Significance testing of the clusters was performed using a null distribution of this test statistic similarly obtained after repeatedly randomly permuting the relevant factor in the GLM and refitting of the model (Bullmore et al. Reference Bullmore, Suckling, Overmeyer, Rabe-Hesketh, Taylor and Brammer1999).
Results
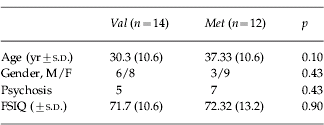
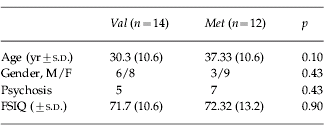
We studied 26 adults with VCFS. Data on age, gender, psychiatric diagnosis and intellectual function are presented in Table 1. Age, psychiatric diagnosis, full scale IQ (FSIQ) and gender did not differ between val- and met-hemizygous groups. However, the women in our sample were significantly older than the men; therefore age was introduced as a covariate in our analyses. Psychiatric diagnosis and FSIQ did not vary as a function of gender. We were unable to acquire MRI scans on seven subjects and neuropsychological data on three subjects. There were no differences in age, psychiatric diagnosis, FSIQ or gender between val- and met-hemizygous subgroups with the MRI or the cognitive data.
Table 1. Characteristics of 26 adults with velo-cardio-facial syndrome
COMT effects on manually traced brain volumes
After accounting for total intracranial volume and age there was a significant COMT effect on volumes of frontal (F=30.09, p=0.000), and temporal lobes (F=10.17, p=0.007), cerebellum (F=8.30, p=0.013); and peripheral CSF (CSF excluding ventricles) (F=10.84, p=0.006) (Fig. 1). There were no gender effects or COMT×gender interactions. No significant COMT, gender or COMT×gender interactions were found in the volume of any other brain region or ventricles (Table 2). After correction for multiple comparisons (p<0.0045) only frontal lobe volumes remained significantly different, with larger volumes in the val-hemizygous group compared with the met-hemizygous group.
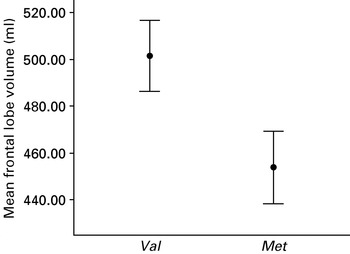
Fig. 1. Mean volumes (ml) of frontal lobes in Met hemizygous and Val hemizygous velo-cardio-facial syndrome people.
Table 2. Manually traced brain and computerized tissue class volumes in Val-hemizygous subjects and Met-hemizygous subjects (corrected for intracranial volume)

CSF, Cerebrospinal fluid.
* p<0.05, ** p<0.01.
COMT effects on total tissue class volumes
Having accounted for total intracranial volume and age, there was no gender effect on total CSF, grey, and white matter volume. There was a significant COMT effect on total CSF volume (p=0.028, F=6.38). In addition there was a significant COMT×gender interaction on total white matter volume (p=0.015, F=8.31) and total CSF volume (p=0.003, F=14.86) (Table 2). After correction for multiple comparisons (p<0.017) only a COMT×gender interaction on CSF remained significant.
COMT effects on regional grey matter distribution
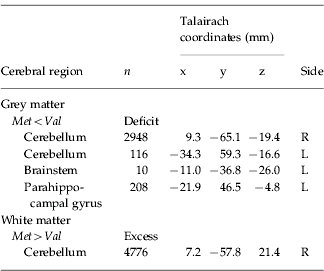
There was a significant difference between the VCFS Val-hemizygous and Met-hemizygous group in grey matter volume at four spatially extensive 3D voxel clusters. The Met-hemizygous group had a significant reduced grey matter volume in left parahippocampal gyrus and brainstem, and bilaterally in cerebellum. There were no significant clusters of excess grey matter in the Met-hemizygous group (Table 3, Fig. 2).
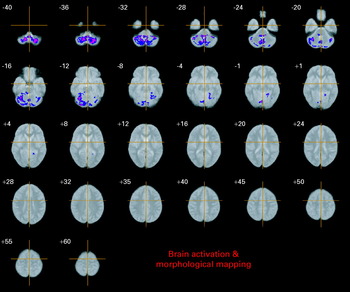
Fig. 2. Relative deficits (blue) and excesses (red) in grey matter volume in Met hemizygous compared with Val hemizygous VCFS people. The maps are oriented with the right side of the brain shown on the left side of each panel. The z coordinate for each row of axial slices in the standard space of Talairach & Tournoux (Reference Talairach and Tournoux1988) is given in millimetres.
Table 3. Regional differences in grey and white matter volume
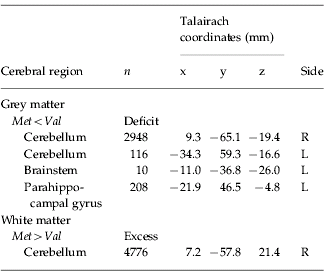
n, Number of voxels in each cluster.
The cluster-wise probability is p=0.001.
COMT effects on regional white matter distribution
The Met-hemizygous group had a significantly increased white matter volume in cerebellum. There were no clusters of reduced white matter in the Met-hemizygous group (Table 3, Fig. 3).

Fig. 3. Relative deficits (blue) and excesses (red) in white matter volume in Met hemizygous compared with Val hemizygous velo-cardio-facial syndrome people. The maps are oriented with the right side of the brain shown on the left side of each panel. The z coordinate for each row of axial slices in the standard space of Talairach & Tournoux (Reference Talairach and Tournoux1988) is given in millimetres.
COMT effects on cognitive variables
Accounting for age, there were significant COMT effects on two subtests of the Continuous Performance Test: Variability of Standard Error (p=0.031, F=5.64), and Standard Error Block Change (p=0.023, F=6.42). Also there was a significant COMT effect on total CPT index score (p=0.019, F=6.85) and on the minimal amount of moves needed (MAM) in the Tower of London task (p=0.019, F=6.96) (Table 4).
Table 4. Neuropsychological test scores for velo-cardio-facial syndrome Met and Val subgroups

Values are group means±s.d.
* p<0.05.
Significant COMT×gender interactions were found for Standard Error Block Change of the CPT (p=0.011, F=8.42), Strategy Formation (p=0.009, F=8.76) and Between Search Errors (p=0.017, F=6.95) on the Golf Spatial Working Memory task, MAM of the Tower of London task (p=0.006, F=10.16), Delayed Logical Memory of Wechsler memory Scale revised (p=0.021, F=6.78), Object Discrimination subtest of VOSP (p=0.018, F=6.79). After Bonferroni correction for multiple comparisons none of the findings remained significant.
Discussion
We found that in adults with VCFS, the COMT Val 158Met polymorphism is associated with significant differences in brain anatomy affecting frontal lobe volume and grey matter density in cerebellum, brainstem and parahippocampal gyrus, and white matter density in cerebellum.
However, our study has limitations. These include the relatively small sample size with insufficient power to look for interactions, and the multiple testing we carried out. Nevertheless, corrections for multiple comparisons were applied for all anatomical and cognitive variables. Also it is unlikely that differences in age, psychiatric comorbidity, and gender can fully account for our findings (as these factors did not differ between Val- and Met-hemizygous groups). In addition, we controlled for gender and age in the statistical analysis. Also, as noted by others (Chow et al. Reference Chow, Mikulis, Zipursky, Scutt, Weksberg and Bassett1999), the effect size for structural brain abnormalities in people with VCFS is relatively large. Nevertheless our findings should be interpreted with caution and further studies of a longitudinal nature using larger samples of people with and without VCFS are required.
Our study is the first to demonstrate that the effect of the COMT Val 158Met polymorphism is not limited to frontal brain regions in people with VCFS. Because of the paucity of dopamine transporters in the prefrontal cortex, COMT is responsible for dopamine degradation in this region (Chen et al. Reference Chen, Lipska, Halim, Ma, Matsumoto, Melhem, Kolachana, Hyde, Herman, Apud, Egan, Kleinman and Weinberger2004). Therefore, effects of the COMT Val 158Met polymorphism on brain volume have previously been hypothesized to be most pronounced in frontal cortex, and this has been the focus of most studies on brain anatomy and COMT Val 158Met polymorphism (Ho et al. Reference Ho, Wassink, O'Leary, Sheffield and Andreasen2005). Our results, using a whole brain anatomy approach, suggest that COMT Val 158Met polymorphism may affect other brain regions as well, including cerebellum, a region particularly compromised in VCFS (Van Amelsvoort et al. Reference Van Amelsvoort, Daly, Henry, Robertson, Ng, Owen, Murphy and Murphy2004a; Simon et al. Reference Simon, Ding, Bish, McDonald-McGinn, Zackai and Gee2005; Campbell et al. Reference Campbell, Daly, Toal, Stevens, Azuma, Catani, Ng, Van Amelsvoort, Chitnis, Cutter, Murphy and Murphy2006). COMT mRNA is expressed in several human brain regions including the frontal, temporal, and parietal lobes, and cerebellum, amygdala, putamen, thalamus and spinal cord (Hong et al. Reference Hong, Shu-Leong, Tao and Lap-Ping1998). Therefore it is not surprising that the Val 158Met polymorphism affects other brain regions than the PFC, which supports our findings. Also, COMT may be crucial for dopaminergic degradation in PFC, but more relevant for regulating norepinephrine (another substrate of COMT) in other brain regions. Both these neurochemical systems affect brain development and function. Thus COMT Val 158Met effects on brain anatomy may not only reflect differences in dopamine degradation but also differences in (nor)epinephrine metabolism (Parini et al. Reference Parini, Coupry, Laude, Diop, Vincent, Sassard and Dausse1988).
The underlying biological mechanism for our finding of larger bulk frontal lobe volumes and increased grey matter densities in Val-hemizygotes is unclear. One possible explanation could be differences in brain maturation between the two genotype groups. Qualitative imaging studies have reported brain anomalies in children and adults with VCFS, for example agenesis of the corpus callosum, white matter hyperintensities and septum pellucidum abnormalities (Mitnick et al. Reference Mitnick, Bello and Shprintzen1994; Chow et al. Reference Chow, Mikulis, Zipursky, Scutt, Weksberg and Bassett1999; Van Amelsvoort et al. Reference Van Amelsvoort, Daly, Robertson, Suckling, Ng, Critchley, Owen, Henry, Murphy and Murphy2001). Quantitative imaging studies have also reported evidence for disturbed brain maturation in people with VCFS. For example, several studies of brain anatomy have reported that people with VCFS compared with controls often have abnormalities in either frontal lobe anatomy (with subtle differences in grey/white matter composition) and/or posterior brain regions including cerebellum (Van Amelsvoort et al. Reference Van Amelsvoort, Daly, Henry, Robertson, Ng, Owen, Murphy and Murphy2004a; Simon et al. Reference Simon, Ding, Bish, McDonald-McGinn, Zackai and Gee2005; Campbell et al. Reference Campbell, Daly, Toal, Stevens, Azuma, Catani, Ng, Van Amelsvoort, Chitnis, Cutter, Murphy and Murphy2006). Normal brain maturation starts from posterior/inferior brain regions and takes place last in frontal regions (Sowell et al. Reference Sowell, Thompson, Holmes, Jernigan and Toga1999) and is associated with an increase in frontal white matter and a decrease in grey matter during adolescence (Nagy et al. Reference Nagy, Westerberg and Klingberg2004). Abnormalities in brain maturation (e.g. programmed cell death) could therefore lead to differences in the relative proportions of grey and white matter and hence frontal lobe volume. Since normal brain maturation is accompanied by increasingly efficient cognitive processing (Levy & Goldman-Rakic, Reference Levy and Goldman-Rakic2000), the finding of decreased efficiency of prefrontal cognitive processing in those possessing the Val allele (Egan et al. Reference Egan, Goldberg, Kolachana, Callicott, Mazzanti, Straub, Goldman and Weinberger2001) perhaps suggests that possessing the Val allele may slow down the maturation process – and hence the relative proportion of grey and white matter and/or bulk volume of frontal regions. A so-called anterior-posterior dichotomy in anatomical differences between people with VCFS and controls has been suggested (Kates et al. Reference Kates, Burnette, Jabs, Rutberg, Murphy, Grados, Geraghty, Kaufmann and Pearlson2001; Van Amelsvoort et al. Reference Van Amelsvoort, Daly, Henry, Robertson, Ng, Owen, Murphy and Murphy2004a; Simon et al. Reference Simon, Ding, Bish, McDonald-McGinn, Zackai and Gee2005), and could also explain differences in brain anatomy within the VCFS population between those hemizygous for Val or Met. How COMT haploinsufficiency in VCFS affects the dopaminergic system is still unknown, although a preliminary open study by Graf et al. (Reference Graf, Unis, Yates, Sulzbacher, Dinulos, Jack, Dugaw, Paddock and Parson2001) suggested increased baseline brain dopamine levels in three of four patients with VCFS and the Met-allele, as measured by the level of a catecholamine metabolite, homovanillic acid, in cerebrospinal fluid compared with calibrated standards and laboratory controls. These preliminary findings suggest that haploinsufficiency in COMT is associated with dysregulation of dopaminergic systems. Results from animal and human studies suggest that dopamine has a trophic action during early brain maturation and later influences prefrontal cortical specification (Nieoullon, Reference Nieoullon2002). Therefore, differences in dopaminergic clearance as a result of Val- or Met-hemizygosity could result in differences in (frontal) brain maturation because of differences in dopamine signalling during early brain development. Our finding of relative cerebellar grey matter increase and white matter decrease in the Val-hemizygotes suggests that dynamic interplay between grey and white matter tissue composition might take place during brain maturation with grey matter volume decrease due to pruning and white matter volume increase during myelination. These findings further support the hypothesis that brain maturation may be slower in val-hemizygotes than in met-hemizygotes.
Our findings on cognitive performance did not survive Bonferroni corrections for multiple comparisons. This is probably partially due to our small sample size. The current literature suggests that cognitive tasks classically thought to be measures of ‘frontal’ regions are modulated by the COMT polymorphism. As mentioned before the results on COMT polymorphism and cognitive function in VCFS are inconsistent also because various study groups have used different cognitive measures and study populations included people of different ages. Future studies with larger sample sizes and of a longitudinal nature should clarify the role of the COMT polymorphism on specific cognitive tasks during lifespan in VCFS.
In conclusion, our results suggest that genetic variation in COMT activity affects brain anatomy in adults with VCFS, and this extends outside frontal brain regions. This suggests variation in COMT activity is implicated in brain development in VCFS. Future studies are required to investigate changes across the lifespan.
Declaration of Interest
None.