Introduction
Recently, there has been an increasing usage of 2-octyl cyanoacrylate (Dermabond; Ethicon, Somerville, New Jersey USA) among physicians due to its speed, convenience, and ease of usage.Reference Perry and Sosin1 Dermabond was first approved by the US Food and Drug Administration in 1998 and is now used by a wide variety of practitioners in a number of clinical settings, ranging from plastic surgeons during cosmetic surgery to emergency room physicians for laceration repairs.Reference Nipshagen, Hage and Beekman2-4 The majority of complications associated with Dermabond involve incorrect application techniques, often resulting in sterile drapes or dressings being stuck to a patient's skin, as well as poor wound edge apposition. Rarely, patients can present with allergic reactions to the chemical compounds.Reference Perry and Sosin1 Despite anecdotal reports among practitioners, there is a paucity of medical literature on the subject, with few other cases reported. Furthermore, nearly all other reported cases were of localized contact dermatitis surrounding the wound, with only one other case of diffuse skin reaction reported.Reference Perry and Sosin1, Reference El-Dars, Chaudhury, Hughes and Stone5-Reference Hivnor and Hudkins7 In this report, the unique case of a patient who suffered a diffuse cutaneous reaction to Dermabond not limited to the skin immediately adjacent to the wound is presented. With the widespread and increasing use of Dermabond among physicians, more allergic reactions are anticipated, along with more patients presenting to the emergency department with these complaints.
Report
A 50-year-old female patient presented with a new diagnosis of atypical ductal hyperplasia of the left breast on core biopsy after routine mammography identified calcifications and a four millimeter mass. The patient had a personal history of left-sided breast cancer, treated five years prior, with no evidence of recurrence up to the time of our encounter. At that time, she underwent a lumpectomy with adjuvant radiation therapy for a 1.1 cm invasive ductal carcinoma. Sentinel node biopsy at that time was negative. The remainder of her past medical history was otherwise unremarkable. She did not have any other prior surgeries, had no documented allergies, was a nonsmoker, and was not actively taking any other medications. On physical examination of her breasts, no suspicious masses, nipple discharge or ulceration, skin edema, and/or nipple retraction were found. Examination of her axillary, cervical, and supraclavicular nodal beds did not demonstrate any palpable nodes.
After consultation with the breast and plastic surgery teams, the patient elected to undergo bilateral skin sparing mastectomies and subsequent breast reconstruction with tissue expanders. The patient tolerated the procedure well and there were no intraoperative complications. The patient spent two nights in the hospital and was discharged home with a routine 7-day course of Cefadroxil. Over the subsequent six months, the patient was seen monthly as her tissue expanders were filled without complications. Eight months after her first surgery, she returned to the operating room for the second stage of the breast reconstruction, an exchange of the tissue expanders for permanent silicone implants. The skin incisions were closed in layers with sutures and Dermabond glue was applied.
On postoperative day seven, she presented to the emergency room with complaints that her right breast had become erythematous over the past two days (Figure 1). She reported the redness began around the incision lines and then began to expand outward from there (Figure 1). Subsequently, she began to develop an itchy, red, maculopapular rash spreading along her abdomen, back, thighs, chest, and upper arms (Figure 2). At that time, she denied any fevers, chills, new medications or food, or recent viral illness. Her incisions were healing well without any evidence of skin necrosis. There was no demonstrable fluid collection around the implant. Additionally, there was no tenderness or fluctuance surrounding the incisions. Her white blood cell count was within the normal range. On presentation, she did not have any difficulty breathing, hemodynamic instability, or signs of airway compromise.
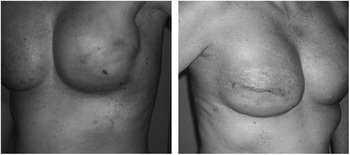
Figure 1 Photographs of Patient Demonstrating Severe Contact Dermatitis at the Incisions and Maculopapular Rash Spreading on the Breast and Chest
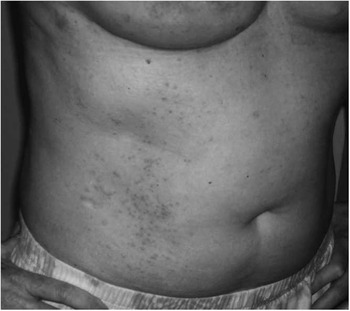
Figure 2 Photograph of the Patient's Right Abdomen and Flank Demonstrating the Spreading Maculopapular Rash
The diagnosis of an allergic reaction to Dermabond was established as infectious etiologies such as cellulitis, subcutaneous abscess, and infection of the implant cavity had been ruled out. Subsequently, the Dermabond was removed from her incisions, the area was washed with soapy water, and she was started on a topical steroid cream. Though systemic steroids were considered by the team, it was not felt they were necessary unless the reaction continued to worsen. Additionally, she was started on a course of diphenhydramine. Within one week after her visit to the emergency room, her rash and symptoms completely resolved and have not returned to date.
Discussion
The main component of the liquid adhesive Dermabond is 2-octyl cyanoacrylate (at a concentration of 94%).Reference Perry and Sosin1 Monomers of this compound polymerize upon contact with the applicator tip, water, and exposure to body temperatures. When fully polymerized, a functional glue is created on the skin.Reference Perry and Sosin1 Several additional components found in Dermabond include plasticizers, which aid in the flexibility of the wound; stabilizers to prolong the shelf life; and polymerization inhibitors to slow the transition of the glue from liquid to solid.4 The polymer is hydrolyzed by the body, creating formaldehyde as one of the breakdown products, which can cause inflammation. The primary contraindication to Dermabond use is an allergy to cyanoacrylate or formaldehyde.
Dermabond allows for the rapid closure of wounds while minimizing local tissue trauma as well as cross- and point-marks associated with sutures.Reference Perry and Sosin1 Issues with Dermabond are commonly attributed to application technique. For example, the skin edges of a wound must be completely opposed to prevent inversion, which could result in wound separation once the Dermabond is removed. Additionally, Dermabond embedded under the skin may result in a foreign body reaction.Reference Dragu, Unglaub and Schwarz8 A true allergy to Dermabond is an extremely rare event and only a few cases documenting an allergy have been reported in the literature, with nearly all cases being limited to a contact dermatitis of the wound.Reference Kanerva, Jolanki and Estlander9-Reference Belsito11 As the indications for Dermabond use expand, a rise in the number of patients like this seen in emergency departments is anticipated.
Once a skin reaction begins, patients, such as the one presented in this case, will re-present to practitioners or to the local emergency department with concern for redness around their wound. The erythema, coupled with rapid onset and progressiveness of the skin changes, would prompt many to make a diagnosis of cellulitis or another infectious process. This misdiagnosis could lead to inappropriate treatment with antibiotics and potential worsening of the patient's condition. The exposure to Dermabond over a week caused an allergic reaction that started out localized around the incision, but subsequently spread to a large percentage of the patient's body surface area.
The distinction between infection and allergic reaction is critical. Steroids are the mainstay of treatment; however, topical petrolatum-based steroids not only treat symptoms but also dissolve the Dermabond. Prompt removal of the glue is crucial to the resolution of symptoms, but care must be taken to not open the incisions. In this case, the correct diagnosis was reached expeditiously, and the patient was successfully treated. Had the patient's condition been misidentified as infectious in nature, she might have gotten worse and incurred potential harmful sequelae.
Conclusion
A true Dermabond allergy is a rare event, but one that needs to be recognized and treated promptly. Care must be taken to discern cause of the skin erythema of an allergic reaction from that of cellulitis and to treat patients’ symptoms while maintaining the integrity of their wounds.
Acknowledgements
The authors would like to thank Tracy Lahair, PA, and Charles Hergrueter, MD for their assistance with this case and for providing the photographs.




