INTRODUCTION
Understanding the mechanism allowing species to coexist in local communities remains one of the major topics in community ecology. Niche theory states that there are limits in similarity of co-occurring species (reviewed in Chase & Leibold Reference CHASE and LEIBOLD2003). The order Chiroptera is well suited to test this fundamental hypothesis, because bats often represent the most species-rich and ecologically diverse group, particularly in the tropics, of locally occurring mammals (Kingston Reference KINGSTON, Kunz and Parsons2009, Rex et al. Reference REX, KELM, WIESNER, KUNZ and VOIGT2008). However, due to their often relatively small size and nocturnal lifestyle, ecological information on the local assemblage is difficult to obtain. As a result, the mechanisms facilitating the local coexistence of bat species are generally poorly understood, which is particularly true for the Old World, including Madagascar.
Research on chiropteran community composition and structure has mainly focused on microhabitat and trophic resource partitioning and revealed that bat assemblages can comprise a variety of different trophic groups with further specializations in foraging strategies, functional morphology, sensory ecology and dietary composition within trophic groups or among congeneric species (Aguirre et al. Reference AGUIRRE, HERREL, VAN DAMME and MATTHYSEN2002, Reference AGUIRRE, HERREL, VAN DAMME and MATTHYSEN2003; Norberg Reference NORBERG, Wainwright and Reilly1994, Siemers & Schnitzler Reference SIEMERS and SCHNITZLER2004). Furthermore, bat species show specific adaptations in echolocation (Schnitzler et al. Reference SCHNITZLER, MOSS and DENZINGER2003) and wing morphology (Norberg Reference NORBERG, Wainwright and Reilly1994) to forage in three-dimensional habitat space and segregate by spatial partitioning.
Recently, considerable advances have been made concerning aspects of the taxonomy and distribution of Malagasy bats, which increased the number of recognized species from 27 (Peterson et al. Reference PETERSON, EGER and MITCHELL1995) to 45, of which 77% are endemic to the island (Goodman Reference GOODMAN2011, Goodman et al. Reference GOODMAN, RAMASINDRAZANA, MAMINIRINA, SCHOEMAN and APPLETON2011, Reference GOODMAN, TAYLOR, RATRIMOMANARIVO and HOOFER2012a). Hitherto, ecological differentiation in assemblages of Malagasy bat species remain unresolved. Here, we focus on one of the more species-rich and intensively surveyed assemblages on the island, from the Ankarana limestone area in the north (Cardiff Reference CARDIFF2006, Goodman et al. Reference GOODMAN, ANDRIAFIDISON, ANDRIANAIVOARIVELO, CARDIFF, IFTICENE, JENKINS, KOFOKY, MBOHOAHY, RAKOTONDRAVONY, RANIVO, RATRIMOMANARIVO, RAZAFIMANAHAKA and RACEY2005, Reference GOODMAN, CARDIFF, RANIVO, RUSSELL and YODER2006, Reference GOODMAN, PUECHMAILLE, FRIEDLI-WEYENETH, GERLACH, RUEDI, SCHOEMAN, STANLEY and TEELING2012b). We aim to indirectly study microhabitat use and diet composition of bat species and thereby illuminate the mechanisms that facilitate their coexistence in this assemblage.
Traditionally, faecal analyses of pellets, as well as analyses of stomach contents, have been used to identify and quantify the proportion of different dietary components of bats (Voigt et al. Reference VOIGT, KELM, BRADLEY, ORTMANN, Kunz and Parsons2009, Whitaker et al. Reference WHITAKER, MCCRACKEN, SIEMERS, Kunz and Parsons2009). These methods have drawbacks in that they only provide a snapshot in time on the feeding behaviour. Recently, the use of stable isotopes has been employed to provide detailed insights into the feeding ecology of bat species (Fleming et al. Reference FLEMING, NUNEZ and STERNBERG1993, Herrera et al. Reference HERRERA, HOBSON, MANZO, ESTRADA, SÁNCHEZ-CORDERO and MÉNDEZ2001, Reference HERRERA, GUTIERREZ, HOBSON, ALTUBE, DIAZ and SANCHES-CORDERO2002; Voigt & Kelm Reference VOIGT and KELM2006, Voigt et al. Reference VOIGT, REX, MICHENER and SPEAKMAN2008) and to assess trophic relationships in communities (Rex et al. Reference REX, CZACZKES, MICHENER, KUNZ and VOIGT2010, Reference REX, MICHENER, KUNZ and VOIGT2011; Voigt Reference VOIGT2010).
Here, we use stable nitrogen and carbon isotope signatures in hair from individuals of 16 sympatric bat species (Appendix 1) as integrated information about assimilated food over several weeks (DeNiro & Epstein Reference DENIRO and EPSTEIN1978, Reference DENIRO and EPSTEIN1981; Eggers & Jones Reference EGGERS and JONES2000) and, thus, indirect indicators of trophic niches of these taxa. We focused on the following hypotheses and predictions: (1) The assemblage is structured into different trophic levels. Since the assemblage contains both largely frugivorous/nectarivorous and insectivorous taxa, we predict that the assemblage has more than one trophic level, i.e. a δ15N range ≥3‰ (McCutchan et al. Reference MCCUTCHAN, LEWIS, KENDALL and MCGRATH2003, Vanderklift & Ponsard Reference VANDERKLIFT and PONSARD2003). (2) The assemblage is trophically structured into different ensembles. Based on field data, the assemblage includes species foraging in different microhabitats. Since δ13C increases with canopy height (Medina & Minchin Reference MEDINA and MINCHIN1980), we predict increased δ13C in species foraging higher in the canopy and in open areas as compared to species foraging in lower portions of the canopy. Furthermore, we expect the centroids (i.e. arithmetic mean of δ13C and δ15N) to differ between ensembles. (3) Bat species are separated into trophic niches. We predict species to occupy different trophic niches, indicated by species-specific isotopic signatures. In particular, we expect species sharing the same microhabitat to show greater differentiation into trophic niches than those of different microhabitats, indicated by larger nearest-neighbour distances. (4) Co-existing congeneric species are trophically more similar as compared with non-congeneric species. Because of phylogenetic inertia and similarity in morphology, we expect interspecific competition among sympatric congeners to be larger than among non-congeners, indicated by low nearest-neighbour distances between these species.
METHODS
Study site
The Ankarana is a large limestone massif deeply sculpted by the action of water, forming a karstic landscape with many caves and crevices, ideal day-roosting sites for numerous species of bats (Cardiff Reference CARDIFF2006). Four distinct habitats occur in the Ankarana: (1) dry deciduous forests, (2) dry forest on limestone, (3) barren areas of rock, and (4) peripheral anthropogenic grassy woodlands. The zone receives approximately 1900 mm of annual rainfall, mostly falling between December and April, resulting in a 7-mo dry season (Cardiff & Befourouack Reference CARDIFF, BEFOUROUACK, Goodman and Benstead2003). During the dry period, virtually no standing water occurs on the ground surface.
Sampling and specimens
The second author has conducted projects with several colleagues on the bat fauna of Ankarana. Most collected specimens were preserved in 12% formaldehyde for approximately 1 mo, washed in flowing water for at least 24 h, transferred to 65–75% ETOH, and stored in glass jars out of direct sunlight. Subsequently, hair samples were clipped from the lower back of catalogued specimens, placed in individual vials, and then air-dried in open vials.
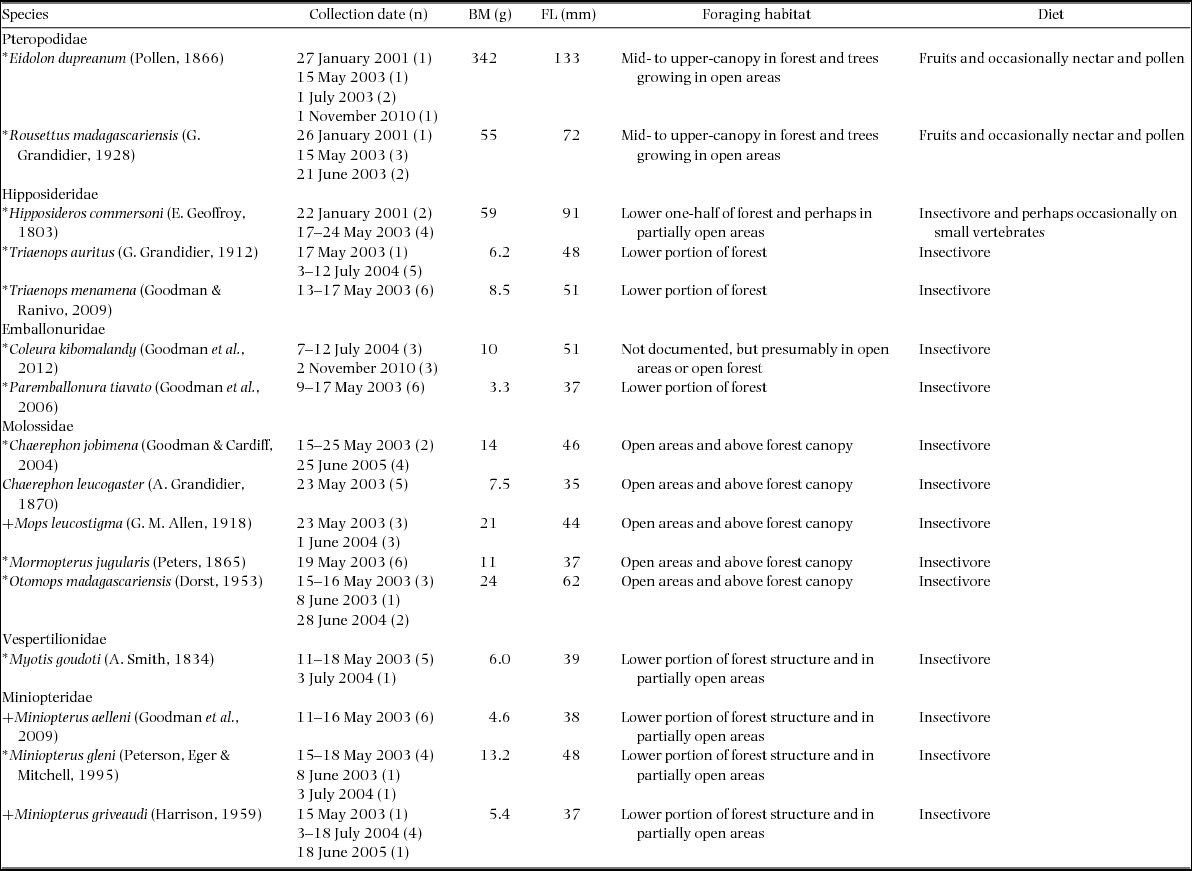
We collected hair samples from specimens of 16 species of six bat families, 12 endemic to Madagascar and three endemic to the Malagasy Region (Madagascar and the Comoros Archipelago) (Appendix 1). These included all of the known bats to occur in the Ankarana, excluding Pteropus rufus (Pteropodidae), the largest frugivore on Madagascar. We obtained six hair samples per species, with the exceptions of Eidolon dupreanum and Chaerephon leucogaster, for which only five samples each were available. The animals representing a given taxon were not necessarily using the same day-roost site. All specimens were from the Ankarana with the exceptions of Mops leucostigma samples collected at the park periphery or a synanthropic roost site at the edge of a nearby village (Ambilobe) and those of C. leucogaster collected from the same synanthropic roost. The M. leucostigma material from the park boundary and the village did not differ in δ15N, but those collected in the village were lower in δ13C (c. 2‰). The C. leucogaster samples from near Ambilobe were similar in δ13C and δ15N to other Molossidae.
There is a very marked dry season at Ankarana, between May and November, presumably causing notable seasonal differences in density and diversity of food resources available to bats, particularly insectivorous species, as known from other sites on Madagascar (Ramasindrazana et al. Reference RAMASINDRAZANA, RAJEMISON and GOODMAN2012). In an attempt to control for seasonality, we have chosen individuals collected during the dry season. In a few cases, this was not possible and for E. dupreanum and Rousettus madagascariensis there is one individual per species from January and for Hipposideros commersoni two individuals from January. No information is available on when Ankarana bat species replace their hair, or in other words, the moment they assimilate the stable isotopes represented in the hair samples. Our assumption is that these different species are not migratory. In addition, samples of leaves of common forest trees (C3 plants) and of arthropods were collected in areas of the Ankarana where bat samples were obtained to establish baseline habitat data for stable isotopes.
Stable isotope analysis
Prior to analyses, all samples were oven-dried at 60°C until weight was constant to remove tissue water. Leaf samples were ground and homogenized with a ball mill. For determination of carbon and nitrogen isotope ratios, 1 mg of either homogenized leaves, parts of arthropods (abdomen, legs) or whole specimens of small arthropods, or whole hairs of bat specimens were enclosed into tin capsules. Mass spectrometry analyses were carried out at the Centre for Stable Isotope Research & Analysis (KOSI) in Göttingen (Germany), using an isotope ratio mass spectrometer (Delta Plus, Finnigan MAT, Bremen, Germany) in an online system after passage through an element analyser (NA 1110, Carlo Erba, Milan, Italy). Since the ratio between the heavy and the light isotopes is small and subject to natural fluctuations, the isotope data are compared with a standard and presented in δ notation calculated as follows:
Where δX is δ15N or δ13C, and R is the respective 15N/14N or 13C/12C ratio. The international standards are atmospheric air for nitrogen and PDB (Pee Dee Belemnite marine carbonate) for carbon. Analytical error was calculated based on the within-run standard deviations of the working standard, acetanilide, and ranged 0.08–0.10‰ for δ15N and 0.06–0.10‰ for δ13C.
Data analyses
First, we analysed δ13C–δ15N bi-plots based on mean values of species to characterize the community as a whole (Layman et al. Reference LAYMAN, ARRINGTON, MONTANA and POST2007). We calculated four different measures of community trophic diversity: (1) total δ15N range signifying the number of trophic levels; (2) total δ13C range indicating variation in basal resources; (3) the total area covered by the community calculated as a minimum convex polygon with ArcView3.3 (Animal movement extension, ESRI) and the standard ellipse (Jackson et al. Reference JACKSON, INGER, PARNELL and BEARHOP2011) indicating the isotope niche space covered by the community; and (4) the mean distance of each species to the community centroid (mean δ15N and mean δ13C over all species) representing average trophic diversity in the community. Further, we calculated two measures to estimate trophic and niche packing parameters: (1) the mean Euclidean distance to nearest neighbours indicating niche packing; and (2) the standard deviations of distances to nearest neighbours reflecting the evenness of species distribution in isotopic niche space. Finally, we assessed whether the packing of species in the community δ13C–δ15N bi-plot deviates from a random pattern using the Clark & Evans (Reference CLARK and EVANS1954) nearest-neighbour analysis for spatial distribution (Krebs Reference KREBS1998). We calculated an index of aggregation (R), based on observed nearest-neighbour distances (NNDobs) in relation to expected nearest neighbour distances of species in the δ13C–δ15N bi-plot area covered by the whole community. The index of aggregation calculation is: R = NNDobs/NNDexp, with NNDobs = ∑NNDi/N and NNDexp = 1/(2√P). The density (P) is given by P = N/area with N = number of species and area = total area covered by the community calculated as a convex hull area. If R = 1, the spatial pattern is random; if R approaches 0, the spatial pattern is clumped; and if R approaches 2.15, the spatial pattern is regular (Krebs Reference KREBS1998).
Second, we used a general hypothesis-testing framework for stable isotope data proposed by Turner et al. (Reference TURNER, COLLYER and KRABBENHOFT2010) and compared differences in centroid location and dispersion metrics across three ensembles in the δ13C–δ15N bi-plot. Our sample contains two species of Pteropodidae, which feed predominantly on fruits. These bats use a variety of habitat types including the forest mid- to upper-canopy and isolated trees in open areas. Further, our sample contains insectivorous species, which use different microhabitats in and around the forest: (1) species foraging in the lower portion of the forest, up to about 5–7 m; and (2) species that forage above the canopy and in open areas. Based on these differences in feeding ecology and microhabitat use, we classified bats into the following ensembles: (1) frugivore mid-upper canopy (n = 2 species); (2) insectivore open space (n = 6 species); and (3) insectivore lower canopy (n = 8 species) (Appendix 1).
To assess whether ensembles occupy different portions of the δ13C–δ15N bi-plot, we compared differences in Euclidean distances between ensemble centroids (i.e. arithmetic mean of δ13C and δ15N per ensemble) for each pairwise combination of ensembles. Further, we assessed differences in ensemble dispersion within isotope space by testing whether (1) mean distances of singular species to ensemble centroids and (2) within-ensemble mean nearest-neighbour distances differ between each pairwise combination of ensembles. Using a residual permutation procedure (RPP), we compared all centroid location and dispersion metrics to null distributions. This procedure shuffles residual vectors of individual observations (δ13C–δ15N pair of one species) to the ensemble centroids and generates null model distributions based on 9999 random permutations of residual vectors (for details see Turner et al. Reference TURNER, COLLYER and KRABBENHOFT2010). The RPP allows for statistical testing of ensemble differences from zero, i.e. the null hypothesis of no difference between the pairs of ensembles. For differences between centroid locations, we calculated the parametric Hotelling's T2 test statistics, which is a multivariate analogue of the t-test. All calculations of centroid location and dispersion metric test statistics are based on Turner et al. (Reference TURNER, COLLYER and KRABBENHOFT2010).
Third, we applied multivariate analysis, which incorporates within-species variation. Based on MANOVA, we test whether species differ in stable isotope signatures. Using univariate ANOVAs and subsequent post hoc Tukey HSD tests, we further examined whether these differences are due to interspecific variation in δ13C or δ15N and whether species pairs differ from each other. In order to test whether the community is isotopically structured into ensembles, we ran further multivariate analyses (1) with ensemble as the only explanatory variable and (2) with ensemble, body mass and forearm length as covariates. Based on univariate ANOVAs, we further tested whether these differences are due to inter-ensemble variation in δ13C or δ15N and used post hoc Tukey HSD tests to examine pairwise differences between ensembles. All statistics are performed in the statistical package R 2.15 (www.r-project.org) and tests were two-tailed with accepted significance levels of P ≤ 0.05.
RESULTS
Plant and arthropod samples
Plant and insect samples were analysed to provide a habitat baseline of isotopic variation. Plant samples (n = 3), collected at sites in the Ankarana in the immediate proximity to bat roosts, had a median of δ13C of −31.2‰ (range = −34.6‰ to −30.7‰) and a median δ15N of 4.0‰ (range = 2.8–4.2‰). Insect samples (small 0.5–1.0 cm, including Diptera and Lepidoptera, n = 41) had a mean (± SD) δ13C of −25.0‰ ± 1.54‰ and δ15N of 7.3‰ ± 2.7‰. Overall, insects showed high variation in both stable isotope ratios, supporting the assumption that isotopic differences between bat species reflect varying prey sources in their diet.
Community overview
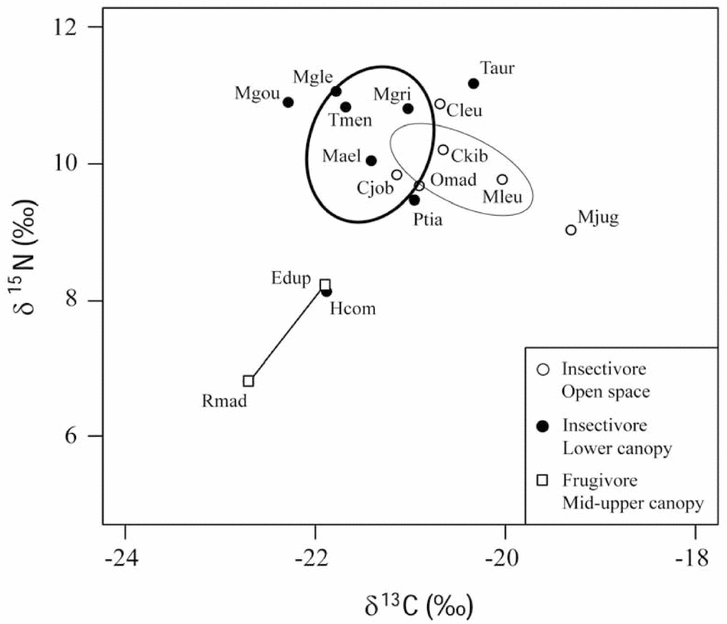
We calculated several measures to describe the trophic community structure based on the δ13C–δ15N bi-plot of species mean values (Figure 1). The mean δ15N of species in the Ankarana bat community was 9.8‰ and the total δ15N range was 4.4‰; hence, the community spans two trophic levels from a δ15N minimum of 6.8‰ for Rousettus madagascariensis, c. 3‰ enriched towards the plants in the habitat, to a maximum of 11.2‰ for Triaenops auritus, c. 4‰ enriched towards the insects in the habitat. For δ13C, the community mean was −21.2‰ and the total range was 3.4‰, with a minimum of −22.7‰ for R. madagascariensis and a maximum of −19.3‰ in Mormopterus jugularis, indicating that consumers include multiple basal resources with varying δ13C in their diets. The total stable isotope area covered by the community was 8.91‰2 based on convex hull and 5.83‰2 based on standard ellipse areas. The mean distance to the community centroid was 1.24‰ (range = 0.05–3.39‰) and the mean nearest-neighbour distance was 0.46‰ (range = 0.06–1.59‰). Species aggregation in the δ13C–δ15N bi-plot was high (R = 1.24), but nearest-neighbour distances did not deviate from a random pattern (Z = 1.85, P = 0.064). For the three cases of species groups of the same genus (Triaenops, Miniopterus and Chaerephon), a congener was never the nearest neighbour in the δ13C–δ15N bi-plot and distances between pairs of congeners were larger than mean + SD of the nearest-neighbour distances (0.86‰). The exception is for M. griveaudi–M. gleni (0.79‰).

Figure 1. Mean δ13C and δ15N of all bat species documented in Ankarana, northern Madagascar. Species belonging to the same ensemble are depicted in the same symbol. Ensembles are largely delineated standard ellipses (bold line: insectivore lower canopy, light line: insectivore open-space) or connected by a solid line (frugivore mid-upper canopy). Key to species – Edup: Eidolon dupreanum, Rmad: Rousettus madagascariensis, Hcom: Hipposideros commersoni, Taur: Triaenops auritus, Tmen: T. menamena, Ckib: Coleura kibomalandy, Ptia: Paremballonura tiavato, Cjob: Chaerephon jobimena, Cleu: C. leucogaster, Mleu: Mops leucostigma, Mjug: Mormopterus jugularis, Omad: Otomops madagascariensis, Mgou: Myotis goudoti, Mael: Miniopterus aelleni, Mglen: M. gleni, Mgri: M. griveaudi.
Dispersion statistic
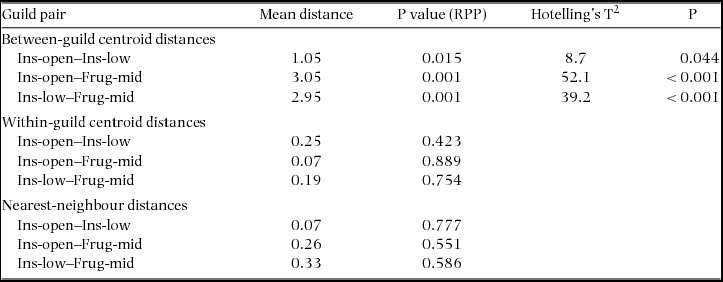
The Euclidean distances between centroids of ensembles differed from zero for all pairwise comparisons of groups (P < 0.05) (Table 1). The mean distances of individual species to the centroid of its ensemble did not differ significantly from zero for any pairwise comparison (all P > 0.4). Further, there were no differences for within-group mean nearest-neighbour distances (all P > 0.5).
Table 1. Mean Euclidean distances between centroids of ensembles, mean Euclidean distances between individual species and centroid of its ensemble, and mean distances between nearest-neighbours within ensembles. Statistical difference from zero was assessed based on residual permutation procedure (RPP) with 9999 random permutations and using the multivariate parametric Hotelling's T2 test statistics. Ins-open: insectivore open space, Ins-low: insectivore lower canopy, Frug-mid: frugivore mid-upper canopy

Multivariate analysis
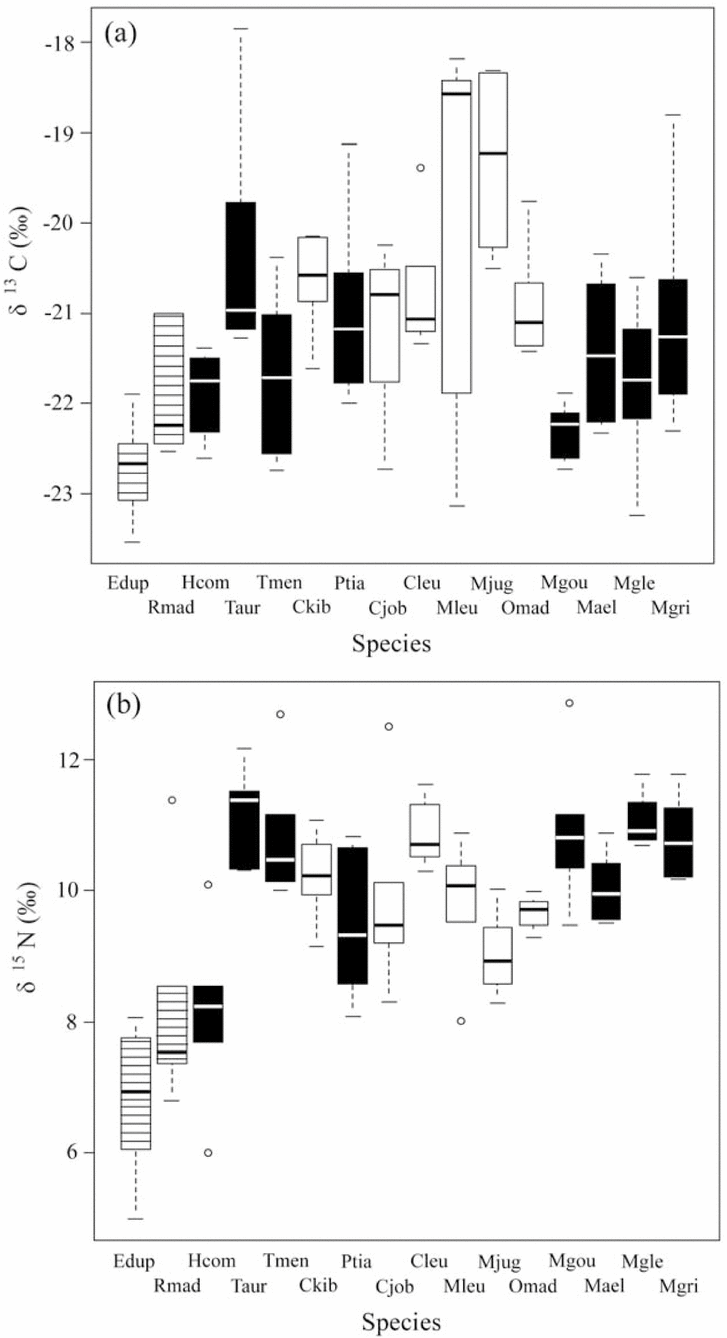
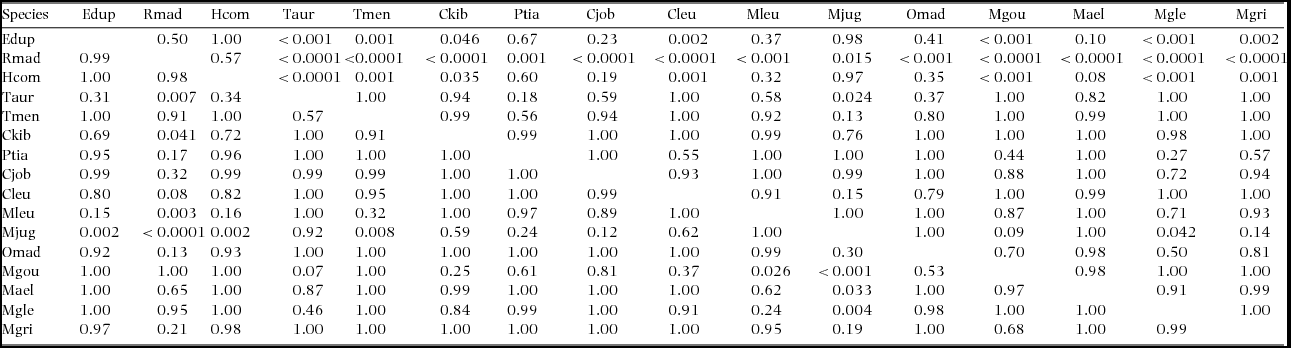
Multivariate analysis revealed an effect of species (MANOVA, Pillai's trace = 1.09, F(15,156) = 6.3, P < 0.0001). Overall, species differed in δ15N (F(15, 78) = 9.60, P < 0.0001) and in δ13C (F(15,78) = 4.58, P < 0.0001) (Figure 2). Post hoc pairwise comparisons showed differences only between some species pairs (Appendix 2). Notably, R. madagascariensis differed from all others in δ15N (all P < 0.02), except Eidolon dupreanum. Eidolon dupreanum differed in δ15N from all Miniopteridae (all P < 0.01), as well as Chaerephon leucogaster, Coleura kibomalandy, Triaenops auritus and T. menamena (all P < 0.05).

Figure 2. Differences in δ13C (a) and δ15N (b) among 16 bat species co-occurring at Ankarana, northern Madagascar. Shown are median, inter-quartile range and range for n = 6 individuals per species, shadings indicate different ensembles (hatched: frugivore mid-upper canopy; black: insectivore open-space; white: insectivore low canopy). See Figure 1 for definition of species acronyms.
There were differences within families for δ15N, such as between Hipposideros commersoni and both Triaenops spp. (all P < 0.001). Across families, Mormopterus jugularis differed in δ15N from Miniopterus gleni (P = 0.042), further H. commersoni differed from C. kibomalandy (P = 0.035), as well as from Myotis goudoti (P < 0.001), Miniopterus gleni (P < 0.001) and M. griveaudi (P < 0.001). For δ13C there were differences across families – Mormopterus jugularis differed from E. dupreanum (P = 0.002), R. madagascariensis (P < 0.0001), H. commersoni (P = 0.002), T. menamena (P = 0.008), Myotis goudoti (P < 0.001), Miniopterus aelleni (P = 0.033) and M. gleni (P = 0.004). Further, R. madagascariensis differed in δ13C from T. auritus (P = 0.007), C. kibomalandy (P = 0.041) and Mormopterus jugularis (P < 0.0001).
Also ensemble differed in isotopic signatures overall (MANOVA, Pillai's trace = 0.57, F(4,182) = 18, P < 0.0001), as well as in δ15N (univariate ANOVA, F(2, 91) = 25.2, P < 0.0001) and in δ13C (F(2,91) = 15.0, P < 0.0001). Post hoc pairwise comparisons revealed that all ensemble pairs differed in δ13C (all P < 0.05). For δ15N, frugivorous mid- to upper-canopy species differed from insectivorous open-space and lower-canopy species (all P < 0.0001), but the two insectivore ensembles were similar (P = 0.28). The inclusion of body mass and forearm length as additional explanatory variables helped to improve model fit (F(4,178) = 4.7, P = 0.001) – ensemble (Pillai's trace = 0.62, F(4,178) = 19.8, P < 0.0001) and forearm length (Pillai's trace = 0.18, F(2,88) = 9.4, P = 0.0002) explain significant parts of the variation but body mass was not significant (Pillai's trace = 0.02, F(2,88) = 0.8, P = 0.44). Overall, the univariate models including species as the only predictor explained more variation (δ15N: R2 = 0.65, δ13C: R2 = 0.47) than models with ensemble as the only predictor (δ15N: R2 = 0.36, δ13C: R2 = 0.25) and the covariance models (δ15N: R2 = 0.45, δ13C: R2 = 0.29).
DISCUSSION
In many tropical forest communities bats are the most species-rich mammalian order (Kingston Reference KINGSTON, Kunz and Parsons2009) and understanding mechanisms allowing their coexistence will provide important insights into community ecology. Classical niche theory (reviewed in Chase & Leibold Reference CHASE and LEIBOLD2003), as well as random processes (Hubbell Reference HUBBELL2001), have only been able to partially explain the structure and composition in New and Old World bat assemblages (Bloch et al. Reference BLOCH, STEVENS and WILLIG2011, Schoeman & Jacobs Reference SCHOEMAN and JACOBS2011, Stevens & Willig Reference STEVENS and WILLIG2000). However, these assemblages are often structured into distinct trophic guilds (Giannini & Kalko Reference GIANNINI and KALKO2004), with partitioning of species along habitat dimensions based on ecosensory and morphological adaptations (summarized in Schnitzler et al. Reference SCHNITZLER, MOSS and DENZINGER2003). Here, we used stable isotope analysis to provide the first insights into the trophic structure of a species-rich Malagasy bat assemblage and possible mechanisms for facilitating the local coexistence of taxa.
Assuming an average trophic enrichment of 3‰ per trophic level (Vanderklift & Ponsard Reference VANDERKLIFT and PONSARD2003), we found that the Ankarana bat assemblage spans two trophic levels with the frugivorous/nectarivorous species Rousettus madagascariensis and Eidolon dupreanum representing the primary consumers. These species are largely distinct from all others at Ankarana in δ15N and the exceptions are best explained by sample size and notable within-species variation. Most important in this regards, is the largest insectivorous species, Hipposideros commersoni, which falls in the same trophic level and is distinct in δ15N from many of the smaller insectivorous bats. This species specializes on Coleoptera, particularly scarabids (Rakotoarivelo et al. Reference RAKOTOARIVELO, RANAIVOSON, RAMILIJAONA, KOFOKY, RACEY and JENKINS2007, Reference RAKOTOARIVELO, RALISATA, RAMILIJAONA, RAKOTOMALALA, RACEY and JENKINS2009; Razakarivony et al. Reference RAZAKARIVONY, RAJEMISON and GOODMAN2005). All other insectivorous taxa form a diverse secondary-consumer level, which is on average 4‰ enriched over the primary level.
Other tropical bat assemblages typically enclose representatives of phytophagous and animalivorous species (Giannini & Kalko Reference GIANNINI and KALKO2004); however, distinct trophic levels are not always found. For example, in a diverse New World assemblage of 67 members of the Phyllostomidae, stable nitrogen isotope analysis revealed that species ranged continuously over three trophic levels including herbivorous, insectivorous and carnivorous/sanguinivorous (Rex et al. Reference REX, CZACZKES, MICHENER, KUNZ and VOIGT2010). Thus, accordingly, the coexistence of bat species at Ankarana can partly be explained by avoidance of feeding competition at different trophic levels.
At Ankarana, species of different ensembles formed distinct clusters in isotopic space. The frugivorous mid-canopy bats were distinct in both isotopes. The insectivorous above canopy/open space bats differed from insectivorous low-canopy bats only in δ13C. This pattern is in accordance with the prediction of the canopy-effect hypothesis, which posits that δ13C in forest plants generally increases as a function of distance from the ground (Medina & Minchin Reference MEDINA and MINCHIN1980). Similar patterns of δ13C and vertical stratification in feeding height have been revealed in Neotropical bats (Rex et al. Reference REX, MICHENER, KUNZ and VOIGT2011, Voigt Reference VOIGT2010) and forest-dwelling rodents (Mauffrey & Catzeflis Reference MAUFFREY and CATZEFLIS2003), a Malagasy tenrec and rodent community (Dammhahn et al. Reference DAMMHAHN, SOARIMALALA and GOODMAN2013) and a complete mammal community in the Congo Basin (Cerling et al. Reference CERLING, HART and HART2004). Thus, the partitioning of microhabitats among insectivorous bat species is a further mechanism decreasing feeding competition and facilitating the coexistence of species with similar diets.
In contrast to our prediction, species utilizing the same microhabitat did not show indications of stronger trophic differentiation than those occupying different microhabitats. Thus, based on stable isotopes these bat species appear to have similar dietary composition. The two frugivorous/nectarivorous species (Eidolon dupreanum and Rousettus madagascariensis) are similar in isotopic signatures. However, these two species differ notably in size (342 g versus 55 g body mass, respectively) and are, thus, not expected to show strong feeding competition (Hutchinson Reference HUTCHINSON1957). Studies of these two taxa in eastern Madagascar revealed that they differ notably in the maximal size of seeds they ingest – E. dupreanum swallows seeds up to 7 mm and feeds almost exclusively on fruits (Picot et al. Reference PICOT, JENKINS, RAMILIJAONA, RACEY and CARRIÈRE2007, Ratrimomanarivo Reference RATRIMOMANARIVO2007) and R. madagascariensis up to 2.5 mm, particularly Ficus fruits, and extensively consumes nectar, flowers and leaves (Andrianaivoarivelo et al. Reference ANDRIANAIVOARIVELO, RAMILIJAONA, RACEY, RAZAFINDRAKOTO and JENKINS2011). Excluding the poorly known emballonurid Coleura kibomalandy, five species of molossid represent the open-space/above-canopy insectivore ensemble in Ankarana. These taxa, which are largely similar in body size (Appendix 1), use narrow-band echolocation to locate large flying insects in clutter-free space and are similar in δ15N and δ13C. Faecal analyses of molossids in eastern Madagascar revealed that they principally feed on Coleoptera, Hemiptera, Lepidoptera and Diptera, with differences in the proportion of different insect groups in the diet of some species (Andrianaivoarivelo et al. Reference ANDRIANAIVOARIVELO, RANAIVOSON, RACEY and JENKINS2006). In particular, Mormopterus jugularis consumed higher proportions of Coleoptera (61%) than Mops leucostigma (40%), but with considerable seasonal variation. On the basis of faecal analyses of individuals captured in another Malagasy dry-forest formation, the largest molossid in the Ankarana sample, Otomops madagascariensis, feeds extensively on Coleoptera and Lepidoptera (> 80%) (Andriafidison et al. Reference ANDRIAFIDISON, KOFOKY, MBOHOAHY, RACEY and JENKINS2007). The low-canopy insectivores are the most species-rich ensemble in the Ankarana bat assemblage, comprising members of four different families and eight different species (Appendix 1). With the exception of H. commersoni, these taxa are very similar in stable isotopes and range in body mass from 3.3 to 13 g with several similarly sized species pairs. Thus, in the Ankarana bat assemblage, niche segregation, as measured by body size or stable isotope variation, does not appear to explain the co-occurrence of the molossid species, as well as of the low-canopy insectivores. Further studies on the feeding ecology of these syntopic species are needed to understand finer details on potential dietary overlap.
Also at the community level, we found only some indication for resource partitioning across species. The conservative post hoc tests differentiated the species-specific isotopic signatures of a few species pairs. Moreover, the community-level analyses based on the bi-plot metrics revealed that the Ankarana assemblage is densely packed in isotope space with many species having similar diets (Layman et al. Reference LAYMAN, ARRINGTON, MONTANA and POST2007), indicating that competitive trophic interactions only partly structure this assemblage.
Theoretically, it has been proposed that congeneric species experience higher competition due to recent ancestry and resulting similarities in ecology, morphology and behaviour (Sfenthourakis et al. Reference SFENTHOURAKIS, TZANATOS and GIOKAS2005). Our results did not support this pattern for the three congeneric species groups in the Ankarana bat assemblage (Appendix 1): (1) Chaerephon was represented by two species, which were similar in isotopic signatures, but differ in body mass and forearm length. The size difference renders increased congeneric feeding competition unlikely between these two species. (2) Three species of Miniopterus co-occur at Ankarana and details of ecological niche partitioning are not known. Miniopterus gleni, the largest, exceeds the other species by c. 30% in forearm length and is more than double in body mass. Thus, reduced feeding competition with the other two coexisting species is expected (Hutchinson Reference HUTCHINSON1957). In contrast, M. aelleni and M. griveaudi are similar in body size and mass but co-occur at various sites in Madagascar and the Comoros (Goodman Reference GOODMAN2011). Ramasindrazana et al. (Reference RAMASINDRAZANA, GOODMAN, SCHOEMAN and APPLETON2011) showed that M. aelleni deviates from the allometric relationship between forearm length and peak frequency within the Miniopteridae, which might indicate echolocation character displacement. Our results indicate that in sympatry M. aelleni and M. griveaudi do not take different arthropod prey based on δ15N and δ13C signatures (Figure 2). (3) Our sample included two species of Triaenops, T. auritus and T. menamena, similar in size and stable isotopic signatures. Faecal analyses of T. furculus, sister species to T. auritus, and T. menamena at a spiny bush site in south-western Madagascar, indicate that they forage in largely the same microhabitats and show broad overlap in the orders of insects they consume, but with some notable seasonal differences (Ramasindrazana et al. Reference RAMASINDRAZANA, RAJEMISON and GOODMAN2012). Since these two species are distinct in the peak frequency of their largely constant-frequency calls, differentiation of prey based on size is likely (Ramasindrazana & Goodman Reference RAMASINDRAZANA and GOODMAN2012).
Stable isotope studies are limited in resolution of dietary composition of the study animal and are based on the assumption that prey sources differ isotopically. Potential insect prey collected at Ankarana showed high variation in both stable isotopes lending further support to this assumption. Thus, low isotopic differentiation of bat species within the Ankarana assemblage can be interpreted as an indication of reduced feeding competition. This conclusion corresponds to similar studies (Stevens & Willig Reference STEVENS and WILLIG2000), which also found low support for competitive interactions in the structuring of bat assemblages. Likewise, combining data on morphology and echolocation in Old World bat assemblages, Schoeman & Jacobs (Reference SCHOEMAN and JACOBS2011) found higher dietary overlap between species within ensembles than expected by chance. Several non-exclusive causes have been presented to explain this apparent deviation from fundamental predictions of ecological niche theory. First, bat assemblages might not reach equilibrium states, because population densities of a given species may be too low (Bloch et al. Reference BLOCH, STEVENS and WILLIG2011). Second, food might not be a limiting resource for insectivorous bats (Fenton Reference FENTON1990), partly because their mobile nature allows them to be more flexible to resource density, size and distribution and, thus, relax local resource competition (Stevens & Willig Reference STEVENS and WILLIG2000). Third, specific combinations of phenotypic characteristics of bats, such as body size, cranial and wing morphology, and echolocation calls, facilitate a variety of dietary specializations (Aguirre et al. Reference AGUIRRE, HERREL, VAN DAMME and MATTHYSEN2002, Norberg Reference NORBERG, Wainwright and Reilly1994, Schnitzler et al. Reference SCHNITZLER, MOSS and DENZINGER2003, Siemers & Schnitzler Reference SIEMERS and SCHNITZLER2004). These different causes could potentially explain the relaxed feeding competition in the Ankarana assemblage.
In conclusion, stable isotopes as an indirect measure of niche differentiation provided some insights into the community ecology of a bat assemblage in northern Madagascar. A combination of mechanisms appeared to facilitate the coexistence of these species: (1) differentiation into two trophic levels and (2) utilization of different microhabitats by insectivorous bats. In contrast to other non-volant mammalian communities (Dammhahn & Kappeler Reference DAMMHAHN and KAPPELER2013, Dammhahn et al. Reference DAMMHAHN, SOARIMALALA and GOODMAN2013), community-wide trophic avoidance among species was less pronounced. This observation is in accordance with other studies of a variety of New and Old World bat assemblages (Schoeman & Jacobs Reference SCHOEMAN and JACOBS2011, Stevens & Willig Reference STEVENS and WILLIG2000), based on different types of data, indicating that competitive interactions appear to be relaxed in the different communities and not a prevailing structuring force. Illuminating alternative mechanisms by studying fine-scale dietary specializations, habitat use, ecomorphology and sensory ecology at local and island-wide scales will be fascinating areas of further research on the bats of Madagascar.
ACKNOWLEDGEMENTS
We thank the Ministère des Forêts et de l'Environnement for providing research authorizations for the capture and collection of animals. The fieldwork was supported by grants from the National Geographic Society (6637-99 & 7402-03), The Volkswagen Foundation and The John D. and Catherine T. MacArthur Foundation. Scott Cardiff, Fanja Ratrimomanarivo, Beza Ramasindrazana, Corrie Schoeman and Peter Taylor helped with some of the fieldwork. We thank Peter M. Kappeler for support and Reinhard Langel (KOSI) for technical help in the lab. Financial support for the isotope analysis was kindly provided by Deutsches Primatenzentrum Göttingen and The Volkswagen Foundation. An earlier version of this manuscript benefited from comments by two anonymous reviewers.
Appendix 1. Collection dates and sample size (n) of hair samples obtained from 94 specimens of all 16 bat species of the Ankarana assemblage included in the stable isotope analyses. Species marked by an asterisk (*) are endemic to Madagascar and those with a plus (+) endemic to the Malagasy Region (Madagascar and the Comoros Archipelago). Information on foraging habitat, diet, body mass (BM) and forearm length (FL) of bat species are largely from Goodman (Reference GOODMAN2011). In cases of species showing sexual dimorphism, we have used the mean body mass value of the two sexes.

APPENDIX 2. Results of post hoc Tukey HSD test for pairwise comparisons between all species. Shown are P-values: upper right for δ15N, and lower left for δ13C. See Figure 1 for definition of species acronyms.