National statistics demonstrate the continuing widespread use of alcohol among adolescents and adults (Center for Behavioral Health Statistics and Quality, 2015). Although on average alcohol use increases over the course of adolescence, typically peaking during emerging adulthood and declining thereafter (Chen & Jacobson, Reference Chen and Jacobson2012), there is substantial heterogeneity in individuals’ trajectories of alcohol use across developmental stages. For example, a number of longitudinal studies have identified distinct trajectories of alcohol use that include (but are not limited to) a non/low use trajectory, a persistent heavy use trajectory, a developmentally limited trajectory, and a late-onset, increasing trajectory (Chassin, Flora, & King, Reference Chassin, Flora and King2004; Flory, Lynam, Milich, Leukefeld, & Clayton, Reference Flory, Lynam, Milich, Leukefeld and Clayton2004; Jackon & Sher, Reference Jackson and Sher2005; Tucker, Ellickson, Orlando, Martino, & Klein, Reference Tucker, Ellickson, Orlando, Martino and Klein2005; Wichers, Gillespie, & Kendler, Reference Wichers, Gillespie and Kendler2013). These different trajectories of alcohol use may have different implications for later outcomes in life; for example, persistent heavy alcohol use is associated with greater risk for long-term physical and psychosocial problems (Chassin, Pitts, & Prost, Reference Chassin, Pitts and Prost2002; Skogen, Knudsen, Hysing, Wold, & Sivertsen, Reference Skogen, Knudsen, Hysing, Wold and Sivertsen2015). Accordingly, identifying factors that differentiate individuals with different alcohol use trajectories is important in preventing adult negative health outcomes. In this study, we examined the associations between individual differences in the serotonin transporter linked polymorphic region (5-HTTLPR) genotype, experience of parenting quality, and their interactions with respect to membership in latent trajectories of alcohol use from early adolescence to young adulthood.
Gene–Environment (G × E) Interaction: 5-HTTLPR and Parenting
Numerous studies have been conducted to understand the etiology of alcohol use, with increasing research attention on how genetic and environmental factors interact in contributing to alcohol use. Twin, adoption, and other genetically informative studies suggest that both genetic and environmental factors contribute to individual variability in alcohol use behaviors (Hopfer, Crowley, & Hewitt, Reference Hopfer, Crowley and Hewitt2003). Moreover, studies suggest that genetic effects on alcohol use may be moderated by environmental factors (van der Zwaluw & Engels, Reference van der Zwaluw and Engels2009), such as parenting behaviors (Dick, Viken, et al., Reference Dick, Viken, Purcell, Kaprio, Pulkkinen and Rose2007). A growing number of studies have examined how specific genetic variants, in conjunction with environmental factors, are associated with alcohol use outcomes. Of particular interest is the functional polymorphism of 5-HTTLPR, which has been implicated in various functions such as mood, affect, and behavioral inhibition (Daw, Kakade, & Dayan, Reference Daw, Kakade and Dayan2002). 5-HTTLPR is located on chromosome 17q12 and consists of a 44-base pair polymorphism, resulting in short and long alleles; the short allele of the 5-HTTLPR has been shown to have lower transcriptional activity of the serotonin transporter than the long allele (Helis et al., Reference Heils, Teufel, Petri, Stober, Riederer, Bengel and Lesch1996; Lesch et al., Reference Lesch, Bengel, Heils, Sabol, Greenberg, Petri and Murphy1996). Animal studies suggested that decreasing serotonergic functioning increased alcohol intake (LeMarquand, Pihl, & Benkelfat, Reference LeMarquand, Pihl and Benkelfat1994), but findings concerning the association between the 5-HTTLPR genotype and alcohol use outcomes in humans have been inconsistent (Feinn, Nellissery, & Kranzler, Reference Feinn, Nellissery and Kranzler2005). In some studies, the short allele of 5-HTTLPR has been associated with higher levels of and larger increase in alcohol consumption in adolescents (Kaufman et al., Reference Kaufman, Yang, Douglas-Palumberi, Crouse-Artus, Lipschitz, Krystal and Gelernter2007; Merenäkk et al., Reference Merenäkk, Mäestu, Nordquist, Parik, Oreland, Loit and Harro2011; van der Zwaluw et al., Reference van der Zwaluw, Engels, Vermulst, Rose, Verkes, Buitelaar and Scholte2010) and higher alcohol consumption and more alcohol use problems in college-age youths and young adults (Covault et al., Reference Covault, Tennen, Armeli, Conner, Herman, Cillessen and Kranzler2007; Herman, Philbeck, Vasilopoulos, & Depetrillo, Reference Herman, Philbeck, Vasilopoulos and Depetrillo2003). In contrast, some studies showed that the homozygous long allele genotype is associated with higher levels of alcohol use (Olsson et al., Reference Olsson, Byrnes, Lotfi-Miri, Collins, Williamson, Patton and Anney2005; Skowronek, Laucht, Hohm, Becker, & Schmidt, Reference Skowronek, Laucht, Hohm, Becker and Schmidt2006) in adolescents. Yet, other studies failed to find an association between the 5-HTTLPR genotype and alcohol use outcomes (Dick, Plunkett, et al., Reference Dick, Plunkett, Hamlin, Nurnberger, Kuperman, Schuckit and Bierut2007). These mixed findings may be in part due to differences in sample characteristics and measurement of alcohol use outcomes. It is also possible that the heterogeneity in patterns of alcohol use contributed to the inconsistent findings from past research. Accordingly, taking into account the heterogeneity of alcohol use across development is important to further clarify the role of 5-HTTLPR in alcohol use. These mixed findings also point to the importance of examining potential moderators of the effect of 5-HTTLPR in relation to alcohol use.
Parenting is an important environmental factor for adolescent development, and parenting behaviors such as parental support, warmth, and involvement during adolescence have been associated with lower alcohol use and related outcomes in adolescents and young adults (Aquilino & Supple, Reference Aquilino and Supple2001; Barnes, Reifman, Farrell, & Dintcheff, Reference Barnes, Reifman, Farrell and Dintcheff2000; Ryan, Jorm, & Lubman, Reference Ryan, Jorm and Lubman2010). Emerging evidence suggests that the associations between 5-HTTLPR and alcohol use outcomes differ as a function of environmental factors such as parenting behaviors (e.g., Brody et al., Reference Brody, Beach, Philibert, Chen, Lei, Murry and Brown2009), suggesting G × E interaction. Different conceptual perspectives regarding the nature of G × E effects have been proposed in the literature, including the diathesis-stress model, where genetic “risk” predispositions increase vulnerability to environmental adversity (Rende & Plomin, Reference Rende and Plomin1992), and the differential susceptibility hypothesis, where individuals with genetic “susceptibility” predispositions are more susceptible to negative environments but are also more responsive to positive environments (Belsky & Pluess, Reference Belsky and Pluess2009). There has been evidence for interactions between 5-HTTLPR and parenting behaviors in relation to alcohol use outcomes that are consistent with the diathesis-stress model. For example, the short allele of 5-HTTLPR was associated with higher rates of increase in substance use including alcohol use in the context of low parental support and involvement in a sample of African American adolescents (Brody et al., Reference Brody, Beach, Philibert, Chen, Lei, Murry and Brown2009). Other studies have suggested that adolescent carriers of the short allele of 5-HTTLPR were more susceptible to negative effects of family conflict on frequency of heavy episodic drinking and intoxication, both concurrently and prospectively (Kim et al., Reference Kim, Park, Glatt, Eckert, Vanable, Scott-Sheldon and Carey2015). To our knowledge, there has not been evidence of 5-HTTLPR × Parenting interaction in relation to alcohol use outcomes supporting the differential susceptibility hypothesis. However, differential susceptibility of the short allele of 5-HTTLPR has been demonstrated in interaction with parenting behaviors for other psychosocial outcomes such as positive affect among adolescents (Hankin et al., Reference Hankin, Nederhof, Oppenheimer, Jenness, Young, Abela and Oldehinkel2011) and longitudinal trajectories of antisocial behaviors from adolescence to young adulthood (Tung & Lee, Reference Tung and Lee2016). Differential susceptibility is generally difficult to detect, and the relatively limited evidence of differential susceptibility may be due to limited consideration of positive environmental factors in G × E research (Roisman et al., Reference Roisman, Newman, Fraley, Haltigan, Groh and Haydon2012).
While evidence of G × E interaction, and specifically 5-HTTLPR × Parenting interaction, in relation to alcohol use outcomes is growing, there is a critical disconnect between the research on G × E interaction in alcohol use and our understanding about the developmental patterns of alcohol use. That is, few studies have explored G × E interaction underlying membership in empirically derived developmental trajectories of alcohol use. Bridging this disconnect to examine G × E interaction in a developmental framework is critical for understanding how genetic predispositions interface with environmental factors to predict different trajectories of alcohol use across development. This is important given that genetic influence may vary over the course of alcohol use (Kendler, Schmitt, Aggen, & Prescott, Reference Kendler, Schmitt, Aggen and Prescott2008), with evidence showing that genetic factors have little influence on the initiation of alcohol use in early adolescence but strongly influence the establishment of alcohol use patterns (Hopfer et al., Reference Hopfer, Crowley and Hewitt2003; Kendler et al., Reference Kendler, Schmitt, Aggen and Prescott2008). Moreover, given that genetic and environmental contributions may differ for different trajectories of risk behaviors (Moffit, Reference Moffitt1993; Wichers et al., Reference Wichers, Gillespie and Kendler2013), accounting for the heterogeneity of developmental trajectories of alcohol use in G × E research may aid in detecting G × E effects that may be trajectory specific. To our knowledge, only one study has examined how specific genetic variants interact with environmental factors to predict different latent trajectories of alcohol use (van der Zwaluw, Otten, Kleinjan, & Engels, Reference van der Zwaluw, Otten, Kleinjan and Engels2014). Using a community sample of Dutch adolescents, van der Zwaluw et al. showed that adolescents’ μ-opioid receptor M1 (OPRM1) genotype interacted with parental alcohol-specific rule-setting behaviors to predict adolescents’ likelihood of following a heavy drinking trajectory relative to the light and moderate drinking trajectories, such that the parental rule-setting behaviors was associated with lower risk for membership in heavy drinking trajectory only for adolescents carrying the risk genotype (AG/GG) of OPRM1. In this study, we extend the existing literature by examining how 5-HTTLPR interacts with parenting quality in predicting latent trajectories of alcohol use across development.
Gender Differences in G × E
Researchers have called for consideration of gender in G × E research and suggested that gender may moderate G × E effects through biological and social environmental pathways (Perry, Reference Perry2013; Salvatore, Cho, & Dick, Reference Salvatore, Cho and Dick2017). Hormonal and other biological differences between men and women may lead to gender differences in gene expression and G × E effects. Gender differences in experiences of and responses to social environments, including social controls and norms related to alcohol use and parenting behaviors, may also contribute to gender differences in G × E effects (Perry, Reference Perry2013). Despite the conceptual plausibility, few studies on candidate gene–environment interaction in alcohol use have considered potential gender differences in G × E effects.
Gender may be particularly relevant in G × E studies that involve 5-HTTLPR. Prior research suggests that gender may condition the interaction effects between 5-HTTLPR and environmental factors on psychosocial outcomes. For example, 5-HTTLPR has been shown to moderate the association between family stress (Hammen, Brennan, Keenan-Miller, Hazel, & Najman, Reference Hammen, Brennan, Keenan-Miller, Hazel and Najman2010) and depression only for females, with the associations being stronger for those carrying the short allele. Similarly, interactions between 5-HTTLPR and childhood maltreatment have been found among females but not males in predicting antisocial behavior (Li & Lee, Reference Li and Lee2010) and marijuana use (Vaske, Newsome, & Wright, Reference Vaske, Newsome and Wright2012) in adolescents, where female carriers of the short allele were more vulnerable to the negative influence of childhood maltreatment. Some studies showed interaction between 5-HTTLPR and environmental factors for males but not for females. For example, the homozygous short (S/S) 5-HTTLPR genotype was associated with increased risk for aggression under stress for male, but not female, college students (Verona, Joiner, Johnson, & Bender, Reference Verona, Joiner, Johnson and Bender2006). Other studies showed interaction effects between 5-HTTLPR and environmental factors for both males and females that differed in nature. In a sample of adults, females with the homozygous short genotype, whereas males with the homozygous long genotype, were at increased risk for depression under stressful life conditions (Brummett et al., Reference Brummett, Boyle, Siegler, Kuhn, Ashley-Koch, Jonassaint and Williams2008). Collectively, these studies demonstrate the importance of considering gender differences in G × E effects. These mixed findings regarding gender differences in G × E effects involving 5-HTTLPR may be in part due to false positive and negative findings because of low power in some studies given the small sample sizes and statistical difficulty in detecting three-way interactions. It is also possible that gender differences in G × E effects involving 5-HTTLPR are specific to the environmental factors and the outcome variables being considered. Thus, it is important to utilize large samples to examine potential gender differences in the interaction effect between 5-HTTLR and environmental factors on alcohol use outcomes. Further, the majority of studies that showed gender difference in G × E effects involving 5-HTTLPR focused on negative environmental factors; thus, it remains unclear how interactions between 5-HTTLPR and positive environmental factors may vary across gender.
The Current Study
Despite growing evidence of G × E interaction in alcohol use, the literature is limited by lack of examination of G × E effects on developmental trajectories of alcohol use over time and limited consideration of gender differences in G × E effects. The goal of this study was to extend the G × E literature on alcohol use by using a developmental perspective to examine how genetic (i.e., 5-HTTLPR) and environmental (i.e., parenting quality) factors may interact in distinguishing individuals’ trajectories of alcohol use from early adolescence to young adulthood. We considered multiple aspects of positive parenting (i.e., parental involvement, parental attachment, and parental warmth) to create a broad index of parenting quality, which capitalizes on previous findings that multiple dimensions of parenting, rather than one single parenting practice, influence adolescent behaviors (Ryan et al., Reference Ryan, Jorm and Lubman2010). Further, we also considered potential gender differences in such G × E effects.
Based on the literature, we expected to identify distinct trajectories of alcohol use from early adolescence to young adulthood, including (but not limited to) a non/low use trajectory, a persistent heavy use trajectory, a developmentally limited trajectory, and a late-onset, increasing trajectory. We expected that parenting quality would be negatively associated with likelihoods for following alcohol use trajectories that indicates higher risk (e.g., persistent heavy use trajectory vs. non/low use trajectory). We hypothesized that carriers of the short allele of 5-HTTLPR would be at greater risk for following risk alcohol use trajectories compared to noncarriers. We also hypothesized that the 5-HTTLPR genotype would moderate the association between parenting quality and alcohol use trajectory membership; however, no specific hypothesis was made regarding the nature of the interaction effects given the alternative conceptual perspectives (e.g., diathesis-stress vs. differential susceptibility) and the mixed findings from previous studies. Likewise, no specific hypothesis was proposed regarding gender differences in the effects of 5-HTTLPR × Parenting Quality interaction in relation to alcohol use trajectory membership, due to limited and mixed evidence from prior research.
Method
Data and procedures
Data for this study came from the National Longitudinal Study of Adolescent to Adult Health (Add Health). Add Health is a nationally representative, prospective study that currently contains four waves of data. In 1994/1995 (Wave 1), a total of 20,745 adolescents in Grades 7–12, aged between 12 and 21, from 132 schools stratified by region, urbanicity, school type, ethnic mix, and size were interviewed. These adolescents were reinterviewed in 1996 (Wave 2, n = 14,738), 2001/2002 (Wave 3, n = 15,197), and 2008 (Wave 4, n = 15,701). Almost all interviews were conducted at participants’ home by trained research assistants. At Wave 4, immediately following the 90-min interview, saliva samples were collected from participants for buccal cell DNA. Saliva samples were then mailed to the Institute for Behavioral Genetics in Boulder, Colorado, where the DNA was extracted, quantified, and genotyped. Details for all data collection procedures have been described elsewhere (Harris, Reference Harris2011).
For the purpose of this study, we included participants who provided genetic data at Add Health Wave 4. Because all participants who provided genetic data at Wave 4 also participated at Wave 1, this approach ensured that every individual in the sample participated in at least two waves of data collection. We excluded individuals aged 12 (n = 78), 20 (n = 155), and 21 (n = 35) at Wave 1 due to the small sample sizes. We also excluded participants who self-identified as American Indian or other race due to small sample sizes in these groups. This approach resulted in a final sample of 13,749 individuals for the current study. Of these participants, 53.3% were female, 56.3% were non-Hispanic White, 21.5% were Black, 16.0% were Hispanic, and 6.1% were Asian.
Measures
Alcohol use
At each wave, participants responded to four questions regarding their frequency and quantity of alcohol use during the past 12 months (on how many days did you drink alcohol, have five or more drinks in a row, and get drunk; and how many drinks did you usually have each time when you had a drink). Participants reported the number of days they drank, had five or more drinks in a row, or got drunk on a 7-point scale. Response categories ranged from 1 (every day or almost every day) to 7 (never). Scores were reverse coded for these items so that higher values indicated more frequent alcohol use. Participants indicated the actual number of drinks they usually had each time, and the numbers were recoded into a 7-point scale to be in the same metric with the other three items, following the approach used by other Add Health researchers (Chen & Jacobson, Reference Chen and Jacobson2012). Given that these four items were modestly to highly correlated (r ranged from .60 to 84) and we wanted to maximize available information on alcohol use to characterize individuals’ drinking patterns, scores were averaged across the four items to create a composite variable that captures frequency and quantity of alcohol use, with higher scores indicating higher levels of alcohol use. Participants who indicated never used alcohol were assigned a score of zero. Cronbach α for this scale was 0.91 at Wave 1, 0.92 at Wave 2, 0.90 at Wave 3, and 0.89 at Wave 4.
Parenting quality
We used three scales developed by other Add Health researchers to measure parenting quality at Wave 1: maternal involvement, maternal attachment, and maternal warmth (Beaver & Belsky, Reference Beaver and Belsky2012; Mogro-Wilson, Reference Mogro-Wilson2008). The maternal involvement scale measured the extent to which mothers were involved in their children's life. Adolescents indicated whether or not they and their residential (biological, step, adoptive, foster, etc.) mother had participated in 10 activities (e.g., played a sport, gone to a movie, talked about a personal problem the adolescent was having, talked about school work, etc.) during the past month. Responses (1= yes, 0 = no) were summed to create a composite scale representing maternal involvement (α = 0.55). The maternal attachment scale asked adolescents two questions on how close they felt to their residential mother and how much they thought their mother cared about them. Responses for each question ranged from 1 (not at all) to 5 (very much). Responses were averaged to create a summary variable representing maternal attachment (α = 0.63). The maternal warmth scale asked adolescents three questions regarding how warm and loving their residential mother was and the overall quality of their relationship with their mother (e.g., most of the time, your mother is warm and loving toward you). Response options to these three items ranged from 1 (strongly agree) to 5 (strongly disagree). Scores were reversed coded and averaged to create a summary variable representing maternal warmth, with higher values indicating higher warmth (α = 0.85). Following the approach used by Beaver and Belsky (Reference Beaver and Belsky2012), we calculated a composite score indexing parenting quality by summing the standardized scores of maternal involvement, maternal attachment, and maternal warmth, weighted by their corresponding factor loadings (.49, .87, and .87, respectively) derived from principle components factor analysis.
5-HTTLPR
At Wave 4, genomic DNA was extracted from buccal cells using standard methods for all participants. We examined the insertion/deletion polymorphism in the 5’ regulatory region of the serotonin transporter gene (5-HTTLPR; Heils et al., Reference Heils, Teufel, Petri, Stober, Riederer, Bengel and Lesch1996); genotyping procedures for this polymorphism has been described in detail elsewhere (Smolen et al., Reference Smolen, Whitsel, Tabor, Killeya-Jones, Cuthbertson, Hussey and Harris2013). In this sample, 19.2% were homozygous for the short allele (S/S), 46.0% were heterozygous (L/S), and 34.8% were homozygous for the long allele. Consistent with prior research (Brody et al., Reference Brody, Beach, Philibert, Chen, Lei, Murry and Brown2009; Tung & Lee, Reference Tung and Lee2016; van der Zwaluw et al., Reference van der Zwaluw, Engels, Vermulst, Rose, Verkes, Buitelaar and Scholte2010; Vaske et al., Reference Vaske, Newsome and Wright2012), we coded participants’ 5-HTTLPR genotypes as 0 (homozygous long) and 1 (heterozygous or homozygous short). Genotype frequencies significantly differed across race (χ2 = 874.55, df = 3, p < .001). Consistent with previous studies (Kaufman et al., Reference Kaufman, Yang, Douglas-Palumberi, Crouse-Artus, Lipschitz, Krystal and Gelernter2007; Vaske et al., Reference Vaske, Newsome and Wright2012), Blacks were more likely to have the homozygous long genotype (55.3%) compared to non-Hispanic Whites (32.5%), Hispanics (24.8%), and Asians (10.7%). Tests of Hardy–Weinberg equilibrium (HWE) indicated that 5-HTTLPR genotype frequencies met the assumption for HWE among non-Hispanic Whites (χ2 = 0.00, p = 1.00), Hispanics (χ2 = 1.88, p = .17), and Asians (χ2 = 0.04, p = .84), but deviated from HWE among Blacks (χ2 = 6.78, p < .01). Of the total sample of 13,749 participants, 21 (0.2%) were missing the genotype information for 5-HTTLPR. Reliability of genotyping was excellent (>95%; Smolen et al., Reference Smolen, Whitsel, Tabor, Killeya-Jones, Cuthbertson, Hussey and Harris2013).
Statistical analyses
Add Health is organized by wave of assessment, and the sample for this study included participants aged between 13 (birth cohort 1982) and 19 (birth cohort 1976) at Wave 1 who became 14–20 years old at Wave 2, 19–25 years old at Wave 3, and 26–32 years old at Wave 4. Given the significant variability in chronological age at each wave and that age is a more informative metric for development than assessment waves, consistent with other longitudinal studies using Add Health (Tung & Lee, Reference Tung and Lee2016), we restructured the four waves of data so that time was represented by age, consistent with an accelerated longitudinal design (Little, Reference Little2013; Singer & Willet, Reference Singer and Willett2003). This approach took into account the wide range of age variation at each wave in Add Health and allowed for examination of developmental trajectories of alcohol use spanning from age 13 (youngest participants at Wave 1 with sufficient data) to age 32 (oldest participants at Wave 4 with sufficient data). This strategy also resulted in a notable amount of missing data that were “planned missingness” or “missing by design,” which is appropriately handled via full information maximum likelihood estimation in Mplus (Little, Reference Little2013; Muthén & Muthén, Reference Muthén and Muthén1998–2012). Table 1 presents the number of participants by wave, cohort, and age.
Table 1. Number of participants by wave, cohort, and age

We conducted growth mixture modeling (GMM) analyses using Mplus version 7.31 to examine developmental trajectories of alcohol use from age 13 to age 32. GMM captures heterogeneity among individuals in their trajectories or growth curves of a certain developmental outcome over time by classifying individuals into latent classes that are indicated by different latent growth parameters (i.e., intercept and slopes; Muthén, & Muthén, Reference Muthén and Muthén2000). GMM estimates a mean latent growth curve for each class and assumes that individuals within the same latent class follow a similar growth curve over time that is distinct from individuals in other latent classes. We estimated a series of growth mixture models that specified different number of latent classes (from 2 to 6) and quadratic growth curves for each class. The most well fitting model was determined via several fit indices, including the Akaike information criterion (AIC), sample size adjusted Bayesian information criterion (adjusted BIC), Lo–Mendell–Rubin likelihood ratio test, and model entropy. A model with lower AIC, adjusted BIC, and higher entropy is preferable (Wang & Bonder, Reference Wang and Bodner2007). In addition, latent class separation (i.e., whether classes can clearly be distinguished from each other) and model interpretability (e.g., class size and meaningfulness of each class) were taken into account to determine the optimal model.
After identifying trajectories of alcohol use using GMM, we conducted a series of multinomial logistic regression models to examine how 5-HTTLPR, parenting quality, and their interactions were associated with the likelihoods of following each trajectory, using the R3STEP command in Mplus, an automatic approach linking covariates to class membership (Muthén & Muthén, Reference Muthén and Muthén1998–2012). We started with a model examining main effects of 5-HTTLPR and parenting quality. We then evaluated the interaction effects between 5-HTTLPR and parenting quality by adding to the main effects model a product term between 5-HTTLPR and mean-centered parenting quality. Following recommendations regarding properly accounting for potential confounders in testing G × E effects (Keller, Reference Keller2014), we included the interaction terms between covariates (e.g., race/ethnicity) and 5-HTTLPR and parenting quality as additional control variables in this model; only the statistically significant ones were retained in the model for the purpose of model parsimony. Finally, we examined gender differences in the effect of 5-HTTLPR × Parenting interaction by testing a three-way interaction between gender, 5-HTTLPR, and parenting quality.
We controlled for self-reported race/ethnicity in all analyses to take into account potential population stratification effects due to differences in allele frequency among people of different ancestries and racial differences in patterns of alcohol use. Although self-reported race does not perfectly represent ancestry, statistically adjusting for race/ethnicity reduces potential bias due to population stratification. Furthermore, controlling for self-reported race is a recommended approach to account for population stratification when more superior approaches such as genomic control and ancestry informative genetic markers are not available (Barnholtz-Sloan, McEvoy, Shriver, & Rebbeck, Reference Barnholtz-Sloan, McEvoy, Shriver and Rebbeck2008). Because prior research suggests that trajectories of alcohol use are similar across gender but there is gender difference in the likelihood of following different trajectories, for example, males are more likely to follow a persistent heavy drinking trajectory than females (e.g., Chassin et al., Reference Chassin, Pitts and Prost2002, Reference Chassin, Flora and King2004), we conducted analyses with males and females combined but included gender as a covariate in the analyses. We accounted for clustering within school using the CLUSTER command in Mplus and took into account all available data in the analyses using full information maximum likelihood estimation.
Results
Preliminary analysis
Add Health participants who were included in the sample for this study reported higher levels of maternal education (t = –5.17, df = 18,411, p < .001) and higher alcohol use at Wave 3 and Wave 4 (t = –4.23, df = 15,161, p < .001 and t = –3.48, df = 15,686, p < .01, respectively) than those who were excluded from this study due to lack of genetic data or age or race/ethnicity. Those included in the final sample were also more likely to be White (χ2 = 172.43, df = 3, p < .001) and female (χ2 = 128.30, df = 1, p < .001). Those included in the final sample did not significantly differ from those excluded in terms of parenting quality (t = –1.54, df = 19,439, p = .12) or alcohol use at Wave 1 (t = 0.60, df = 13,560, p = 55) or Wave 2 (t = –1.91, df = 14,734, p = .06). As such, in addition to race, maternal education and gender were included as covariates in all analyses. Descriptive statistics and bivariate correlations between the study variables are presented in Table 2.
Table 2. Descriptive statistics and bivariate correlations

Note: aPoint serial correlation coefficients are presented for gender. bChi-square or F statistics are presented for race. **p < .01.
Identifying trajectories of alcohol use
We evaluated a series of growth mixture models specifying two to six classes of alcohol use trajectories. Fit indices for the models are presented in Table 3. The four-class model had smaller AIC and adjusted BIC than the three-class model and higher entropy compared to the five- and six-class models. In addition, the alcohol use trajectories identified in the four-class model were distinguishable from each other, interpretable from a substantive standpoint, and of reasonable sizes. In consideration of the balance of model fit, parsimony, and interpretability of the classes, we adopted the four-class solution as the optimal model. Figure 1 presents the four distinct trajectories of alcohol use (i.e., non/light alcohol use, late-onset heavy alcohol use, developmentally limited alcohol use, and persistent heavy alcohol use) identified from the selected optimal model. An estimated 58.2% of the sample were classified as non/light drinkers who followed a trajectory characterized by none or light alcohol use over time. Of the participants, 24.5% were classified as late-onset heavy drinkers. These individuals engaged in light to moderate alcohol use during adolescence but increased their alcohol use over time and engaged in relatively heavy drinking in young adulthood. In addition, 12.2% of the participants were classified as developmentally limited drinkers. These individuals followed a trajectory characterized by moderate alcohol use in early adolescence, increase in alcohol use throughout adolescence (reaching peak use around age 21), and decline in alcohol use during young adulthood. Finally, 5.1% of the participants were classified as persistent heavy drinkers who followed a trajectory of persistent heavy alcohol use from early adolescence to young adulthood.

Figure 1. Estimated means of alcohol use from early adolescence to young adulthood by latent trajectories.
Table 3. Fit indices for unconditional growth mixture models for alcohol use with two to six classes

Note: AIC, Akaike information criterion. Adjusted BIC, adjusted Bayesian information criterion. LMR LRT, Lo–Mendell–Rubin likelihood ratio test.
Predicting trajectories of alcohol use from 5-HTTLPR, parenting quality, and gender
Table 4 presents coefficients from the multinomial logistic regression models predicting trajectories of alcohol use. In terms of main effects (see Step 1 in Table 4), consistent with our hypothesis, the short allele of 5-HTTLPR was associated with higher likelihood of following the persistent heavy drinkers trajectory relative to the developmentally limited drinkers trajectory and higher likelihood of following the late-onset heavy drinkers trajectory relative to the developmentally limited drinkers trajectory and the non/light drinkers trajectory. Also consistent with hypothesis, parenting quality was associated with lower likelihood of following the persistent heavy and developmental-limited drinkers trajectories compared to the non/light drinkers trajectory; lower risk of following the persistent heavy drinkers trajectory and higher likelihood of following the late-onset trajectory, relative to the developmentally limited trajectory; and lower likelihood of following the persistent heavy drinkers trajectory relative to the late-onset drinkers trajectory. Parenting quality did not significantly differentiate individuals’ likelihoods of being in the late-onset versus non/light drinkers trajectory.
Table 4. Coefficients from multinomial logistic regression models predicting trajectories of alcohol use

Note: Multinomial logit estimates and odds ratios (in parentheses) are presented. Gender is coded 1 = male, 0 = female. Non-Hispanic White was the reference group for race. Persistent, persistent heavy drinkers. Non/light, non/light drinkers. Late onset, late-onset heavy drinkers. Developmental, developmentally limited drinkers. Hisp, Hispanic. ap = .072, bp = .081, cp = .051, *p < .05, **p < .01, **p < .001. Interactions between race and 5-HTTLPR and interactions between maternal education and 5-HTTLPR/parenting quality were also considered as covariates in the analysis but were excluded from the final models presented here because they were not significantly associated with membership in any alcohol use trajectories.
As for G × E effects, our results indicated that there was no significant two-way interaction effect between 5-HTTLPR and parenting quality in predicting trajectories of alcohol use (see Step 2 in Table 4). However, the three-way interaction between gender, 5-HTTLPR, and parenting quality significantly predicted individuals’ likelihood of following the persistent heavy drinkers trajectory relative to the non/light drinkers trajectory (marginally significant, p = .051) and the developmentally limited drinkers trajectory (p = .034; see Step 3 in Table 4). These three-way interactions were evident after properly controlling for several important covariates and potential confounder effects. We ran additional analyses to probe the interaction effect, following the approach suggested by Aiken and West (Reference Aiken and West1991). Results indicated that the interaction effect between 5-HTTLPR and parenting quality in predicting the likelihood of following the persistent heavy drinkers trajectory relative to the non/light drinkers trajectory was signfiicant for males (B = –0.245, SE = 0.100, p = .015), but not for females (B = 0.034, SE = 0.109, p = .756). The pattern of interaction effects for males is illustrated in Figure 2a. For males, parenting quality was associated with lower risk of following the heavy drinkers trajectory compared to the non/light drinkers trajectory for those carrying the short allele of 5-HTTLPR (B = –0.272, SE = 0.056, p < .001), but not for those not carrying the short allele (B = –0.030, SE = 0.084, p =.721). For females, parenting quality was associated with lower risk of following the persistent heavy drinkers trajectory relative to the non/light drinkers trajectory, both for those carrying the short allele (B = –0.301, SE = 0.054, p < .001) and those not carrying the short allele of 5-HTTLPR (B = –0.335, SE = 0.108, p = .002), with no significant difference in magnitude of the associations as a function of the 5-HTTLPR genotype.

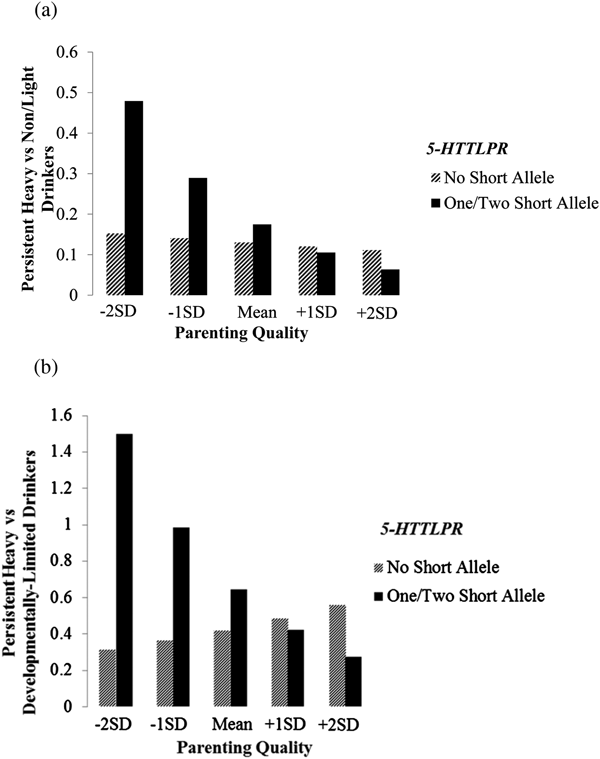
Figure 2. Interaction between 5-HTTLPR and parenting quality in predicting trajectories of alcohol use for males. Values on the y-axis are likelihoods (odds ratios) of membership for the persistent heavy drinking trajectory relative to the comparison trajectories.
Similarly, the interaction between 5-HTTLPR and parenting quality was significant for males (B = –0.333, SE = 0.125, p = .008) but not for females (B = 0.035, SE = 0.119, p = .766), in predicting the likelihood of following the persistent heavy drinkers trajectory relative to the developmentally limited drinkers trajectory. The pattern of interaction effects for males is illustrated in Figure 2b. Parenting quality was associated with lower likelihood of following the persistent heavy drinkers trajectory for males carrying the short allele of 5-HTTLPR, but not for males without the short allele of 5-HTTLPR. This association was not evident for females regardless of their 5-HTTLPR genotypes (B = –0.139, SE = 0.113, p = .218 for females without the short allele; B = –0.099, SE = 0.064, p = .125 for females carrying the short allele).
Because of the conflicting literature and possibility of chance findings regarding G × E interaction effects involving 5-HTTLPR, replication is important to increase confidence in our results. We used a k-fold cross-validation approach to check robustness of our results. This approach involves randomly partitioning the sample into k unique and equal-sized validation samples, using the remaining k – 1 partitions to validate model estimations, and repeating the process for k times. Given the relatively large sample size in the current study, we performed a 5-fold cross-validation analysis to examine whether the genetic and gene–environment interaction effects replicated across the five validation samples. In order to use all available data to derive reliable trajectories of alcohol use, we used a two-step approach (Clark & Muthén, Reference Clark and Muthén2009). That is, we first conducted an unconditional growth mixture model to derive class membership for alcohol use trajectories within the whole sample. We then conducted a series of multinomial logistic regressions with class membership as the dependent variable to test for genetic and gene–environment interaction effects within each of the validation samples. Results indicated that the three-way interactions between 5-HTTLPR, parenting quality, and gender found in the whole sample were generally replicated in the five validation samples; despite some small differences in magnitude of effects and the significance levels, the direction of the effects were consistent. Results from the cross-validation analyses are summarized in supplementary materials (online-only Supplemental Tables 1–5).
Discussion
Using a large and nationally representative sample of adolescents followed prospectively (Add Health), we examined how adolescents’ 5-HTTLPR genotype and perceived maternal parenting quality independently and interactively associated with different developmental trajectories of alcohol use from early adolescence to young adulthood and whether and how gender may moderate these associations. Consistent with previous research, we identified four distinct trajectories of alcohol use: a non/light drinking trajectory, a developmentally limited drinking trajectory, a late-onset drinking trajectory, and a persistent heavy drinking trajectory. Findings indicated that 5-HTTLPR and maternal parenting quality independently and interactively predict membership in latent trajectories of alcohol use; the interaction effect was evident for males but not for females.
Our findings of distinct trajectories of alcohol use from early adolescence to young adulthood build on the literature to emphasize the heterogeneity in alcohol use patterns over time. The late-onset alcohol use trajectory identified in this study had an inflection point of alcohol use in the middle 20s. We note that others have reported that alcohol use typically peaks in the early 20s instead of the middle 20s (e.g., Chen & Jacobson, Reference Chen and Jacobson2012; Dick et al., Reference Dick, Cho, Latendresse, Aliev, Nurnberger, Edenberg and Kuperman2014). This difference in the identification of inflection point of alcohol use between our findings and previous reports may be in part due to methodological differences: those reports typically considered the overall trajectory of alcohol use, whereas we examined the distinct trajectories of alcohol use. It is also important to note that our data included ages spanning from 13 and 32 years of age. It is possible that the difference in peak of alcohol is in part due to the differences in the included age ranges across studies.
Consistent with our hypothesis, higher maternal parenting quality was associated with a lower likelihood of membership in the persistent alcohol use trajectory group relative to any other alcohol use trajectory and a lower likelihood of membership in the developmentally limited drinking trajectory relative to the non/light drinking trajectory. This is consistent with previous findings that suggest a positive role of supportive parenting in reducing risk for alcohol use across development (e.g., Ryan et al., Reference Ryan, Jorm and Lubman2010). We note that participants included in the current analysis reported higher maternal education than the excluded participants. Because maternal education was positively correlated with maternal parenting quality in the current study (see Table 2), exclusion of adolescents whose mothers obtained lower levels of education may have attenuated the effects of parenting quality on membership into the different trajectories of alcohol use. However, it is reassuring to find the expected effect of maternal parenting quality despite the potential attenuation effect.
Somewhat unexpectedly, maternal parenting quality was associated with lower likelihood of membership in the late-onset heavy drinking trajectory relative to the developmentally limited trajectory, and maternal parenting quality did not differentiate membership in the late-onset heavy drinking trajectory and the non/light drinking trajectory. We note that individuals who were classified in the late-onset heavy drinkers group engaged in light or low levels of alcohol use during adolescence whereas individuals who followed the developmentally limited drinking trajectory engaged in moderate levels of alcohol use during adolescence. Perhaps maternal parenting quality assessed during adolescence in this study is particularly relevant for protecting against alcohol use during adolescence and thus is associated with a lower likelihood of membership in trajectories that involve higher levels of alcohol use during adolescence. Given that the late-onset heavy drinkers did not differ much from the non/light drinkers in terms of alcohol use during adolescence, it is thus not surprising that maternal parenting quality during adolescence did not differentiate membership in these trajectories. Perhaps other environmental factors that are salient to young adulthood such as employment and romantic relationships are more relevant for potentially differentiating membership in the late-onset heavy drinking trajectory versus the non/light drinking trajectory.
Findings indicated that the short allele of 5-HTTLPR was associated with higher risk for membership in the persistent heavy drinking trajectory and the late-onset heavy drinking trajectory. This is consistent with our hypothesis and prior research that the short allele of 5-HTTLPR is associated with higher risk for alcohol use and related outcomes (Kaufman et al., Reference Kaufman, Yang, Douglas-Palumberi, Crouse-Artus, Lipschitz, Krystal and Gelernter2007; Merenäkk et al., Reference Merenäkk, Mäestu, Nordquist, Parik, Oreland, Loit and Harro2011; van der Zwaluw et al., Reference van der Zwaluw, Engels, Vermulst, Rose, Verkes, Buitelaar and Scholte2010). Our findings extended the literature to provide support for the association between 5-HTTLPR and latent trajectories of alcohol use over time. Prior findings regarding the effect of 5-HTTLPR and alcohol use outcomes have been mixed. Our findings that 5-HTTLPR was associated with risk for membership in the persistent heavy and late-onset heavy drinking trajectories but not the developmentally limited trajectory suggest the importance of considering the heterogeneity of alcohol use behaviors in genetic association studies. It is likely that 5-HTTLPR may be more relevant in influencing “risky” alcohol use over time than low risk, developmentally limited alcohol use; mixed findings from prior research may be in part due to differences in sample characteristics, particularly in terms of drinking patterns, across studies.
Furthermore, findings indicated that 5-HTTLPR moderated the association between maternal parenting quality and membership in alcohol use trajectories, but the moderation effect was evident for males only. Consistent with previous research suggesting that carriers of the short allele of 5-HTTLPR are more susceptible to influences of environmental factors including parenting behaviors (Brody et al., Reference Brody, Beach, Philibert, Chen, Lei, Murry and Brown2009; Kim et al., Reference Kim, Park, Glatt, Eckert, Vanable, Scott-Sheldon and Carey2015), we found that maternal parenting quality was associated with membership in the persistent heavy drinking trajectory only for carriers of the short allele of 5-HTTLPR (among males). A closer examination of the interaction effect indicated that compared to male noncarriers of the short allele, male carriers of the short allele of 5-HTTLPR were at much higher risk for membership in the persistent heavy drinking trajectory relative to the developmentally limited trajectory and the non/light drinking trajectory when parenting quality was low, but were at somewhat lower risk when parenting quality was high, a pattern of interaction effect suggestive of differential susceptibility. Prior research has shown evidence of differential susceptibility of the short allele of 5-HTTLPR to the influence of parenting behaviors in relation to developmental outcomes such as positive affect and antisocial behaviors (Hankin et al., Reference Hankin, Nederhof, Oppenheimer, Jenness, Young, Abela and Oldehinkel2011; Tung & Lee, Reference Tung and Lee2016). Here we extend the literature by providing additional preliminary support for differential susceptibility of the short allele of 5-HTTLPR to the influence of maternal parenting quality in relation to trajectories of alcohol use from early adolescence to young adulthood.
One notable finding that emerged from the current analyses is that gender conditioned the interaction between 5-HTTLPR and maternal parenting quality in relation to trajectories of alcohol use. Previous findings regarding gender differences in the interaction between 5-HTTLPR and environmental factors have been mixed. A number of previous studies have found interaction between 5-HTTLPR and negative environmental factors (e.g., childhood maltreatment and negative life events) in relation to psychosocial outcomes such as depression and antisocial behaviors for females but not for males (Hammen et al., Reference Hammen, Brennan, Keenan-Miller, Hazel and Najman2010; Li & Lee, Reference Li and Lee2010; Vaske et al., Reference Vaske, Newsome and Wright2012), suggesting that female carriers of the short allele of the 5-HTTLPR are particularly sensitive to environmental stressors. In this study, we focused on a measure of positive environment (i.e., parenting quality) and found that the interaction between 5-HTTLPR and maternal parenting quality in relation to membership in the persistent heavy drinking trajectory was evident for males but not for females. Though preliminary, perhaps gender differences in G × E effects involving 5-HTTLPR are dependent on the environmental factors under consideration and it may be that male carriers of the 5-HTTLPR are particularly sensitive to the influence of positive environmental factors whereas female carriers of the 5-HTTLPR are more sensitive to environmental stressors. Gender differences in interaction between 5-HTTLPR and positive environmental factors have rarely been examined, and thus future research is needed to explore this possibility and replicate our preliminary findings.
Alternatively, genetic risk and G × E effects on alcohol use outcomes may be manifested differently for males and females, due to potential gender differences in social control for alcohol use. While alcohol use is common for both males and females during adolescence and young adulthood, there may be more social expectation for females than for males to “mature out” of alcohol use as they transition into adulthood and assume responsibilities associated with family such as motherhood. In the present study and others (e.g., Chassin et al., Reference Chassin, Pitts and Prost2002, Reference Chassin, Flora and King2004), females were much less likely to follow the persistent heavy alcohol use trajectory than males. This greater social control for alcohol use among adult females may have constrained the expression of 5-HTTLPR × Parenting effects on females’ likelihoods of following the persistent heavy drinking trajectory in this study.
In the current study, maternal parenting quality was on average slightly higher for males than for females. The influence of maternal parenting quality on alcohol use trajectories appeared to be stronger for females than for males overall, as suggested by the significant two-way interactions between parenting quality and gender (Table 4, Step 2). However, these differences in the levels of maternal parenting quality and its effects on alcohol use trajectories between male and female offspring were accounted for in the analysis, and thus should not confound the findings regarding gender differences in G × E effects. Future research is needed to better understand the mechanisms underlying gender differences in G × E effects.
It is noteworthy that the effects of 5-HTTLPR and 5-HTTLPR × Parenting Quality interaction on trajectories of alcohol use varied depending on the nature of the trajectory. Overall, significant genetic and G × E effects were largely found for trajectories that are considered as more problematic, such as the persistent heavy drinking trajectory and the late-onset heavy drinking trajectory. This is consistent with previous research examining genetic effects on latent trajectories of alcohol use (van der Zwaluw et al., Reference van der Zwaluw, Otten, Kleinjan and Engels2014) and marijuana use (Vaske, Boisvert, Wright, & Beaver, Reference Vaske, Boisvert, Wright and Beaver2013), as well as Moffit's (Reference Moffitt1993) proposition that problematic developmental trajectories such as life-course persistent antisocial behavior are more likely to be influenced by individual predispositions such as genotypes, whereas trajectories that are developmentally limited or less problematic are more likely to be influenced by environmental factors. These findings also highlight the utility of using a longitudinal design and taking into account the heterogeneity in trajectories of alcohol use over time in understanding G × E effects on alcohol use. Our findings suggest that the effects of 5-HTTLPR (and its interaction with other factors) on alcohol use may vary as a function of individuals’ trajectories over time and across different developmental stages. For example, we found a significant Gender × 5-HTTLPR × Parenting interaction effect in differentiating individuals’ likelihood of following the persistent heavy use trajectory relative to the developmentally limited alcohol use trajectories. If we just focus on adolescence (e.g., by analyzing data cross-sectionally), the persistent heavy users and the developmentally limited users in the current study would have looked relatively similar in their levels of alcohol use, and the genetic effect may not be found.
Our findings should be interpreted in the context of several limitations of this study. First, we used a biallelic genotyping of the 5-HTTLPR based on long and short variants. Although this approach is commonly used in previous studies (Brody et al., Reference Brody, Beach, Philibert, Chen, Lei, Murry and Brown2009; Tung & Lee, Reference Tung and Lee2016; Vaske et al., Reference Vaske, Newsome and Wright2012), it has been suggested that a single nucleotide polymorphism (SNP rs25531, A/G) in the long variant of the 5-HTTLPR might have functional significance such that the LA allele is associated with higher transcriptional activity, whereas the LG allele has transcriptional activity no greater than the short allele (Hu et al., Reference Hu, Oroszi, Chun, Smith, Goldman and Schuckit2005). This study did not examine effects of 5-HTTLPR using this triallelic genotyping based on LA, LG, and short variants because triallelic genotyping of 5-HTTLPR was not available in the data set used for this study. Future studies need to consider triallelic genotyping of 5-HTTLPR to replicate findings from this study. Second, participants reported on both perceived parenting quality and alcohol use, and thus our findings may be subject to influences of shared method variance. However, the temporal separation between parenting quality (at Wave 1) and alcohol use (at Waves 1, 2, 3, and 4) reduced the method bias to some extent (Podsakoff, MacKenzie, & Podsakoff, Reference Podsakoff, MacKenzie and Podsakoff2012). Third, this study focused on parenting behaviors of mothers. We note that maternal and paternal parenting may differentially influence male versus female adolescents’ alcohol use (Webb, Bray, Getz, & Adams, Reference Webb, Bray, Getz and Adams2002). Unfortunately, we were not able to examine the role of paternal parenting in the current study due to the lack of reliable data. Future studies need to examine how fathers’ parenting quality may interact with adolescents’ genetic predispositions (e.g., 5-HTTLPR) to predict trajectories of alcohol use over time. Fourth, although we chose to focus on examining the role of parenting (and its interaction with 5-HTTLPR) in the current study, we recognize that peers also play an important role in influencing alcohol use during adolescence and young adulthood. There has been increasing evidence that peer factors (e.g., peer deviance) moderate genetic effects on alcohol use and related outcomes (Harden, Hill, Turkheimer, & Emery, Reference Harden, Hill, Turkheimer and Emery2008, Mrug & Windle, Reference Mrug and Windle2014, Salvatore et al., Reference Salvatore, Aliev, Edwards, Evans, Macleod, Hickman and Dick2014). Future research is warranted to examine whether and how peer factors may interact with 5-HTTLPR to influence longitudinal trajectories of alcohol use. In addition, as with in all genetic analyses, there is concern for population stratification. Ancestry informative genetic markers were not available. Following recommendations of best practices, we attempted to adequately account for potential biases due to population stratification by statistically controlling for self-report race, Race × 5-HTTLPR, and Race × Parenting in our analyses, but it is possible that potential biases still remain. Fifth and finally, we only examined the effect of one single genetic variant. Complex behaviors like alcohol use are polygenic in nature and influenced by many genes with small effects (Plomin, Haworth, & Davis, Reference Plomin, Haworth and Davis2009). Future research can extend the current study to use a genomewide polygenic risk score approach to capture genetic risk for alcohol use and examine how aggregate genetic risk may interact with important environmental factors to influence trajectories of alcohol use over time.
In conclusion, despite these limitations, this study advanced research on etiology of alcohol use in several ways. First, using an accelerated longitudinal design of a large-scale, nationally representative sample, this study identified distinct trajectories of alcohol use across a wide developmental life span (age 13 to age 32). Second, our findings provided support for the independent effects and the interaction between genetic variants (i.e., 5-HTTLPR) and environmental factors (i.e., parenting quality) in predicting membership of alcohol use trajectories across development. These findings highlighted the importance of considering the heterogeneity in developmental trajectories of alcohol use in the study of G × E in alcohol use. Third, this study considered a positive environmental factor (in contrast to the focus of negative environmental factors in the majority of prior G × E research) and provided evidence suggestive of differential susceptibility of the short allele of 5-HTTLPR. Fourth and finally, this study demonstrated the importance of considering gender differences in G × E effects in relation to alcohol use outcomes and suggested that G × E effects on trajectories of alcohol use may differ across adolescent gender. Future studies need to consider the heterogeneity of alcohol use trajectories and potential gender differences in understanding how genetic and environmental factors interplay in predicting alcohol use across development.
Supplementary Material
To view the supplementary material for this article, please visit https://doi.org/10.1017/S095457941800024X.