INTRODUCTION
Microalgal mass culture technologies have failed to produce bulk volumes of microalgal biomass at low cost due to contamination by biological pollutants (Zou & Richmond, Reference Zou and Richmond1999; Hu & Gao, Reference Hu and Gao2003; Aslan & Kapdan, Reference Aslan and Kapdan2006; Chisti, Reference Chisti2007; Park et al., Reference Park, Craggs and Shilton2011; Day et al., Reference Day, Slocombe and Stanley2012; Peng et al., Reference Peng, Lan, Zhang, Sarch and Laporte2015). Of these biological pollutants, protozoa are the common predatory species in the mass cultivation of microalgae (Rosetta & McManus, Reference Rosetta and McManus2003; Frederiksen et al., Reference Frederiksen, Edwards, Richardson, Halliday and Wanless2006; Li et al., Reference Li, Zhang, Wu and Zheng2006; Lurling & Beekman, Reference Lurling and Beekman2006).
Recently, it has been recognized that many microalgae have a defence against predator grazing (Wang et al., Reference Wang, Zhang, Chen, Wang and Liu2013). Previous investigations have demonstrated that the defence is a passive response to increased algal population density by excretion of chemicals with a change in physical properties (Wang et al., Reference Wang, Zhang, Chen, Wang and Liu2013). However, as regards multivariate approaches to identifying their ability of defence little information has been documented.
As a primary component of microbiota, protozoa play a crucial role in transferring carbon and energy from low tropic levels (bacteria and microalgae) to high tropic levels in microbial food webs (Norf et al., Reference Norf, Arndt and Weitere2009a, Reference Norf, Arndt and Weitereb; Xu et al., Reference Xu, Zhang, Jiang and Yang2014). With short generation times, relative immobility, rapid responses to environmental changes and ease of sampling, they have been widely used as a robust bioindicator for bioassessment or bioassay, especially at community level (Xu et al., Reference Xu, Zhang, Jiang and Yang2014).
In this study, the defence effects of microalgae on protozoan grazing were studied at community level using an artificial substratum method. The main objectives of this study were: (1) to reveal the defence effect of microalgae on protozoan grazing, (2) to demonstrate the relationships between variations in species richness, taxonomic distinctness of protozoan communities and algal concentrations, and (3) to evaluate the feasibility of community-based bioassay for identifying the defence against protozoan grazing.
MATERIALS AND METHODS
Protozoan sample collection
Samples of protozoan communities were collected in coastal waters of the Yellow Sea, northern China (Figure 1). The glass slide systems were designed, deployed, anchored and sampled as described by Xu et al. (Reference Xu, Zhang, Jiang, Zhu, Al-Rasheid, Warren and Song2011b). A total of 20 microscopy glass slides were used as artificial substrata for collecting the protozoa at a depth of 1 m below the water surface. Two PVC frames were used to hold the 20 glass slides and all slides were collected at the exposure time of 14 days. The glass slides were transferred into Petri dishes containing in situ water and then stored in a cooling box before transporting to the laboratory within 2 h for testing of protozoan assemblages (Xu et al., Reference Xu, Zhang, Jiang and Yang2014).

Fig. 1. Sampling station, which was located in the harbour of the Olympic Sailing Center (OSC), a coastal area of the Yellow Sea, near Qingdao, northern China.
After 3-day domestication under laboratory conditions in an illumination culture cabinet (temperature 21.6°C and illumination 3960 LUX), a total of 18 glass slides with protozoan communities were used as test communities.
Experimental designation
Two algal species Nannochloropsis oceanica and Chlorella sp. were used as test microalgae, which were obtained from the Laboratory of Applied Microalgae Biology, Ocean University of China.
All bioassay experiments were conducted in Petri dishes during a period of 9 days. For each of two test microalgae, five treatments with a same gradient of concentrations were designed as 100 (treatment 1 as a control), 104 (treatment 2), 105 (treatment 3), 106 (treatment 4) and 107 (treatment 5) cell ml−1, respectively. For each of both controls and a total of eight treatments, one glass slide with protozoan communities was transferred into a Petri dish with 20 ml filtered seawater (FSW) without and with test microalgae, respectively. In each treatment, two replicates were used as parallel tests.
Identification and enumeration
Protozoa identification and enumeration were conducted following the methods outlined by Xu et al. (Reference Xu, Zhang, Jiang, Zhu, Al-Rasheid, Warren and Song2011b). Taxonomic classification of protozoa was based on the published references such as Song et al. (Reference Song, Warren and Hu2009). The taxonomic scheme used was according to Lynn (Reference Lynn2008).
The enumeration of protozoa in vivo was conducted at a 100-fold magnification under an inverted microscope (Xu et al., Reference Xu, Zhang, Jiang, Zhu, Al-Rasheid, Warren and Song2011b). For recovering all species colonizing the glass slides, the whole slide (17.5 cm2) was examined to record both occurrences and individual abundances, using bright field illumination.
Data analysis
Four taxonomic diversity/distinctness measures were summarized using four taxonomic relatedness parameters: taxonomic diversity (Δ), taxonomic distinctness (Δ*), average taxonomic distinctness (Δ+) and variation in taxonomic distinctness (Λ+).They were computed following the equations:
where x i (i = 1, 2, …, S) denotes the abundance of the ith species; N is the total number of individuals in the sample; ω ij is the ‘distinctness weighting’ given to the path length linking species i and j (i < j); S is the number of species(Warwick & Clarke, Reference Warwick and Clarke1995).
The distinctness weightings used in this study were according to Clarke & Warwick (Reference Clarke and Warwick1998): ω = 1 (species in the same genus), 2 (same family but different genus), 3 (same order but different family), 4 (same class but different order) and 5 (same phylum but different class). The distinctness of two species connected at the highest taxonomic level was set equal to 100 (Warwick & Clarke, Reference Warwick and Clarke1998, Reference Warwick and Clarke2001). A regional master list was compiled using the data from Song et al. (Reference Song, Warren and Hu2009), in which a total of 375 protozoa species was recorded from local areas of the Yellow Sea, near Qingdao, China.
Multivariate analyses of variations in the protozoan communities were analysed using the PRIMER v7.0.11. Bray–Curtis similarity matrices were used for biological community analysis (Xu et al., Reference Xu, Zhang, Jiang and Yang2014). The variations in protozoan community structure at five concentration levels of both algal species were summarized using the submodule CAP (canonical analysis of principal coordinates) on Bray–Curtis similarities from the transformed species-abundance data (Anderson et al., Reference Anderson, Gorley and Clarke2008). Vector overlay of Pearson correlations of dominant species with the two CAP axes is also shown in CAP plots (Anderson et al., Reference Anderson, Gorley and Clarke2008; Jiang et al., Reference Jiang, Xu, Hu, Zhu, Al-Rasheid and Warren2011, Reference Jiang, Yang, Min, Kang and Lee2013). Ellipse tests were conducted to determine the significant departure at different concentration levels of the microalgae with an expected trait hierarchy using the submodule TAXDTEST (Clarke & Gorley, Reference Clarke and Gorley2015).
RESULTS
Species composition of protozoan community used
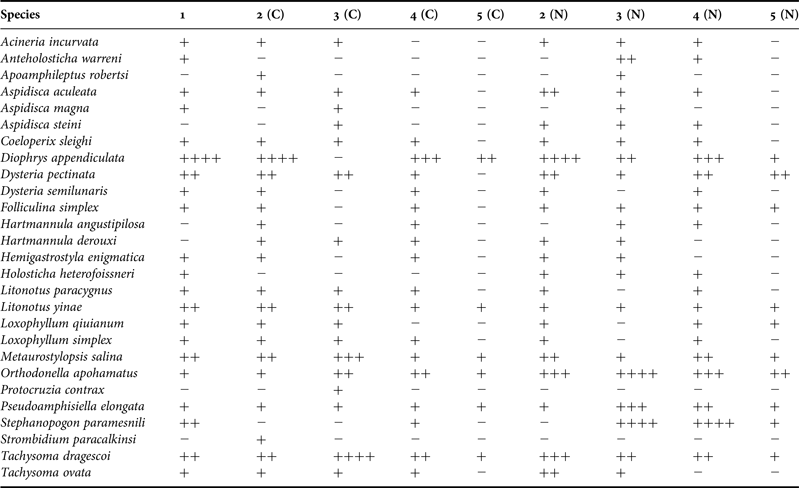
The protozoa species with occurrence and abundance are presented in Table 1. In the protozoan samples used, a total of 25 protozoan species (mainly ciliates) were identified (Table 1).
Table 1. Protozoan (mainly ciliate) species identified in samples used and average abundance (ind. cm−2) in five (1, 2, 3, 4 and 5) treatments with five (100, 104, 105, 106 and 107 cell ml−1) concentrations of two algal species Nannochloropsis oceanica and Chlorella vulgaris, respectively. Protozoan abundance: + = 0–1, ++ = 1–10, +++ = 10–100, ++++ = 100–1000, +++++: over 1000 ind cm−2). C = Chlorella vulgaris; N = Nannochloropsis oceanica.
Variations in species number and abundance of protozoan samples
The species number and abundance of the protozoan samples in five treatments are shown in Figure 2. In both N. oceanica and C. sp. treatments, the minimum values of species number were recorded in treatment 5 (Figure 2A), while abundances were highest in treatment 1 and 3, and lowest in treatment 5, respectively (Figure 2B).
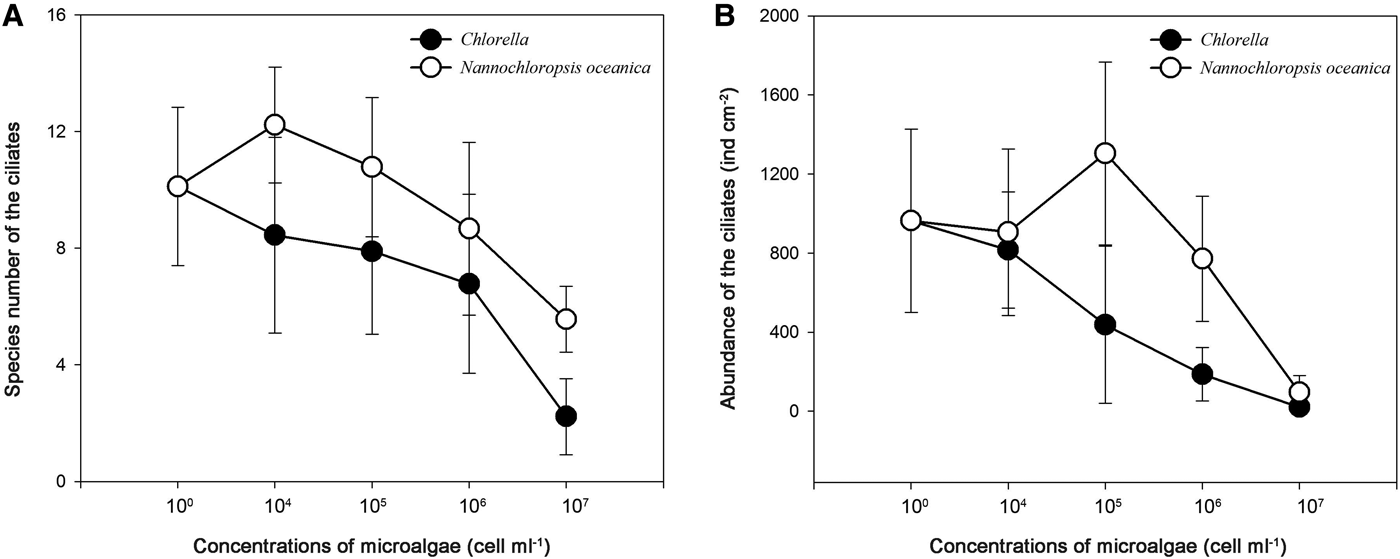
Fig. 2. Variations in species number (A) and abundance (B, ind. cm−2) of protozoan communities in five treatments (1–5).
Variations in community structure of protozoan samples
The relative abundance of protozoan communities at different concentration levels of two microalgae are shown in Figure 3. In treatments of C. sp., three structural community types of the protozoan assemblages could be recognized: (1) those dominated by Diophrys appendiculata and, to a lesser extent, Litonotus yinae (e.g. treatment 1 and 2); (2) those dominated by Tachysoma dragescoi and, to a lesser extent, Metaurostylopsis salina (treatment 3); and (3) those dominated by Diophrys appendiculata and, to a lesser extent, Orthodonella apohamatus (treatment 5) (Figure 3A).

Fig. 3. Variations in relative species number (A) and relative abundance (B, ind. cm−2) of protozoan communities in five treatments (1–5).
In treatments of N. oceanica, five structural community types could be distinguished: (1) those dominated by Diophrys appendiculata and, to a lesser extent, Litonotus yinae (treatment 1); (2) those dominated by Diophrys appendiculata and, to a lesser extent, Orthodonella apohamatus, Tachysoma dragescoi and Metaurostylopsis salina (treatment 2); (3) those dominated by Orthodonella apohamatus and, to a lesser extent, Stephanopogon paramesnili (treatment 3); (4) those dominated by Stephanopogon paramesnili and, to a lesser extent, Diophrys appendiculata (treatment 4); and (5) those dominated by Dysteria pectinata and, to a lesser extent, Orthodonella apohamatus (treatment 5) (Figure 3B).
Based on CAP ordinations, discrimination among 45 data of each treatment set of two microalgae showed a clear variation in community structure of the protozoa along the gradient of algal concentrations (Figure 4A, C). For example, in the treatments of C. sp., the first canonical axis (CAP 1) separated protozoan communities of treatment 5 (right side of figure) from other four treatments (left side of figure); the second canonical axis (CAP 2) discriminated data points of treatments 3 and 4 from those of treatments 1 and 2 (upper and lower parts of Figure 4A), respectively. A similar pattern was also found in the treatments of N. oceanica (Figure 4C).

Fig. 4. Canonical analyses of principal coordinates (CAP) for protozoan communities (A, B), with correlations of top seven species of contributors to the protozoan communities (C, D) with the CAP axes, in five treatments (1–5) of Chlorella sp. (A, C) and Nannochloropsis oceanica (B, D).
Vector overlay of Pearson correlations of top seven typical species with the CAP axes is shown in Figure 4B, D. For example, in the treatments of C. sp., vectors for two species (Diophrys appendiculata and Stephanopogon paramesnili) pointed towards data points of treatments 1 and 2; five (Orthodonella apohamatus, Metaurostylopsis salina, Dysteria pectinata, Litonotus yinae, Tachysoma dragescoi) and vectors for the remaining three species pointed towards the datapoint clouds of treatments 3 and 4 (Figure 4B). However, a different case was found in the treatments of N. oceanica (Figure 4D).
Variations in taxonomic diversity/distinctness of protozoan communities
In treatments of C. sp., except for the taxonomic diversity (Δ), the taxonomic distinctness (Δ*) and the average taxonomic distinctness (Δ+) of the protozoan samples generally represented a sharp decrease in treatment 5, while those in treatments of N. oceanica levelled off within the gradient of algal concentrations (Figure 5A–C). However, the variation in taxonomic distinctness (Λ+) generally showed a clear decreasing trend along the gradient of algal concentrations in treatments of both microalgae (Figure 5D).
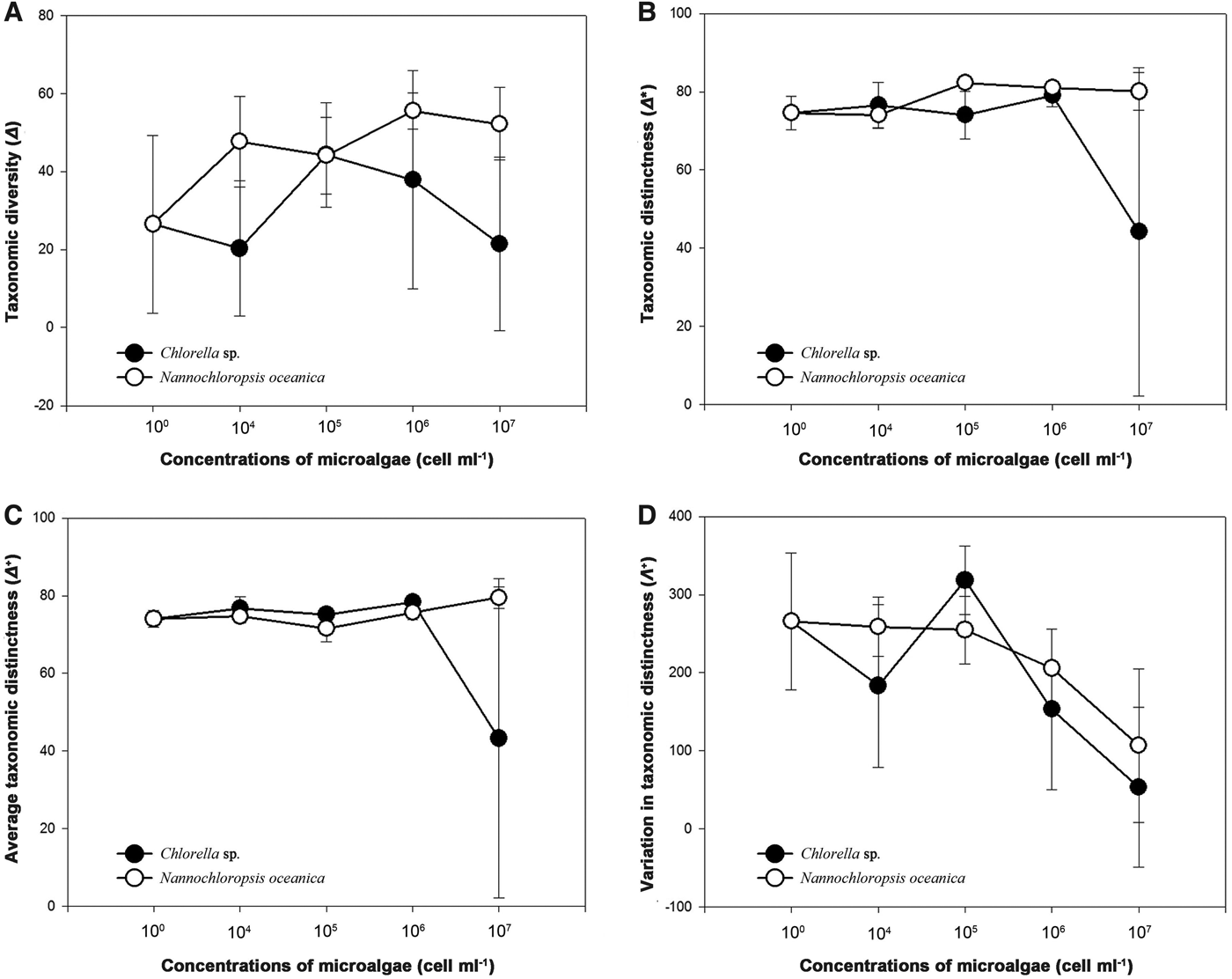
Fig. 5. Variations in taxonomic diversity (Δ, A), taxonomic distinctness (Δ*, B), average taxonomic distinctness (Δ+, C) and variation in taxonomic distinctness (Λ+, D) of the protozoan communities in five treatments (1–5) of both Chlorella sp. and Nannochloropsis oceanica.
The ellipse plots of 95% probability regions, with a range of two sub-list sizes (10 and 20) for the pair-wise (Δ+, Λ+) values of the small species pool at all five treatments, are shown in Figure 6. It is shown that the Δ+ − Λ+ data points of treatment 5 showed a different behaviour: 5 and 8 data were outside the 10 sub-size contours in treatments of C. sp. (Figure 6B–E) and N. oceanica (Figure 6F–I), respectively. It should be noted that no samples of the protozoa departed from the expected range of 10-species sub-list

Fig. 6. Ellipse plots of 95% probability regions with a range of two sub-list sizes (10 and 20) for the pair-wise (Δ+, Λ+) values of the ciliate samples in five treatments (1–5) of both Chlorella sp. (A–E) and Nannochloropsis oceanica (A, F–I), showing the departure of the protozoan samples from an expected range of 10-species sub-list.
DISCUSSION
Multivariate approaches are a powerful tool to detect changes in community structure (Clarke & Ainsworth, Reference Clarke and Ainsworth1993; Jiang et al., Reference Jiang, Wu and Shen2007, Reference Jiang, Xu, Hu, Zhu, Al-Rasheid and Warren2011; Xu et al., Reference Xu, Jiang, Al-Rasheid, Al-Farraj and Song2011a). In this study, CAP ordination demonstrated that the variations in protozoan community structure are related to the algal concentration gradient. A significant variation in community pattern of protozoa was found to be driven by algal concentrations, during which both species richness and abundance of protozoa decreased with an increase of algal concentrations. These findings suggest that two microalgae N. oceanica and C. sp. have strong defence effects on protozoan grazing.
Taxonomic diversity/distinctness have been widely used to summarize the internal trait of taxonomic relatedness pattern of a community (Warwick & Clarke, Reference Warwick and Clarke1995, Reference Warwick and Clarke2001). Compared with traditional biodiversity measures (e.g. species diversity), they have desirable properties such as less dependence on environment and sample and high sensitivity to microalgae concentration (Warwick & Clarke, Reference Warwick and Clarke1995, Reference Warwick and Clarke2001). In this study, the taxonomic distinctness and average taxonomic distinctness (Δ* and Δ+) of protozoa communities represented a high sensitivity to high concentration of microalgae. Furthermore, ellipse tests demonstrated an increasing trend of departure from the expected taxonomic pattern with increase of algal concentrations. Thus, it is suggested that this approach may be used as a potential tool for identifying defence of microalgae against protozoa grazing.
In summary, both N. oceanica and C. sp. represented a significant defence effect against protozoan grazing, especially at high density levels of the algae. Species richness, abundance and taxonomic distinctness of the protozoan showed a sharp decrease at high concentration level (107 cell ml−1) of both algae. A significant variation in community structure of the protozoa was found to be driven by the gradient of the algal concentrations. The paired biodiversity indices of the protozoan communities showed an increasing trend of departure from the expected taxonomic pattern with increase of algal concentrations. Based on the results, we suggest that the community-based bioassay might be used as a feasible tool for identifying defence against protozoan grazing of microalgae.
ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China (No. 31672308).









