The world breaks every one and afterward many are strong at the broken places.
—Ernest Hemingway, A Farewell to Arms, 1929, p. 249It has long been recognized that the experiences of parents and ancestors influence future generations (Harper, Reference Harper2005). Many cultural practices and rituals function to transmit historical knowledge and experience across generations. The Jewish Passover seder gathers family and friends for a meal during which the biblical story of the escape from bondage in Egypt is retold and freedom is celebrated. Children are specifically instructed to consider what the story means to them and about them. When survivors and witnesses to communal trauma, such as genocide, die off, cultural memory is enshrined and conveyed through memorials, museums, the arts, and memorialized anniversaries and rituals. Cultural trauma has been defined as occurring “when members of a collectivity feel they have been subjected to a horrendous event that leaves indelible marks upon their group consciousness, marking their memories forever and changing their future identity in fundamental and irrevocable ways” (Alexander, Reference Alexander2004). The shared experience of cultural trauma, targeting a cultural or ethnic group, becomes part of the story the community tells about the world, about itself, and about its survival (Volkan, Reference Volkan2001). It is hoped that the lessons of the past will be passed on, although the nature of the lesson may be a matter of disagreement. While the historical events may be in some way fixed, they are also open to reinterpretation and new meaning for future generations.
In the fields of psychology and psychiatry, both the experience and the developmental sequelae of trauma have primarily been conceptualized and studied as individual-level phenomena. The Diagnostic and Statistical Manual of Mental Disorders (DSM-5) defines trauma as “exposure to actual or threatened death, serious injury, or sexual violence,” including war, physical assault, threatened or actual sexual violence, terrorism, natural or human-made disasters, and severe accidents (American Psychiatric Association, 2013). Diagnoses such as posttraumatic stress disorder (PTSD) or depression following trauma are, of course, applied only to individuals. However, when trauma exposure is communal, it is not clear whether it requires different conceptual models, and whether the effects will be similar to any other trauma exposure, with comparable psychological, neuroendocrine, neurological, and molecular correlates. Clearly, the impact of cultural trauma must be evaluated not only at the individual level but also at the meso-level community and macro-level societal levels (Bronfenbrenner, Reference Bronfenbrenner1977, Reference Bronfenbrenner2009). Consideration of the effects of cultural trauma thus requires an interdisciplinary and multilevel approach, and cultural trauma has been approached from anthropological, sociological, psychological, historical, literary, political, and religious frameworks (Argenti & Schramm, Reference Argenti and Schramm2009; Danieli, Reference Danieli1998; Doucet & Rovers, Reference Doucet and Rovers2010; Hooker & Czajkowski, Reference Hooker and Czajkowskin.d.; Moon, Reference Moon2009; Prager, Reference Prager2003; Schwab, Reference Schwab2010). The integration of these with biological analyses poses a significant and important challenge for the interdisciplinary field interested in the intergenerational effects of trauma.
Recent advances in molecular biology have facilitated investigations of the intergenerational transmission of trauma-related effects through epigenetic mechanisms. Epigenetics is the study of mechanisms that modify gene expression, thus shaping phenotypic outcome, but do not alter the underlying DNA sequence (Goldberg, Allis, & Bernstein, Reference Goldberg, Allis and Bernstein2007). Epigenetics has been described as the means by which the environment “gets under the skin,” facilitating or suppressing the expression of genes, which are themselves fixed and immutable (McEwen, Reference McEwen2012). The idea that such adaptations would be passed to the next generation was once rejected as an example of the failed Lamarckian theory of evolution. However, converging evidence, primarily in animal models but also in humans, indicates that subtle adaptations at the molecular level may not be completely erased in gametes and at conception, but may be conserved in offspring (Bale, Reference Bale2014; Yehuda & Lehrner, Reference Yehuda and Lehrner2018).
This article will review some of the challenges in studying cultural trauma (vs. individual-level trauma), as well as research on the biological sequelae of trauma and PTSD and the intergenerational transmission of biological, psychological, and behavioral effects of trauma. Putative epigenetic mechanisms for such transmission, through social, intrauterine, and gametic pathways, will be presented. Finally, the interpretations and implications of this body of research will be discussed, with an emphasis on alternative narratives of cultural specificity, growth and resilience, and continued plasticity.
Challenges in Studying Cultural Trauma
The experience of cultural trauma has profound consequences for the targeted community. These consequences may include geographical dispossession, loss of material possessions, dispersion of family and social networks, financial and educational hardship, and inadequate access to resources. The material and concrete challenges that ensue following cultural traumas, such as genocide, slavery, and forced separation of families, reshape the community and the lives of survivors and future generations. Psychological consequences, such as distressing nightmares or flashbacks, hypervigilance, profound grief and horror, and dissociative numbing, coexist with the need to come to terms with the nature of humanity, one's place in a community, and what it means to be part of a people, not just an individual entity. These challenges undoubtedly shape the lives of survivors and future generations, but it is not clear whether there are universal effects of cultural traumatic experiences. Attempting to trace the effects of cultural trauma on later generations is fraught with both political implications and methodological challenges.
Long-standing political and policy debates in many countries hinge on disagreements about whether historical traumas continue to influence structural inequalities and mental health problems in those communities. Other discussions include considerations of whether these histories of cultural trauma warrant some form of reparation (e.g., debates about reparations for slavery in the United States, for the lost generation in Australian Aboriginal communities, and for forced family separation in Native American/First Nation communities). These debates have been recapitulated in the academic literature and public policy discussions aimed at addressing putative cultural aspects of cycles of poverty (e.g., Leacock, Reference Leacock1971). For example, arguments about the intergenerational effects of trauma have been used to explain cultural practices and gaps in achievement, but many deny the continued relevance of historical experiences. This denial, in turn, is viewed as blaming the victim, and as resulting in revictimization (e.g., Ryan, Reference Ryan1976; Wright, Reference Wright1993). Scientific inquiry about psychological and biological transmission of the effects of cultural trauma cannot help but wade into these heated political and cultural debates.
Psychological and mental health consequences of cultural trauma have also been considered from both individual- and community-level perspectives (de Jong, Reference de Jong2006; Steel, Silove, Phan, & Bauman, Reference Steel, Silove, Phan and Bauman2002). However, while community- and cultural-level factors may be investigated as influences on mental health and development, conceptualizations of the mental health of a community qua community are rare. For example, there are literatures investigating cultural influences on mental health treatments disparities, or neighborhood effects (e.g., levels of violence) on individual-level mental health indicators (Brach & Fraserirector, Reference Brach and Fraserirector2000; Fowler, Tompsett, Braciszewski, Jacques-Tiura, & Baltes, Reference Fowler, Tompsett, Braciszewski, Jacques-Tiura and Baltes2009). However, when a trauma is experienced at the group level, this raises the question of whether mental health and behavioral consequences can be considered community-wide, and if so, what the implications of this community-wide effect is for the individual survivor (Chaitin & Steinberg, Reference Chaitin and Steinberg2008; de Tubert, Reference de Tubert2006). Consideration of community-level effects thus complicates research on the developmental effects of trauma.
Methodologically, there are many challenges to taking a developmental psychopathology approach to the study of cultural trauma. As a communal event, trauma is experienced by an entire community across age and developmental stage. This makes it difficult to recruit adequate samples of survivors at potentially important developmental stages, and creates a confound for studying children and adolescents in that they are subsequently being raised by adults who are themselves affected by the event, in a community shaped by the event, and so on. Furthermore, it is difficult to bound what is likely a somewhat chronic and diffused context of trauma, as those who have fled genocide subsequently face the stressors of being refugees, and are vulnerable to further traumatization. If cultural trauma influences multiple levels of the individual's ecosystem, it is difficult to parse specific influences. There is also the issue of how analysis at the cultural level might account for individual differences in the response to trauma. If the traumatic experience is chronic, such as in communities facing years of attempted genocide or war zone exposure, isolating developmental influences is difficult as survivors are aging throughout the exposure. In addition, in some cases opportunities to conduct research are only available years or decades after the trauma, rather than longitudinally following trauma. Prospective research on trauma at the individual level is notoriously difficult, as it is hard to define a cohort prior to exposure with adequate power to study traumatic sequelae longitudinally, but prospective research on communal trauma is difficult to even conceptualize. Finally, in the case of historical trauma, as described above it is difficult to disentangle the effects of legacies of trauma from the effects of compounding and ongoing trauma and stress to individuals and communities that remain under physical threat and are structurally disadvantaged, such as Native American and First Nation communities, and African American communities in the United States.
The Holocaust as a Case Study of Cultural Trauma
Research about the effects of cultural trauma cannot help but be part of a larger narrative about the meaning of the event for survivors and the community. In the case of the Holocaust, many members of the targeted community felt it was extremely important to demonstrate that the Nazis had failed in their agenda to destroy the Jewish community, and that living well was the best revenge. After all, Jews had a long history of surviving oppression, and the Holocaust provided another instance not only of their victimization but also of their resilience. Some research with survivors identified psychological resilience following the Holocaust, with robust well-being and quality of life and normal adjustment (Dimsdale, Reference Dimsdale1974; Leon, Butcher, Kleinman, Goldberg, & Almagor, Reference Leon, Butcher, Kleinman, Goldberg and Almagor1981). However, for others, the traumas they experienced left lasting psychological scars that impacted their functioning and quality of life, scars that their children felt, observed, and at times suffered from (Danieli, Reference Danieli1998). Research with survivors documented these impacts, including psychiatric symptoms, poor adjustment, and impaired functioning (Chodoff, Reference Chodoff1963; Dor-Shav, Reference Dor-Shav1978; Eitinger, Reference Eitinger1961; Niederland, Reference Niederland1981). Some hypothesized that survivors may show resilience and normal functioning under regular daily circumstances, but be more vulnerable and emotionally reactive when faced with intensely stressful or threatening situations (van IJzendoorn, Bakermans-Kranenburg, & Sagi-Schwartz, Reference van IJzendoorn, Bakermans-Kranenburg and Sagi-Schwartz2003).
Research that took a developmental approach suggested that understanding the impact of surviving the Holocaust requires consideration not only of the experience itself but also of the developmental stage at the time of the trauma and the ways in which the trauma exposure may have conferred vulnerability to later stressors. For Holocaust survivors living in the United States, as with survivors of other forms of trauma, the presence of PTSD in late life (mean age = 70 years) was associated not only with exposure to the trauma of genocide but also with cumulative lifetime trauma and more recent stress (Yehuda, Kahana, Schmeidler, & Southwick, Reference Yehuda, Kahana, Schmeidler and Southwick1995). Symptom profiles also appeared to vary depending on age of exposure (Yehuda, Schmeidler, Siever, Binder-Brynes, & Elkin, Reference Yehuda, Schmeidler, Siever, Binder-Brynes and Elkin1997). Thus, the impacts of cultural trauma must be considered in light of the developmental stage of the survivor, and may potentiate the deleterious effects of later adverse experiences.
Observations of PTSD symptoms and increased vulnerability to PTSD among Holocaust survivors raised questions of whether these survivors of cultural trauma would demonstrate the same alterations in neuroendocrine markers observed in combat veterans and civilians with PTSD. Holocaust survivors were found to have signs of heightened sensitivity of the neuroendocrine stress response, including low urinary cortisol excretion (Yehuda, Bierer, Andrew, Schmeidler, & Seckl, Reference Yehuda, Bierer, Andrew, Schmeidler and Seckl2009; Yehuda, Kahana, Binder-Brynes, & Southwick, Reference Yehuda, Kahana, Binder-Brynes and Southwick1995; Yehuda, Morris, Labinsky, Zemelman, & Schmeidler, Reference Yehuda, Morris, Labinsky, Zemelman and Schmeidler2007) and increased glucocorticoid receptor responsiveness (as assessed by the response to the low-dose dexamethasone suppression test; Yehuda, Halligan, Grossman, Golier, & Wong, Reference Yehuda, Halligan, Grossman, Golier and Wong2002), compared to demographically comparable Jewish controls. Age of exposure to the physical and emotional stressors of the Holocaust (including nutritional deprivation) has been associated with both psychological symptom profiles and cortisol metabolism, indicative of trauma's role in early developmental programming of the hypothalamic–pituitary–adrenal (HPA) axis and other stress sensitive pathways (Yehuda et al., Reference Yehuda, Bierer, Andrew, Schmeidler and Seckl2009).
Intergenerational Transmission of Trauma Effects
Whereas the intergenerational effects of cultural trauma have long been the subject of research in disciplines ranging from anthropology to political science (Argenti & Schramm, Reference Argenti and Schramm2009; Galtung, Reference Galtung1990; Martino, Reference Martino, Yovanovich and Huras1980; Mendeloff, Reference Mendeloff2009; Sullivan, Reference Sullivan1986), the psychological and psychiatric literatures turned to this issue in the wake of converging clinical observations, demands for clinical services, and social organizations of Holocaust survivor offspring (Danieli, Reference Danieli and Figley1985; Solkoff, Reference Solkoff1981; Steinitz, Reference Steinitz1982). As Holocaust survivors aged, their adult offspring began to explore the legacy of the Holocaust, not only politically and culturally, but also for their own development and psychological functioning, through literature, film, theater, music, and memoir (Aarons, Reference Aarons2016; Goldberg, Reference Goldberg2015; McGlothlin, Reference McGlothlin2006; Spiegelman, Reference Spiegelman2003). A movement of “second-generation” survivors grew, and many felt that through their parents they had also been deeply and directly affected by the cultural trauma of the Holocaust. The population of Holocaust survivors was relatively unique in that the entire religious group was targeted, regardless of sex, age, temperament, or other risk factors, and the Holocaust ended after the war and so could be chronologically bounded. Despite the dispersion of survivors, they tended to settle in Jewish communities in the United States and Israel and could thus be identified and recruited for study. Furthermore, the second generation was composed of adults who were interested in research questions about intergenerational transmission of trauma effects and of age to consent and participate in such research. A research literature followed, chasing questions of whether and how a trauma not directly experienced may have had intergenerational influences, affecting the development of second and even third generations of offspring.
Research regarding the degree to which Holocaust offspring experienced mental health problems yielded mixed results, paralleling political and cultural debates about the impact of the Holocaust on the community. Some found resilience and high levels of functioning, and others found problems such as depression, anxiety, PTSD, and personality disorders. A large meta-analysis of 32 samples (n = 4,418) indicated that sampling differences in subpopulations of offspring may explain the divergent findings (van IJzendoorn et al., Reference van IJzendoorn, Bakermans-Kranenburg and Sagi-Schwartz2003). Among nonclinical samples, there was no evidence for transmission of effects of trauma to offspring, but among clinical samples, effects of parental trauma were more pronounced.
Although evidence regarding specific mental health and functional outcomes has been mixed, the experience of feeling affected by parental trauma has been widely described. As with survivors, some research has suggested that offspring may be more vulnerable should they experience extreme stress or threat. For example, an important study of Israeli combat soldiers following the Lebanon War found that those with Holocaust survivor parents were more likely to develop PTSD (Solomon, Kotler, & Mikulincer, Reference Solomon, Kotler and Mikulincer1988). Furthermore, one epidemiological study of middle-aged Holocaust offspring found that although offspring, especially those with two survivor parents, reported higher life satisfaction, quality of life, and optimism and hope compared with controls, they also experienced more physical health problems, including high blood pressure and cholesterol, and sleeping problems (Shrira, Palgi, Ben-Ezra, & Shmotkin, Reference Shrira, Palgi, Ben-Ezra and Shmotkin2011; but see Radomislensky & Shemesh, Reference Radomislensky and Shemesh2007, for epidemiological data that did not find physical health differences in offspring). These results are consistent with biological findings of altered HPA axis reactivity among offspring of Holocaust survivors (Yehuda, Schmeidler, Wainberg, Binder-Brynes, & Duvdevani, Reference Yehuda, Schmeidler, Wainberg, Binder-Brynes and Duvdevani1998), alterations that are strongest for those with parental PTSD (Yehuda et al., Reference Yehuda, Bierer, Schmeidler, Aferiat, Breslau and Dolan2000; Yehuda, Blair, Labinsky, & Bierer, Reference Yehuda, Blair, Labinsky and Bierer2007; Yehuda, Halligan, & Bierer, Reference Yehuda, Halligan and Bierer2002; Yehuda, Teicher, et al., Reference Yehuda, Teicher, Seckl, Grossman, Morris and Bierer2007). Taken together, the research literature and cultural products created by Holocaust survivor offspring suggest that the legacy of cultural trauma extends beyond the survivors, deeply affecting their offspring. Research on the intergenerational impact of other genocides, colonization, war, and slavery reflects the widespread understanding that such traumas resonate across generations and throughout communities. For example, see discussions relating to First Nation, Native American, Australian Aboriginal, and New Zealand Maori communities (Brave Heart, Reference Brave Heart1998; Evans-Campbell, Reference Evans-Campbell2008; Gone, Reference Gone2013; Pihama et al., Reference Pihama, Reynolds, Smith, Reid, Smith and Nana2014; Raphael, Swan, & Martinek, Reference Raphael, Swan, Martinek and Danieli1998); the legacy of slavery among African Americans (DeGruy, Reference DeGruy2017; Eyerman, Reference Eyerman2001); and the effects of genocide and war in Cambodian (Field, Muong, & Sochanvimean, Reference Field, Muong and Sochanvimean2013; Münyas, Reference Münyas2008), Armenian (Azarian-Ceccato, Reference Azarian-Ceccato2010; Esmaeili, Reference Esmaeili2011; Karenian et al., Reference Karenian, Livaditis, Karenian, Zafiriadis, Bochtsou and Xenitidis2011), Rwandan (Perroud et al., Reference Perroud, Rutembesa, Paoloni-Giacobino, Mutabaruka, Mutesa, Stenz and Karege2014; Roth, Neuner, & Elbert, Reference Roth, Neuner and Elbert2014), Palestinian (Barron & Abdallah, Reference Barron and Abdallah2015), Ukrainian (Bezo & Maggi, Reference Bezo and Maggi2015), Sierra Leonean (Betancourt, McBain, Newnham, & Brennan, Reference Betancourt, McBain, Newnham and Brennan2015), and Croatian communities (Svob, Brown, Takšić, Katulić, & Žauhar, Reference Svob, Brown, Takšić, Katulić and Žauhar2016).
What Is Meant by “Transmission”?
The idea that trauma may have intergenerational effects has recently shown broad cultural resonance, with discussions of the topic in news reports, social media, and entertainment. A Google scholar search for “intergenerational transmission of trauma” yields approximately 37,000 results; “transgenerational transmission of trauma” yields over 15,000. This concept is distinct from that of the intergenerational cycle of abuse or violence, which is a widely researched (but only weakly supported) hypothesis regarding the perpetuation of child abuse and maltreatment across generations within a family (Widom, Reference Widom1989). Following reports that some offspring of traumatized parents reported experiencing their own distress and psychiatric symptoms, initial investigations into these effects focused on psychodynamic, parenting, family systems, and learning theory explanations (Abrams, Reference Abrams1999; Barocas & Barocas, Reference Barocas and Barocas1980; Danieli, Reference Danieli1998). All of these have in common the assumption that the effects of parental experiences of trauma on their children are mediated by parental symptoms and behaviors. Similar to the concept of vicarious or secondary traumatization used to describe effects of combat exposure on soldiers’ children (Rosenheck, Reference Rosenheck1986), it was thought that offspring were affected not directly by the trauma but by the effects of the trauma on the parent's emotional state and behavior, which in turn affected their own development, emotional state, and behavioral repertoire.
The introduction of possible biological mechanisms through which parental trauma might influence offspring shifted the focus of investigation from psychological and behavioral levels of analysis to neuroendocrine and molecular levels. However, biological findings have raised similar questions about the origin and nature of intergenerational effects, with significance for the interpretation of results. In the popular press, such biological findings have been reported to represent the transmission of the trauma itself across generations (for example, a 2016 magazine headline stating “Trauma From Slavery Can Actually Be Passed Down Through Your Genes: You Can Get PTSD From Your Ancestors”; Blades, Reference Blades2016). Many reports in the popular press have suggested that memories and trauma are “inherited” (Yehuda, Lehrner, & Bierer, Reference Yehuda, Lehrner and Bierer2018). These interpretations seem to represent a slippage from a psychological and cognitive level (i.e., the perception and experience of life threat or the memory of a lived event) to a biological level that treats these as equivalent. Transmission of an effect of trauma at the molecular level is not the same as transmission of an experience of trauma, whatever that would mean. As biological research on intergenerational effects of trauma expands, it becomes increasingly important to be clear about what is being transmitted, and how such transmission might occur.
The Biological Impact of Trauma Survival and PTSD
Research on the biological consequences of trauma exposure emerged from the field of stress studies, which had identified and characterized the activation of the HPA axis as central to the acute stress response (Smith & Vale, Reference Smith and Vale2006). Briefly, the perception of threat triggers a neuroendocrine cascade that is ultimately resolved by the release of glucocorticoids, which returns the organism to homeostasis once the threat has been removed and safety reestablished (De Kloet, Joëls, & Holsboer, Reference De Kloet, Joëls and Holsboer2005). The hypothalamus releases corticotropin-releasing hormone, which stimulates the pituitary to release adrenocorticotropic hormone. Adrenocorticotropic hormone travels to the adrenal cortex, activating the adrenal glands to produce cortisol (a glucocorticoid). As cortisol is released into the system, it acts through negative feedback on the pituitary and hypothalamus to shut down the cycle. The HPA axis is also regulated through neuronal, brain, and sympathetic and parasympathetic systems, leading to a highly adaptive stress response system. Glucocorticoids have wide-ranging effects through the brain and body, influencing memory, inflammatory and immune function, and other disease processes. The experience of chronic stress, and chronic activation of the stress-response system, has been associated with increased allostatic load and increased rates of morbidity and mortality (Chrousos, Reference Chrousos2009; Juster, McEwen, & Lupien, Reference Juster, McEwen and Lupien2010; McEwen, Reference McEwen1998; McEwen & Wingfield, Reference McEwen and Wingfield2003).
The nature of the stress-response system, and its facilitation of emotional, cognitive, and behavioral responses to promote survival (e.g., fight, flight, or freeze), is one of rapid and flexible response to acute and time-limited threat, followed by a return to baseline once the threat is over. However, some trauma survivors continue to act and feel as if the trauma has not resolved, as if they have been fundamentally altered by an experience that continues to be ever present instead of receding into the past. Consistent with this clinical presentation, initial research on the effects of trauma anticipated signs of an activated HPA axis, as if the survivor was in a state of chronic stress response. However, the unexpected finding was that trauma survivors with PTSD appeared to have lower, rather than higher, levels of cortisol (Glover & Poland, Reference Glover and Poland2002; Mason, Giller, Kosten, Ostroff, & Podd, Reference Mason, Giller, Kosten, Ostroff and Podd1986; Yehuda, Kahana, Binder-Brynes, et al., Reference Yehuda, Kahana, Binder-Brynes and Southwick1995; Yehuda et al., Reference Yehuda, Southwick, Nussbaum, Wahby, Giller and Mason1990; Yehuda, Teicher, Trestman, Levengood, & Siever, Reference Yehuda, Teicher, Trestman, Levengood and Siever1996), initially indicating, possibly, an underperforming rather than hyperactive HPA axis. Research with Holocaust survivors, combat veterans, and civilians exposed to trauma has since consistently identified HPA axis alterations in association with PTSD (De Kloet et al., Reference De Kloet, Vermetten, Geuze, Kavelaars, Heijnen and Westenberg2006; Morris, Compas, & Garber, Reference Morris, Compas and Garber2012). Furthermore, these individuals also demonstrate high glucocorticoid receptor number and sensitivity (Yehuda, Boisoneau, Mason, & Giller, Reference Yehuda, Boisoneau, Mason and Giller1993; Yehuda, Golier, Yang, & Tischler, Reference Yehuda, Golier, Yang and Tischler2004; Yehuda, Halligan, Grossman, et al., Reference Yehuda, Halligan, Grossman, Golier and Wong2002; Yehuda, Lowy, Southwick, Shaffer, & Giller, Reference Yehuda, Lowy, Southwick, Shaffer and Giller1991; Yehuda, Southwick, et al., Reference Yehuda, Southwick, Krystal, Bremner, Charney and Mason1993), and low glucocorticoid receptor gene (NR3C1) methylation (Yehuda et al., Reference Yehuda, Flory, Bierer, Henn-Haase, Lehrner, Desarnaud and Meaney2015), contributing to an enhanced negative feedback sensitivity in the HPA axis. Elevated catecholamine levels (norepinephrine and epinephrine; Kosten, Mason, Giller, Ostroff, & Harkness, Reference Kosten, Mason, Giller, Ostroff and Harkness1987; Liberzon, Abelson, Flagel, Raz, & Young, Reference Liberzon, Abelson, Flagel, Raz and Young1999; Yehuda, Siever, et al., Reference Yehuda, Siever, Teicher, Levengood, Gerber, Schmeidler and Yang1998; Yehuda, Southwick, Giller, Ma, & Mason, Reference Yehuda, Southwick, Giller, Ma and Mason1992; Young & Breslau, Reference Young and Breslau2004) in conjunction with suppressed cortisol levels have been hypothesized to result in a prolonged stress response state, with inadequate levels of cortisol failing to suppress the HPA axis (Mason, Giller, Kosten, & Harkness, Reference Mason, Giller, Kosten and Harkness1988). The organism is thus exposed to catecholamines for an extended period, with potential consequences for conditioned learning, avoidance behavior, memory, and hyperarousal (Yehuda, Reference Yehuda2009).
These initially unexpected biological observations are consistent with epidemiological data that has consistently showed that while the majority of adults worldwide have experienced at least one traumatic incident, only a minority subsequently develop PTSD (Breslau, Reference Breslau2009; Galea, Nandi, & Vlahov, Reference Galea, Nandi and Vlahov2005; Kessler et al., Reference Kessler, Berglund, Demler, Jin, Merikangas and Walters2005; Kessler, Sonnega, Bromet, Hughes, & Nelson, Reference Kessler, Sonnega, Bromet, Hughes and Nelson1995; Sorel, Reference Sorel2010). Taken together, the biological and epidemiological data support the hypothesis that PTSD is not the predominant reaction to trauma; rather, most people recover from the experience (Yehuda & McFarlane, Reference Yehuda and McFarlane1995). However, the natural recovery process fails for a minority of survivors, who become stuck in a posttraumatic state that includes alterations in HPA axis functioning (Yehuda, McFarlane, & Shalev, Reference Yehuda, McFarlane and Shalev1998). This is not to say that all survivors are not deeply affected by their experiences, but that only a subset develop a lasting, maladaptive disorder following such an experience.
Biological Findings in Offspring of Trauma Survivors
Given these observations in PTSD, and the fact that the HPA axis is sensitive to developmental programming (Weaver, Reference Weaver2007), initial biological investigations of offspring focused on HPA axis parameters. A body of research has grown showing that offspring of trauma survivors and combat veterans, even offspring without PTSD, have HPA axis alterations similar to those observed in samples with PTSD, such as lower cortisol levels and higher glucocorticoid receptor sensitivity (Lehrner et al., Reference Lehrner, Bierer, Passarelli, Pratchett, Flory, Bader and Makotkine2014; Yahyavi, Zarghami, Naghshvar, & Danesh, Reference Yahyavi, Zarghami, Naghshvar and Danesh2015; Yehuda et al., Reference Yehuda, Bierer, Schmeidler, Aferiat, Breslau and Dolan2000; Yehuda, Blair, et al., Reference Yehuda, Blair, Labinsky and Bierer2007; Yehuda, Teicher, et al., Reference Yehuda, Blair, Labinsky and Bierer2007). Although it is methodologically more challenging to establish parental mental health status than simply exposure to the Holocaust, in studies that have been able to evaluate parental symptoms, biological findings in offspring have been specifically associated with parental PTSD (Lehrner et al., Reference Lehrner, Bierer, Passarelli, Pratchett, Flory, Bader and Makotkine2014; Yehuda et al., Reference Yehuda, Daskalakis, Lehrner, Desarnaud, Bader, Makotkine and Meaney2014; Yehuda, Schmeidler, et al.,Reference Yehuda, Schmeidler, Wainberg, Binder-Brynes and Duvdevani1998; Yehuda, Teicher, et al., Reference Yehuda, Blair, Labinsky and Bierer2007). In addition, there have been different patterns observed in association with maternal versus paternal PTSD. The neuroendocrine patterns in offspring described above appear to be associated with maternal PTSD (Lehrner et al., Reference Lehrner, Bierer, Passarelli, Pratchett, Flory, Bader and Makotkine2014; Yehuda et al., Reference Yehuda, Daskalakis, Lehrner, Desarnaud, Bader, Makotkine and Meaney2014; Yehuda, Teicher, et al., Reference Yehuda, Blair, Labinsky and Bierer2007).
Potential Epigenetic Mechanisms for Transmission of the Effects of Trauma
With observations of both psychological and biological differences in offspring whose parents had PTSD compared with controls, the question of potential mechanism(s) for transmission of these effects takes on new relevance. As the field has seen rapid advances in methods for studying molecular processes, there has been heightened interest and research on potential epigenetic mechanisms for intergenerational transmission of trauma effects. Epigenetic marks consist of chemical modifications of chromatin (chromosomal material, including protein, RNA, and DNA) that are induced by environmental factors. These alterations usually affect transcriptional activity, leading some to describe epigenetics as the means by which the environment “turns genes on and off.” Epigenetic mechanisms include DNA methylation, usually on cytosine residues of CpG islands, modifications of histone proteins, and noncoding RNA, with functional interactions across these pathways (Goldberg et al., Reference Goldberg, Allis and Bernstein2007). DNA methylation suppresses gene transcription and thus silences the gene. These marks are considered generally stable, but there are “erasers,” such as DNA and histone demethylases, as well, and some modifications are quite dynamic (Daxinger & Whitelaw, Reference Daxinger and Whitelaw2012). The field of epigenetics has generated great interest by offering a mechanism through which the environment shapes us, representing a rejection of genetic determinism.
The concept of epigenetic transmission or inheritance warrants clarification (see Figure 1). Parental, or intergenerational, effects are those induced by the offspring's direct exposure to the trauma, such as in utero exposure, which may affect both the embryo or fetus and its developing germline (Heard & Martienssen, Reference Heard and Martienssen2014). Transgenerational effects are those observed in generations not directly exposed to the triggering environment. For this reason, effects are only considered transgenerational if observed in F3 females (F0 is the exposed mother), because the F1 female fetus and her germ cells would be exposed to the trauma or effects of the trauma in utero. F3 would therefore be the first generation not directly exposed to the trauma. In males, effects observed in F2 would be considered transgenerational, because spermatogenesis occurs during adolescence rather than in the fetus, and thus F1 germ cells would not be directly exposed to the trauma in utero (Dias & Ressler, Reference Dias and Ressler2014; Heard & Martienssen, Reference Heard and Martienssen2014).
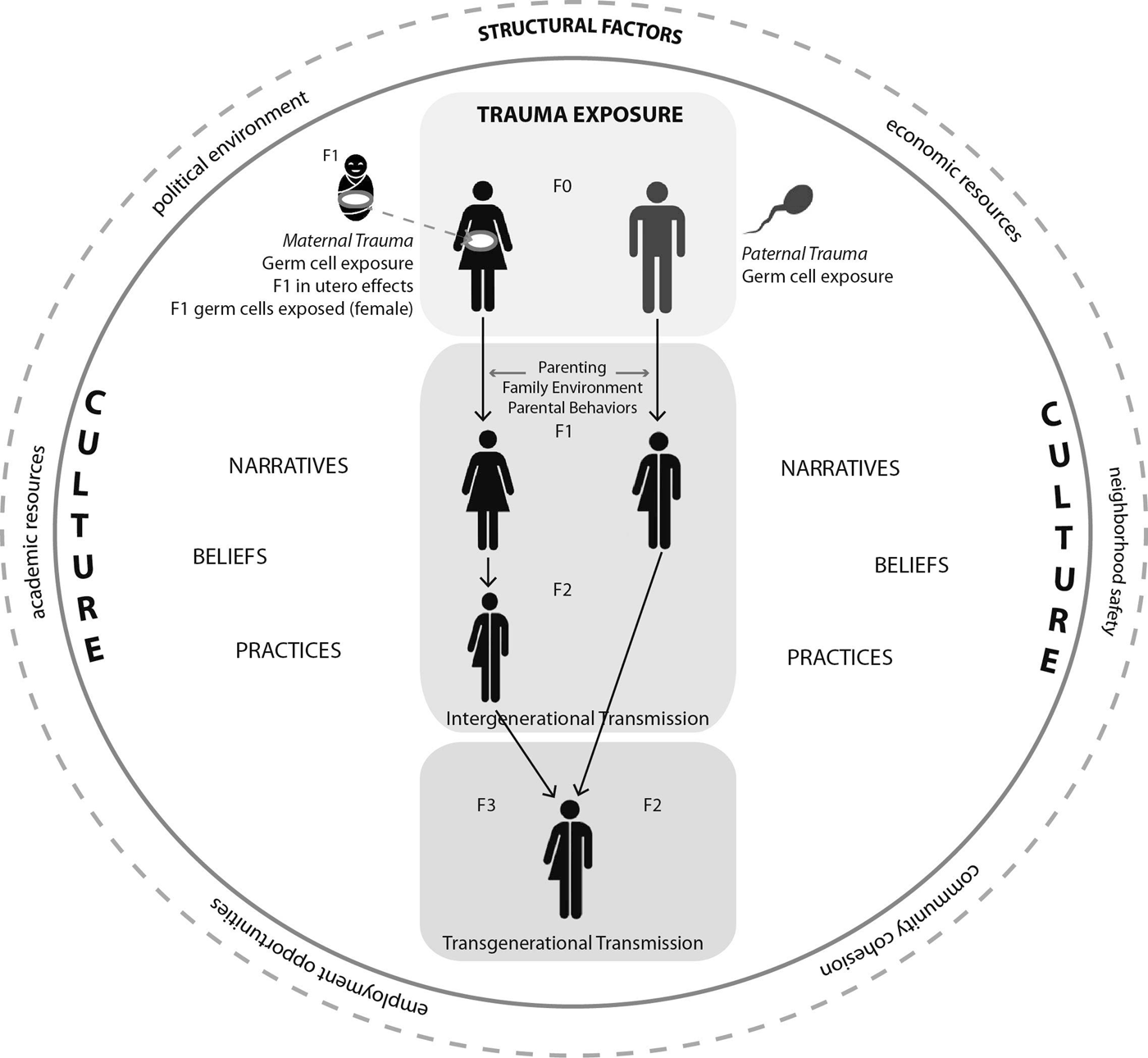
Figure 1. Intergenerational transmission of biological effects of trauma. There are multiple potential pathways for the biological transmission of trauma effects across generations. Preconception trauma exposure (F0) may affect the epigenetic status of maternal oocytes or paternal sperm; this may be developmentally dependent, particularly in females. These effects may be conserved and manifest during embryogenesis, resulting in inheritance of those effects by offspring. In the case of mothers exposed to trauma during pregnancy, the fetus (F1) and its developing germline may be affected in utero. In the case of an exposure during pregnancy, the future offspring of the fetus (F2) will have been directly exposed to the grandmaternal (F0) stress, and as a result only the third generation (F3) can be considered to evidence transgenerational transmission that was not an effect of direct exposure. In the case of paternal exposure, F2 can be considered to reflect a true transgenerational effect. Effects of parental trauma exposure may also be transmitted through parenting, family environment, and parental behaviors. The experience and transmission of trauma effects are embedded within a larger cultural context that includes narratives, beliefs, and practices. The effects of trauma are also felt and transmitted within a sociostructural context that includes access to resources, relative safety of the neighborhood, and the larger political environment.
Epigenetic mechanisms have been associated with parenting and social influences, in utero transmission, and transmission through gametes (sperm or oocyte; for review, see Yehuda & Lehrner, Reference Yehuda and Lehrner2018). In other words, social interactions such as parenting may shape the epigenome (the sum of all epigenetic information attached to the genome) of the offspring, a form of “transmission” in which the social context, rather than parental biology, transfers information that is biologically encoded in offspring. Myriad forms of social information, including parent-offspring interactions, social learning, and symbolic cultural communication, can lead to the transmission of epigenetic variations (Jablonka & Raz, Reference Jablonka and Raz2009). The intrauterine environment can also affect the offspring epigenome, through fetoplacental interactions that are influenced by maternal stress, physical condition, and mental state. In this case, the offspring is directly exposed during gestation to an aspect of the maternal experience, mediated by the placenta. These are all forms whereby parental experiences may influence offspring biology through epigenetic mechanisms, and represent intergenerational epigenetic effects.
Inheritance of epigenetic marks from the parent to the offspring, representing inter- or transgenerational epigenetic inheritance, requires transmission through the gametes. In multicellular organisms this occurs when epigenetic marks are conserved through meiosis (cell division creating gametes), gametogenesis, and early embryogenesis, which involve significant demethylation and restructuring of chromatin (Heard & Martienssen, Reference Heard and Martienssen2014; Jablonka & Raz, Reference Jablonka and Raz2009). Despite these processes, methylation marks are not completely erased, and remethylation processes also occur. Furthermore, some RNAs may also be transmitted through the germline (Jablonka & Raz, Reference Jablonka and Raz2009).
Transmission of Trauma Effects Through Parental Care
In seminal work with rats, Meaney and colleagues (Meaney, Aitken, Bodnoff, Iny, & Sapolsky, Reference Meaney, Aitken, Bodnoff, Iny and Sapolsky1985; Meaney, Aitken, Bodnoff, Iny, Tatarewicz, et al., Reference Meaney, Aitken, Bodnoff, Iny, Tatarewicz and Sapolsky1985; Meaney, Aitken, van Berkel, Bhatnagar, & Sapolsky, Reference Meaney, Aitken, van Berkel, Bhatnagar and Sapolsky1988) demonstrated that variations in maternal care, operationalized as licking and grooming of pups, altered DNA methylation in the glucocorticoid receptor gene (nr3c1) in the hippocampus of the offspring (Weaver et al., Reference Weaver, Cervoni, Champagne, D'Alessio, Sharma, Seckl and Meaney2004). As adults, the rats showed alterations in HPA axis functioning, including altered basal and stress induced corticosterone levels, higher glucocorticoid receptor sensitivity, and more hippocampal glucocorticoid receptors. This work demonstrated that maternal behavior can affect offspring at the level of DNA chemistry in the brain, and that methylation at identified regions in the glucocorticoid receptor gene promoter shapes the development of the glucocorticoid-mediated stress response system.
These findings were translated to humans in a study of the hippocampal glucocorticoid receptor in suicide victims, which found higher methylation at the glucocorticoid receptor 1F promoter in postmortem tissue in victims with a history of childhood abuse (McGowan et al., Reference McGowan, Sasaki, D'alessio, Dymov, Labonté, Szyf and Meaney2009). Glucocorticoid receptor methylation in white blood cells of healthy children and adults has also been associated with childhood adversity and negative parenting styles (Tyrka et al., Reference Tyrka, Parade, Eslinger, Marsit, Lesseur, Armstrong and Seifer2015, Reference Tyrka, Parade, Welch, Ridout, Price, Marsit and Carpenter2016; Tyrka, Price, Marsit, Walters, & Carpenter, Reference Tyrka, Price, Marsit, Walters and Carpenter2012). In adult offspring of Holocaust survivors, maternal and paternal PTSD had interacting effects on glucocorticoid receptor methylation in blood, such that offspring with maternal PTSD had lower methylation of the exon 1F promoter region, but those with only paternal PTSD showed higher levels (Yehuda et al., Reference Yehuda, Daskalakis, Lehrner, Desarnaud, Bader, Makotkine and Meaney2014). There have been no studies to date of epigenetic effects of cultural trauma at the community level, as contextual factors have been measured as mediated through parental behavior and biology.
Prenatal In Utero Trauma Effects on Offspring
The differential effects of parental sex suggest the possibility that the intrauterine environment or the gametes contribute to transmission of trauma effects. The HPA axis is developed by 22 weeks of gestation, and is sensitive to developmental programming throughout gestation. Fetoplacental interactions may buffer, or expose, the developing fetus to maternal glucocorticoids related to stress, physical adversity, or psychiatric symptoms, particularly through placental 11B-hydroxysteroid dehydrogenase type 2 (11β-HSD2), an enzyme that converts cortisol to inactive cortisone (Nugent & Bale, Reference Nugent and Bale2015). Influences during gestation may include maternal state, environmental exposures, developmental stage of the fetus, and even the sex of the fetus (Carpenter, Grecian, & Reynolds, Reference Carpenter, Grecian and Reynolds2015; Oberlander et al., Reference Oberlander, Weinberg, Papsdorf, Grunau, Misri and Devlin2008). For example, a prospective study of infants born to pregnant female survivors of the World Trade Center attacks found low cortisol levels in association with maternal PTSD, an effect that was strongest for those exposed in the third trimester (Yehuda et al., Reference Yehuda, Engel, Brand, Seckl, Marcus and Berkowitz2005). Offspring of mothers with PTSD also showed signs of anxiety and disrupted behavior, as well as being rated by their mothers as having greater distress to novelty. There is also a growing body of work with rodent models documenting the intergenerational effects of prenatal environmental exposures to biological toxins and maternal stress (Gapp, von Ziegler, Tweedie-Cullen, & Mansuy, Reference Gapp, von Ziegler, Tweedie-Cullen and Mansuy2014). Taken together, this literature provides evidence that in utero exposure to maternal trauma may affect the developing HPA axis of the fetus.
The developmental origins of health and disease field investigates fetal origins of myriad adult disorders, based on findings of the influence of prenatal and even preconception factors on health outcomes, including psychiatric and medical conditions (Gillman, Reference Gillman2005). Epidemiological studies of intergenerational effects of two large-scale famines have documented health and disease outcomes in offspring over multiple generations. Supporting the hypothesis that there are developmentally sensitive windows during gestation, both phenotypic outcomes and epigenetic marks were observed in adults exposed to famine at conception and during the first half of gestation, but not in those exposed in the third trimester or postnatal period (Heijmans et al., Reference Heijmans, Tobi, Stein, Putter, Blauw, Susser and Lumey2008; Ravelli, Stein, & Susser, Reference Ravelli, Stein and Susser1976). DNA methylation mediated the association of prenatal famine exposure and adult metabolic health, with additional CpG sites identified among those exposed early in gestation (Tobi et al., Reference Tobi, Slieker, Luijk, Dekkers, Stein, Xu and Heijmans2018). Exposure to famine during pregnancy was associated with neonatal adiposity and poorer health in offspring of the exposed female fetus, providing the first human evidence that starvation during pregnancy can affect health outcomes in grandchildren (Painter et al., Reference Painter, Osmond, Gluckman, Hanson, Phillips and Roseboom2008). This literature supports hypotheses that aversive environmental stimuli or deprivation during pregnancy may have effects on health outcomes over two generations, and that these effects may be partly mediated by epigenetic alterations in the offspring.
Regarding trauma exposure specifically, there is evidence that prenatal exposure to violence has effects on offspring methylation, although these studies are not powered to analyze gestational age of exposure. Higher methylation of the glucocorticoid receptor gene has been observed in adolescent offspring of mothers who experienced prenatal intimate partner violence (Radtke et al., Reference Radtke, Ruf, Gunter, Dohrmann, Schauer, Meyer and Elbert2011), newborns of women exposed to severe prenatal stress, particularly war zone stressors, in the Democratic Republic of Congo (Mulligan, D'Errico, Stees, & Hughes, Reference Mulligan, D'Errico, Stees and Hughes2012), and young adult offspring of Tutsi widows exposed to the Rwandan genocide during the second and third trimesters of pregnancy (Perroud et al., Reference Perroud, Rutembesa, Paoloni-Giacobino, Mutabaruka, Mutesa, Stenz and Karege2014). These observations are directionally different than those observed in older Holocaust offspring, raising questions of whether preconception exposure, age of offspring, prior maternal trauma exposure, or concurrent psychiatric symptoms influence the degree and directionality of DNA methylation. It is unclear whether and to what degree the influence of prenatal stress and trauma exposure on fetal development is mediated through maternal psychiatric symptoms or subjective distress, which may affect intrauterine signals during gestation. Furthermore, HPA axis sensitization may lead to changes in functioning over time as the system attempts to adapt or becomes exhausted. For example, among individuals with PTSD, higher basal cortisol has been observed proximally to trauma exposure, but lower HPA axis activity has been observed related to more distal trauma exposure (Morris et al., Reference Morris, Compas and Garber2012).
Transmission of the Effects of Trauma Through Gametes
The foregoing describes the transmission of effects of trauma as mediated by social processes (e.g., parental behavior) or in utero influences. Trauma that occurred prior to conception may also affect offspring through alterations in parental germ cells that are conserved during fertilization and embryogenesis. As noted above, initial understanding of DNA methylation, based on animal research, was that epigenetic marks were erased during comprehensive reprogramming in gametes and in the early embryo (Daxinger & Whitelaw, Reference Daxinger and Whitelaw2012). However, since the late 1980s evidence has accrued that parental epigenetic states can be transmitted through the gametes and maintained across more than one generation (Daxinger & Whitelaw, Reference Daxinger and Whitelaw2012).
There are no studies to date of trauma-induced epigenetic marks in oocytes, which although present since birth are sensitive to environmental influences, particularly during childhood (Cortessis et al., Reference Cortessis, Thomas, Levine, Breton, Mack, Siegmund and Laird2012; Faulk & Dolinoy, Reference Faulk and Dolinoy2011). The age of maternal Holocaust exposure was associated with cortisol levels and metabolism in offspring, independently of maternal PTSD, with the strongest effect for women who were children during the war, suggesting a possible gametic effect (Bader et al., Reference Bader, Bierer, Lehrner, Makotkine, Daskalakis and Yehuda2014). There is data from animal models showing that methylation status in dams can affect offspring phenotype, suggesting that epigenetic marks may survive oogenesis and affect the epigenetic state of offspring (Jaenisch & Bird, Reference Jaenisch and Bird2003).
There is a growing literature on transmission of environmental effects through sperm, which is more easily isolated from confounding influences than oocytes. Spermatogenesis begins at puberty and continues throughout the life span (Desai, Ludgin, Sharma, Anirudh, & Agarwal, Reference Desai, Ludgin, Sharma, Anirudh, Agarwal, Falcone and Hurd2017). There are no known investigations of transgenerational effects of trauma through human sperm, but there are observational studies linking environmental exposures in men, including diet and famine, smoking, alcohol consumption, toxins, and stress, with both biological and behavioral outcomes in offspring, in some cases with demonstrated epigenetic marks in paternal sperm (Anway, Cupp, Uzumcu, & Skinner, Reference Anway, Cupp, Uzumcu and Skinner2005; Friedler, Reference Friedler1996; Schagdarsurengin & Steger, Reference Schagdarsurengin and Steger2016; Yehuda & Lehrner, Reference Yehuda and Lehrner2018). For example, studies have shown that adult offspring of fathers exposed to famine had higher body mass index and obesity rates (Veenendaal et al., Reference Veenendaal, Painter, Rooij, Bossuyt, Post, Gluckman and Roseboom2013). Observations of paternal transmission in humans have been extended to three generations. Parental and grandparental diet and food availability prior to puberty has been linked with different sex-specific risk and protective factors in children and grandchildren (Kaati, Bygren, Pembrey, & Sjöström, Reference Kaati, Bygren, Pembrey and Sjöström2007). Smoking, alcohol dependence, and opioid dependence have all been linked with changes in DNA methylation and chromatin in sperm (Chorbov, Todorov, Lynskey, & Cicero, Reference Chorbov, Todorov, Lynskey and Cicero2011; Hamad, Shelko, Kartarius, Montenarh, & Hammadeh, Reference Hamad, Shelko, Kartarius, Montenarh and Hammadeh2014; Ouko et al., Reference Ouko, Shantikumar, Knezovich, Haycock, Schnugh and Ramsay2009). In at least one study, paternal smoking effects on offspring were limited to cases of prepubertal smoking in fathers (Pembrey et al., Reference Pembrey, Bygren, Kaati, Edvinsson, Northstone, Sjöström and Golding2006).
This body of epidemiological and associational research provides support for the hypothesis that trauma effects could be transmitted through gametes in humans. However, the majority of this work has been conducted in animals for methodological reasons, such as the ability to control other environmental stimuli, including in utero or early developmental effects. A body of experimental research with rodents has documented effects of paternal stress or environmental insults, using a variety of paradigms across developmental stages, on offspring behavior, neurology, and sperm (Bale, Reference Bale2014; Franklin et al., Reference Franklin, Russig, Weiss, Gräff, Linder, Michalon and Mansuy2010; Yehuda & Lehrner, Reference Yehuda and Lehrner2018), although not all stressors have been shown to affect offspring through sperm (Dietz et al., Reference Dietz, LaPlant, Watts, Hodes, Russo, Feng and Nestler2011). Epigenetic mechanisms may include DNA methylation, oxidative damage to sperm DNA, histone modifications, and changes in small noncoding RNAs, including microRNA (Heard & Martienssen, Reference Heard and Martienssen2014; Yehuda & Lehrner, Reference Yehuda and Lehrner2018). Overall, this body of research suggests that stress to males across the life span can lead to phenotypic and biological changes in offspring mediated by epigenetic changes in sperm (Rodgers, Morgan, Bronson, Revello, & Bale, Reference Rodgers, Morgan, Bronson, Revello and Bale2013).
Permanence Versus Plasticity of the Epigenome
Because epigenetic marks are relatively stable (vs. transient hormonal responses to stress, for example), trauma-induced changes and their transmission have often been considered permanent. However, given that epigenetic processes represent a fundamental biological mechanism of plasticity and adaptability, this assumption bears examination. Over generations, such changes may lead to more extreme phenotypes or more reliable transmission if the environmental contexts endure, or conversely, may fade away over generations if the contexts change (Jablonka & Raz, Reference Jablonka and Raz2009). These questions are more easily pursued with animals, as the shorter life span facilitates study of transgenerational effects, and breeding and environmental influences can be controlled.
A new line of research has emerged investigating possibilities for preventing the transmission of trauma-induced epigenetic effects. Gapp et al. (Reference Gapp, Bohacek, Grossmann, Brunner, Manuella, Nanni and Mansuy2016) found that environmental enrichment (including sensory, motor, and cognitive stimuli) after weaning in male mice prevented behavioral and biological effects associated with early stress in male offspring. Specifically, hippocampal DNA methylation and glucocorticoid receptor gene expression levels associated with paternal stress were reversed in the adult offspring. Stressed males exposed to an enriched environment and their offspring performed similarly to controls in response to stressful challenges in adulthood, whereas without enrichment fathers and offspring showed behavioral effects of early paternal stress. Furthermore, offspring of fathers exposed to enrichment after early stress failed to show alterations in glucocorticoid receptor expression and DNA methylation associated with early stress exposure, indicating a normalization of gene expression following enrichment. The researchers analyzed sperm to isolate the mechanism of this transmission, and found that DNA methylation was altered in sperm cells of stressed males, but that these effects were no longer found in sperm of fathers raised in the enriched environment. It is of interest to note, however, that the behavioral alterations of offspring associated with paternal early life stress conferred resilience to aversive conditions and more active coping in responding to aversive stimuli (Gapp et al., Reference Gapp, Bohacek, Grossmann, Brunner, Manuella, Nanni and Mansuy2016).
Implications of Potential Epigenetic Transmission of Trauma Effects
As research on intergenerational inheritance of environmentally induced epigenetic marks grows, a landscape of post-Lamarckian, non-Mendelian heritability unfolds to great, if sometimes overstated, excitement (Goldberg et al., Reference Goldberg, Allis and Bernstein2007; Lim & Brunet, Reference Lim and Brunet2013; Miller, Reference Miller2010; Yehuda, Lehrner, & Bierer, Reference Yehuda, Lehrner and Bierer2018). There has been much excitement about the possibility that what happens to individuals, societies, and cultures, can have long-lasting effects on the way genes function. However, the study of epigenetic influences of environmental stimuli, especially in early life, has been primarily conducted in animals. It remains to be seen if these observations will be reliably observed in humans. If so, it will be important to determine whether epigenetic marks in blood cells have functional significance. Furthermore, epigenetic research on behavioral or psychiatric indicators in humans has relied almost exclusively on peripheral samples such as blood rather than brain tissue. Because methylation patterns are tissue specific, it is not clear whether and to what extent methylation patterns observed in peripheral tissue reflect activity in the brain or other tissue of interest. While there is some support from rats that there may be overlapping patterns of gene expression related to stress across both blood and brain, with convergent glucocorticoid signaling pathways, this has not yet been examined with respect to methylation patterns (Daskalakis, Cohen, Cai, Buxbaum, & Yehuda, Reference Daskalakis, Cohen, Cai, Buxbaum and Yehuda2014). Finally, the role of genetic factors in epigenetic changes associated with trauma requires further investigation.
When the environmental context is traumatic (when it involves genocide, violence, and oppression), it may be natural to assume that any adaptations to such contexts must be harmful, as it is difficult to conceptualize benefits from such traumas. Interpretations of biological transmission tend to fall under the (usually unspecified) cumulative stress model, in which the impact of early life adversity and maternal stress is assumed to confer vulnerability to later insults. There is a strong body of literature in animals documenting such effects in response to maternal stressors (Daskalakis, Oitzl, Schächinger, Champagne, & de Kloet, Reference Daskalakis, Oitzl, Schächinger, Champagne and de Kloet2012). However, from a biological perspective, changes in response to environmental stimuli are not inherently “good” or “bad.” It may make evolutionary sense for parents to pass on adaptations to threat to their offspring; whether these adaptations serve offspring depends on the environmental contexts the organism encounters across its life span.
Epigenetic mechanisms facilitate adaptive response and flexibility to environmental perturbations with possible evolutionary significance (Jablonka & Raz, Reference Jablonka and Raz2009). Heritable epigenetic changes in response to traumatic environments would be evolutionarily advantageous, preparing offspring for events that are unpredictable but intense and potentially recurring, especially when the offspring is born into more favorable conditions (Harper, Reference Harper2005) These epigenetic accommodations to external stimuli are relatively stable, rather than transient, but they are not permanent and are sensitive to subsequent remodeling. They are part of a dynamic system that continues to evolve, maximizing the organism's ability to thrive. It is teleological to argue that the existence of such mechanisms necessarily indicates adaptivity, but the intergenerational conservation of information relevant to environmental contexts is certainly likely to generate a wider range of phenotypes, thus expanding the community's ability to adapt and respond to changing environments. The intergenerational transmission of effects of trauma is not synonymous with permanent damage in offspring; it just implies change as a result of environmental exposure.
It has been argued that inheritance of trauma effects may be detrimental in the case of an environmental mismatch, where the offspring is primed for threat but the sociocultural context has changed (Santarelli et al., Reference Santarelli, Lesuis, Wang, Wagner, Hartmann, Labermaier and Schmidt2014; Schmidt, Reference Schmidt2011). This is consistent with epidemiological research indicating that offspring conceived during famine but raised in contexts of food abundance demonstrate poorer health outcomes. There is also the possibility that inherited effects remain dormant in the absence of a relevant environmental trigger. In this case, offspring may not show phenotypic similarity with the parent (such as psychiatric or behavioral indicators), but may have increased vulnerability or reactivity when confronted with relevant stimuli. Jablonka and Raz (Reference Jablonka and Raz2009) have termed this “epigenetic recall,” similar to a memory trace that requires reinduction for memory reconstruction. This possibility is consistent with research on Holocaust offspring showing healthy functioning in general, but poor responses to severe stressors (van IJzendoorn et al., Reference van IJzendoorn, Bakermans-Kranenburg and Sagi-Schwartz2003).
Support for the hypothesis that the potential adaptiveness or harmfulness of early trauma depends on subsequent environmental demands comes from the animal work that initially identified effects of maternal care on offspring DNA methylation. As adults, the offspring of low licking and grooming mothers showed deficits in neural plasticity and memory under baseline conditions (Champagne et al., Reference Champagne, Bagot, van Hasselt, Ramakers, Meaney, De Kloet and Krugers2008). However, under stressful or high corticosterone (the animal homologue of cortisol) conditions, the animals displayed enhanced plasticity and memory, whereas animals who received high licking and grooming demonstrated the opposite results. A match/mismatch hypothesis has been proposed to explain how early life experiences, and by extension, inheritance of biological alterations associated with parental experience, may confer either vulnerability or enhanced coping and plasticity depending on later life contexts (Daskalakis et al., Reference Daskalakis, Oitzl, Schächinger, Champagne and de Kloet2012).
Conclusions and Future Directions
There is a general consensus that parental and communal trauma affect development, but how this happens and what it means remain open questions. The emergence of epigenetics as a potential biological mechanism for transmission of parental experiences has thrust these questions to the forefront of many academic and popular culture forums. The effects of parental trauma may influence male and female gametes prior to conception, fetal development in utero, and postnatal parenting and family environment, all of which may shape offspring biology and phenotype. It is strange, however, that the possibility of conservation and transmission of learning related to trauma from parent to offspring is most commonly assumed to convey vulnerability and damage. Transmission of cultural memory through rituals, symbols, and practices serves to transmit learning and meaning, to allow future generations to understand the world and to respond adaptively. Few argue that such memory is damaging, poses a threat to healthy development, or requires preventive interventions (although see Linden & Rutkowski, Reference Linden and Rutkowski2013, for an argument in favor of “beneficial forgetting”). In many traditions, transmission of memory of important cultural events aims to convey a sense of identity, to forewarn and forearm future generations against threat, and to celebrate resilience and perseverance. It is interesting that the possibility that such memory might also be conserved at a molecular level, shaping expression of the genome, frequently becomes mired in a narrative of damage.
The cultural narrative about what is being transmitted and what it means profoundly shapes the nature of the experience of offspring of trauma survivors (Mohatt, Thompson, Thai, & Tebes, Reference Mohatt, Thompson, Thai and Tebes2014). Western conceptions of mental health and wellness, and definitions of “normalcy,” are culture bound, and reflect expectations of safety, trust, optimism, and happiness, among other things. To be affected by experiences that undermine these expectations can be interpreted as “maladaptive” or reflecting mental illness, and the sociocultural conditions that gave rise to such experiences and that may continue to shape experiences of later generations can be ignored. Yet these assumptions are widely unexamined in relation to discussions of the effect of cultural trauma on development and future generations.
Gone and Kirmayer (Reference Gone, Kirmayer, Millon, Krueger and Simonsen2010) provide an instructive example from O'Nell's (Reference O'Nell1996) anthropological work among the Salish Indians of Montana regarding the meaning ascribed to feeling the intergenerational effects of centuries of conquest, racism, and oppression. Although members of this community described aspects of depression such as feeling bereaved, aggrieved, and worthless, only feeling worthless was associated with suicide, whereas feeling bereaved was understood as appropriate given the losses of the community over the previous century. Feeling bereaved was respected as a sign of maturity among elderly members, who were seen to grieve appropriately for the many losses experienced by the community (Gone & Kirmayer, Reference Gone, Kirmayer, Millon, Krueger and Simonsen2010). In this case, whereas one interpretation might be that intergenerational transmission puts members of this community at risk for depression, the lived narrative is one that values the experience of those who continue to bear a mantle of grief over the community's history of trauma.
The concept of being strengthened by adversity, and even trauma, has deep roots in Western paradigms as well. The idea that good can come from suffering is embedded in Judeo-Christian theology. More recently, the field of psychology has expanded the study of trauma to include not only posttraumatic maladjustment but also posttraumatic growth (Calhoun & Tedeschi, Reference Calhoun and Tedeschi2014), defined as psychological change following challenging or traumatic events, which may manifest as an “increased appreciation for life in general, more meaningful interpersonal relationships, an increased sense of personal strength, changed priorities, and a richer existential and spiritual life” (Tedeschi & Calhoun, Reference Tedeschi and Calhoun2004). One hypothesized mechanism for this growth is the development of a trauma narrative that acknowledges the reality of the trauma, allows the individual to let go of beliefs or schemas that are inconsistent with this reality, and generates new worldviews that allow for continued movement toward values and goals. The development of acceptance, of facing existential questions about life, and the perspective that comes with posttraumatic growth has also been framed as the development of wisdom (Linden, Baumann, Lieberei, Lorenz, & Rotter, Reference Linden, Baumann, Lieberei, Lorenz and Rotter2011). Although the concept of posttraumatic growth has been primarily applied to individuals, in their seminal paper delineating the concept, Tedeschi and Calhoun (Reference Tedeschi and Calhoun2004) address the application of posttraumatic growth to communities and societies that have experienced shared and widespread trauma. They suggest that such experiences can be recognized as “turning points” and form the basis of shared social narratives that can transform the community's sense of identity, principles, and values, and understanding of the world.
The introduction of epigenetics into the conversation about the intergenerational transmission of trauma effects has the potential to highlight the phenotypic diversity, potential adaptiveness, and divergent possibilities inherent in epigenetic inheritance. Too often the discussion in effect becomes a mirror of the old nature versus nurture debate. On the one hand, the possibility of epigenetic transmission appears to reject genetic determinism in favor of the power of environmental influences. On the other hand, it often reifies a kind of environmental determinism, implying that the experiences of parents determine the biology and therefore available repertoire of offspring. Evidence that cultural trauma in past generations may leave traces in the epigenome may serve to validate offspring experiences or to imply a legacy of damage. As with the experience of trauma itself, the narrative we tell about its meaning has much power in determining the consequences.



