Introduction
In the early 1990s, advances in the biotechnology of fish reproduction were consolidated by using efficient protocols for semen cryopreservation. Currently, enhancing reproductive biotechnologies for the cryopreservation of fish embryos is a promising field in aquaculture science and fish farming technology.
Cryopreservation has the objective of both maintaining the quiescent cell metabolism and preserving the fish embryos for a long time. Effective cryopreservation protocols could enlarge the farming acreage, improve the management of endangered species, preserve rare species (Wildt et al., Reference Wildt, Seal, Rall, Cloud and Thorgaard1993), supply fish embryos to farmers in the periods of irregular spawning (Janik et al., Reference Janik, Kleinhans and Hagedorn2000), increase the commercial production (Ballou, Reference Ballou1992), and maintain the genetic diversity. Hagedorn et al. (Reference Hagedorn, Hsu, Kleinhans and Wildt1997a) highlighted the importance of the egg size, vitello granules, permeability of embryo membranes, and eggs highly sensitive to low temperatures as the main characteristics hindering the cryopreservation.
Currently, every fish species is cryopreserved using intracellular, extracellular or both types of compounds before submitting the embryos to subzero temperatures. Intracellular cryoprotectants are organic solutes with a low molecular weight that protect the organelles without significant toxicity during the freezing period because of their easy permeation throughout the membranes. Methanol, ethanol, propylene glycol ethylene glycol, dimethyl sulphoxide (DMSO) and glycerol, for example, have these activities. Otherwise, the extracellular cryoprotectants are macro-molecules or sugars with high molecular weight that are capable of reducing the probability of ice formation (Fornari et al., Reference Fornari, Ribeiro, Streit, Vargas and Moraes2010). Sucrose, glucose, lactose and trehalose have these activities (Denniston et al., Reference Denniston, Michelet, Godke, Tiersch and Mazik2000). Usually, compounds with fast permeation throughout the membranes are the best cryoprotectants because they reduce the time of exposure before the freezing period and prevent osmotic injuries (Kasai, Reference Kasai1996). The strategic application of safe cryoprotectants and the combination of both types of substances have been used to reduce the morphological injuries (Kasai, Reference Kasai1996; Vajta, Reference Vajta2000).
In Brazil, the pacu (Piaractus mesopotamicus) is a fast growing and rustic neo-tropical species that is adapted easily to farming conditions and artificial feeding. Currently, the pacu is the second native species raised under on-farm conditions because of its large acceptance by consumers and the excellent farming characteristics for large acreages (Castagnolli & Zuim, Reference Castagnolli and Zuim1985; IBAMA, 2007). The pacu has been an excellent model to participate in this type of investigation because of its rheophilic behaviour. Thus, the objective of this study was to evaluate morphological injuries on P. mesopotamicus embryos submitted to freezing and thawing after the application of intra- and extracellular cryoprotectants.
Materials and methods
Location
The experiment was conducted in the Fish Farming Station at the Universidade Estadual de Maringá (UEM/CODAPAR), Estação de Hidrologia e Aqüicultura at DUKE ENERGY INTERNATIONAL – Geração Paranapanema, Salto Grande (SP), Laboratory of Histochemical Analyses in the Department of Morphology and Physiology, Brasil in collaboration with the Complexo Central de Apoio à Pesquisa (UEM-COMCAP).
Fish embryos
Ten 4-year-old males and females of pacu (P. mesopotamicus) were selected randomly from the parental stock raised at the Duke Energy Station. At 9.5 h of incubation, when 80% of the eggs developed blastopore closure, a sample of 1500 fertile eggs was collected in the incubator, excess water was eliminated, and then 40 fish embryos were immersed into their respective cryoprotectant solution (treatment).
Cryoprotectant solutions
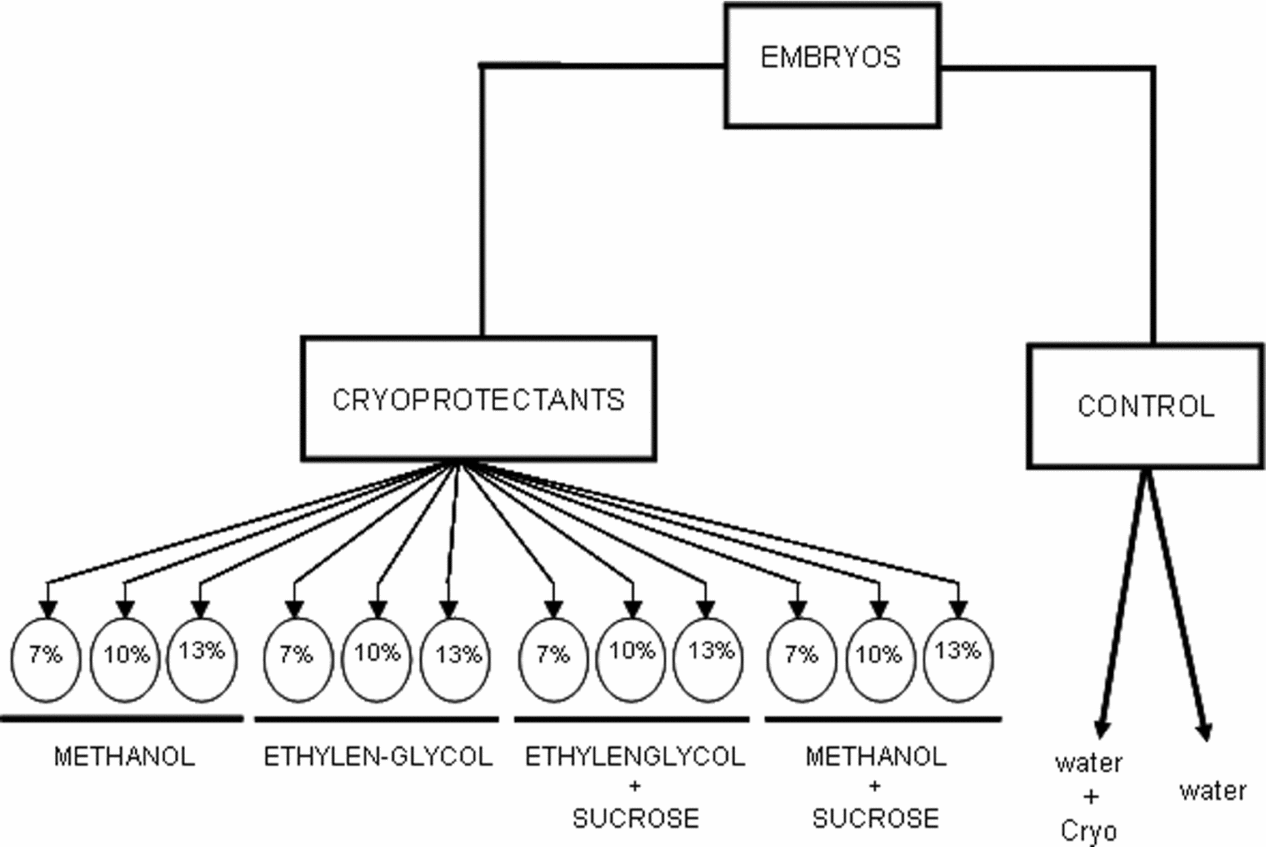
Methanol and ethylene glycol, both at 7, 10 and 13% plus 0.1 M sucrose for 10 min (Zhang & Rawson, Reference Zhang and Rawson1995) and two control treatments with only distilled water or aqueous sucrose solution were prepared under a temperature of 27.5 ± 1.0°C (Fig. 1).

Figure 1 Treatments in which the embryos of pacu (P. mesopotamicus) were incubated for 9 h, and immersed for 10 min into intracellular cryoprotectants (methanol and ethylene glycol) plus two controls with the extracellular 0.1 M sucrose or only water.
Cryopreservation
After every treatment, the embryos were frozen in liquid N2. Every treatment had 40 fish embryos distributed in four palettes of 0.5 ml with 10 embryos submitted to the Bio-cool (BIO-COOL III FITSSYSTE MS, INC) under a temperature of –7°C. The freezing curve was established at 1°C/min down to –33°C. Soon after, they were stored in liquid N2 for 40 days and thawed using the following thawing protocol. Every palette was submitted to the thawing process using water bath at 45°C for 10 min. Soon after, excess cryoprotectant was gradually eliminated and the embryos were rehydrated. During the thawing process, all cryoprotected embryos were submitted to concentrations from 6, 4, 2 to 1% methanol plus ethylene glycol and finally distilled water for 30 s. One pallet from every treatment was incubated at 27°C for 15 h to verify larvae development.
Morphological evaluation
Optical microscopy
Soon after rehydration, the embryos were immersed into ‘Bouin’ solution, treated with 2-hydroxyethyl methacrylate (Leica historesin). Three embryos per treatment were sectioned in serial slices measuring 3 μm in thickness (six cuttings per embryo) and dyed using the hematoxylin–eosin (H-E) method. The morphological analysis was carried out using an optical microscope Olympus model CBA, and the images were photographed by a high resolution Olympus camera, model Q-Color 3 assembled under the microscope Olympus Bx41.
Scanning electron microscopy (SEM)
Four embryos from T1 (water), T2 (sucrose solution), T3 (10% methanol plus sucrose), T4 (10% methanol), and T5 (10% ethylene glycol plus sucrose) were prepared for scanning electron microscopy. Furthermore, two embryos without the freezing treatments and two embryos frozen and thawed were prepared for scanning microscopy. These embryos were fixed in 2.5% glutaraldehyde, 0.1 M of the cacodylate buffer at pH 7.2 and stored in the refrigerator for dehydration at 30, 50, 70, 80, 90, 95% ethanol for 30 min, and more two baths with 100% ethanol for 30 min.
Next, the samples were submitted to the Critical Point Dryer (BAL-TEC CDP 030) which uses liquid and gaseous CO2 for total dehydration. Finally, they were coated with gold–palladium alloy in the metal stubs of the Metal Desk II Denton vacuum, and the scanning electron micrographic images were used to detect the normal development or the injuries that were photographed with the scanning electron microscope JEOL (JSM-540).
Results and Discussion
The embryos showed severe symptoms of morphological injuries during the thawing and rehydration processes. Fish embryos from the controls with only distilled water or sucrose solutions did not overcome the hydration process and were completely destroyed by the cryoprotectant solutions. The difficulties of achieving a sufficient number of embryos for the historesin treatments were the same as those reported by Robles et al. (Reference Robles, Cabrita, Real, Alvarez and Herraez2003) for Scophthalmus maximus and Ninhaus-Silveira et al. (Reference Ninhaus-Silveira, Foresti and Azevedo2006) for Prochilodus lineatus. None of the eggs hatched after the incubation period of 15 h because almost of them died, and the whitish vitello indicated the presence of ice formation. All thawed embryos had the chorion, blastoderm, vitello and vitelline syncytial layer morphologically injured.
Chorion
The chorion shape was irregular and showed ruptures in all the embryos (Fig. 2A,B). This membrane has been the main barrier to impede cryoprotectant permeation (Hagedorn et al., Reference Hagedorn, Kleinhans, Artmov, Artmov and Pilatus1998). As the embryos of Prochilodus lineatus, for example, developed very well when the chorion was eliminated by the pronase enzyme (Ninhaus-Silveira et al. Reference Ninhaus-Silveira, Foresti, Azevedo and Augustine2007), this membrane might be unnecessary because it just protects the embryo against mechanical impacts.

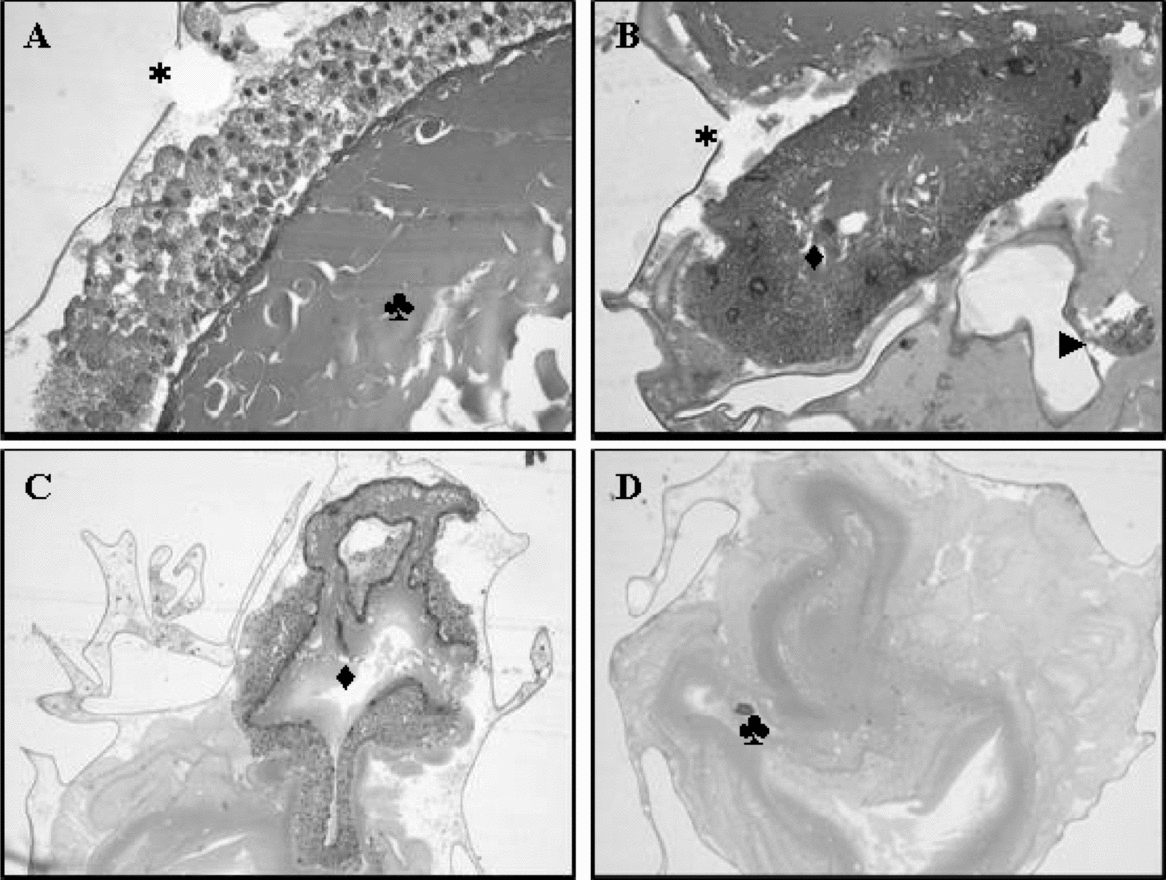
Figure 2 Frozen embryos of Piaractus mesopotamicus. (A) Cellular mass appearance of the vitello (♣), chorion disruption (*) (×40). (B) CSV involving parts of the vitello (♦), small portion of the blastoderm (►) (×20). (C) CSV with different thickness (♦) (×10). (D) Absence of the blastoderm, and the cellular mass appearance of the vitello (♣) (×20). Haematoxylin–eosin staining.
Vitello
The vitello was seen as a mass of cells with amorphous appearance, and its contents leaked because the granules lost their individualization. Furthermore, parts of the vitello were spread all over the morphological structures of the embryos, and there was no precise site to observe this compartment (Fig. 2A,D). We hypothesized that the lesions in the cell membranes of the vitello was incited by their sensitiveness to subzero temperatures, insufficient volume and time of permeation, and the unsafe levels of the intracellular cryoprotectants, for example.
These responses contrast with those from Brachydanio rerio that tolerated subzero temperatures after treatment with DMSO and propylene glycol where the embryos had the vitello with normal morphology, intact membranes and full contents (Hagedorn et al. Reference Hagedorn, Kleinhans, Artmov, Artmov and Pilatus1998). These authors identified that the main local of damage could be the vitello after identifying the barrier to the DMSO and propylene glycol and quantifying the parameters for water and solute permeation through volumetric changes detected by optical microscope and nuclear magnetic resonance spectroscope. In the present experiment, therefore, the vitello was destroyed by ice formation because the inner protection failed in preserving this compartment.
Syncytial vitelline layer (SVL)
Irregular size and shape was detected in the membranes of the syncytial vitelline layer. They were found at atypical locations as below the vitello or mixed in its parts (Fig. 2B,C) as reported by Hagedorn et al. (Reference Hagedorn, Kleinhans, Artmov, Artmov and Pilatus1998). These authors who used the vitrification protocol with high concentration of cryoprotectants and ultra-fast exposure time observed morphological modifications during the freezing, and saw organelles and membranes destroyed after the thawing. Only some membranes and nuclei with dense and inactive chromatin were not found to have their full shape.
The syncytial layer of the vitello had a more electron-dense cytoplasm, compact and collapsed reticules, nucleus with disorganized chromatin, and ruptures in the plasmatic membrane close to the endoderm, and consequently damaged microvilli.
The embryos under freezing had unstructured cytoplasm, a destroyed endo-membranes net, altered chromatin nucleus but had preserved mitochondria as reported by Ninhaus-Silveira et al. (Reference Ninhaus-Silveira, Foresti, Azevedo and Augustine2007) who evaluated the syncytial vitelline layer of curimbatá (P. lineatus) for ultrastructural modifications of frozen embryos.
Blastoderm
Nuclei with altered shape were the most frequent injury in the blastoderms, but in some embryos there were no nuclei or they had irregular shapes. They were found in atypical regions while in others they were absent or in small quantities with cytoplasm presence, but without nuclei (Figure 2B,D). Hagedorn et al. (Reference Hagedorn, Kleinhans, Artmov, Artmov and Pilatus1998) verified that the blastoderm of zebra fish embryos was destroyed because of the insufficient entrance of the cryoprotector into the cells.
Cryopreservation X injuries
As Hagedorn et al. (Reference Hagedorn, Hsu, Kleinhans and Wildt1997a, Reference Hagedorn, Kleinhans, Weidt and Rallb) and Streit Jr. et al. (Reference Streit, Digmayer, Ribeiro, Sirol, Moraes and Cock2007) observed less toxicity at 10% methanol plus sucrose, scanning electron microscopy was only evaluated on the embryos submitted to this concentration. Figure 3A–D was obtained from embryos submitted to intra- and extracellular cryoprotectants only, and Fig. 4A–D was obtained from cryoprotected embryos after freezing and thawing. The chorion was torn in most of the embryos (Fig. 4A–D), but in some of them it started to pull apart even in the beginning of the hydration period, and we detected only chorion scraps at the end. The structural alterations in the vitello of several embryos were leaked granules (Fig. 4A,C). The partial preservation of the vitello structure can be related to insufficient permeation of cryoprotectants due to ice formation and rupture of the vitello. Figure 4C shows an embryo completely destroyed and where parts of the chorion are spread. In this case, identification of the preserved structures was impossible.

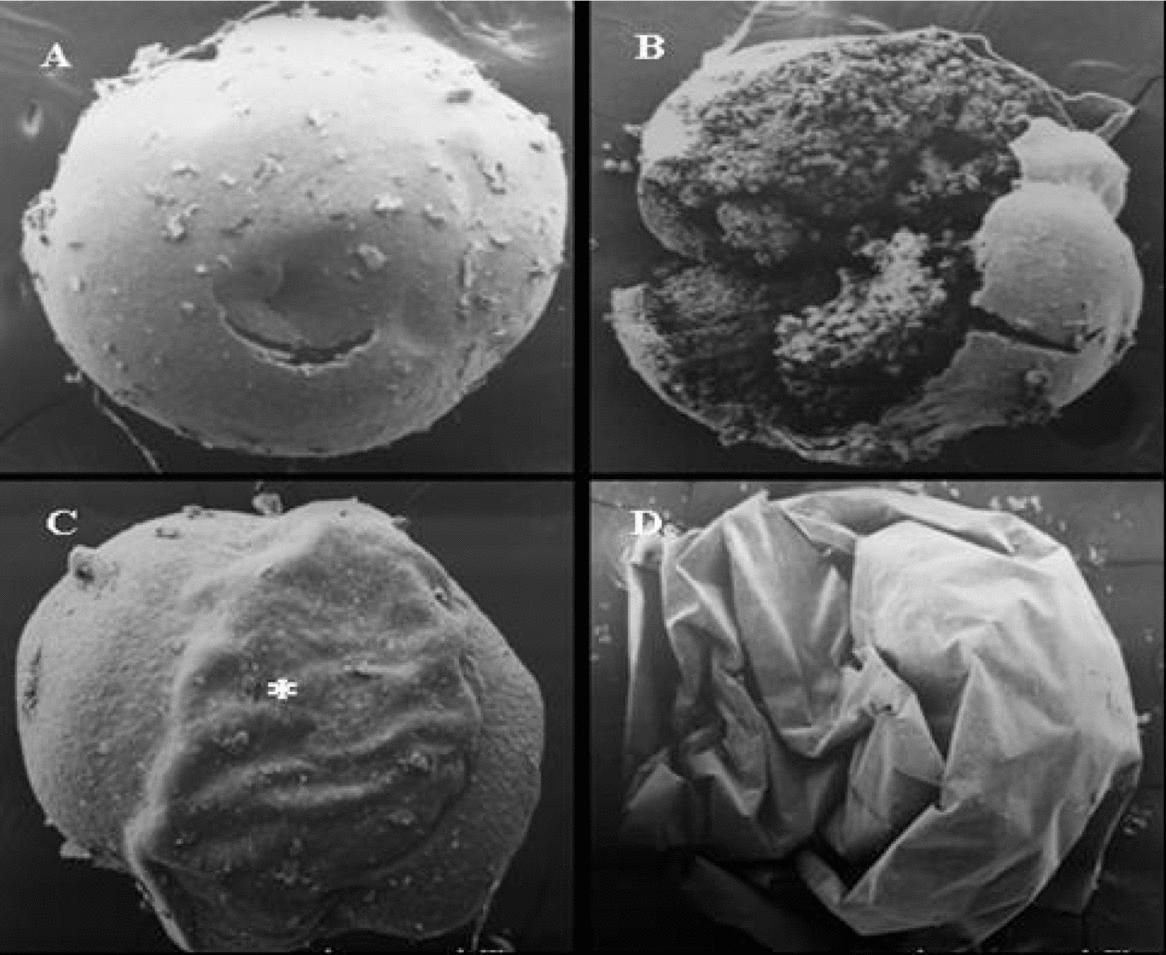
Figure 3 Scanning electron micrographic image of non-frozen pacu embryos. (A) Evidence of the normal vitello with a small hole made by the manipulation (×39). (B) Mechanical damage in the vitello showing the granules of the vitello (×80). (C) (*) Evidence of the embryo involving the vitello (×40). (D) Pacu embryo involved by the chorion (×80).

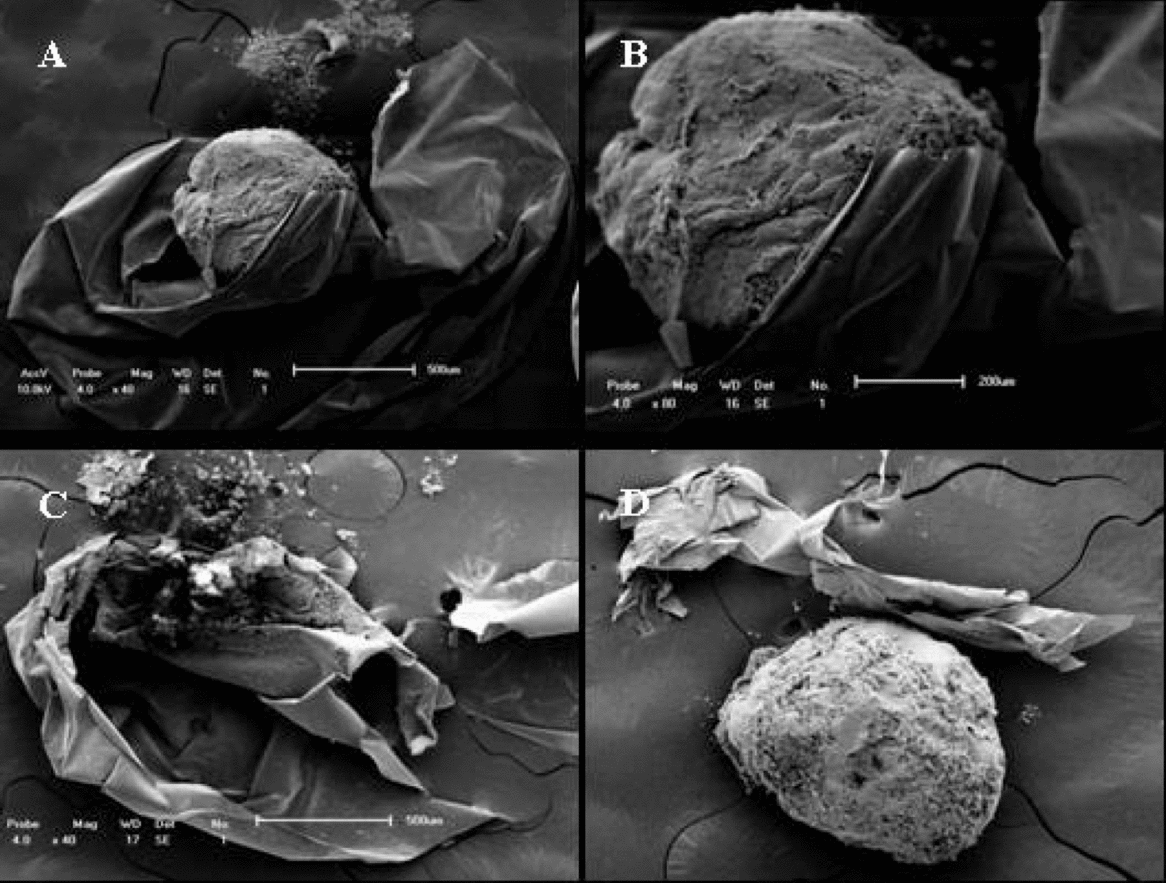
Figure 4 Scanning electron micrographic images of frozen embryos of P. mesopotamicus. (A) Vitello involved by the disrupted chorion with the leaked granules (×40). (B) Vitello involved by the disrupted chorion (×80). (C) Evidence of the destroyed embryo (×40). (D) Destroyed chorion (×40).
Ice crystals were observed even before the rehydration process because of the whitish appearance of the embryos. This fact is corroborated by Mazur (Reference Mazur1984), who reported the lethality of intracellular ice formation about –10°C in treatments without cryoprotection, and Hagedorn et al. (Reference Hagedorn, Peterson, Mazur and Kleinhans2004) who reported temperatures ranging from –20 to –30°C for treatments under cryoprotection.
In Prochilodus lineatus, Ninhaus-Silveira et al. (Reference Ninhaus-Silveira, Foresti, Azevedo and Augustine2007) observed an opaque vitello during the hydration period because of intra-embryonic ice formation (Zhang et al. Reference Zhang, Rawson and Morris1993; Zhang & Rawson, Reference Zhang and Rawson1996; Fornari et al. Reference Fornari, Ribeiro, Streit, Vargas and Moraes2010). This fact may indicate the absence of cryoprotectant penetration or concentration and time incapable of protecting the embryo. As the cryopreservation can be extremely lethal to the cellular organization, Dobrinsky (Reference Dobrinsky1996) reported that the intracellular ice can injure the plasmatic membrane and the liquid N2 can denature intracellular functions, organelles, destroy the cytoskeleton with severe modification in the intracellular shape and, finally, the complete cell destruction.
Therefore, preventing ice formation is the prime condition for successful cryopreservation. Presence of intracellular ice formation and embryo survivorship are negatively correlated, and reinforce the presence of ice as the main factor capable of hampering embryo survival (Harvey, Reference Harvey1983).
Conclusion
The present protocols did not avoid intracellular ice formation, and these morphological injuries during the freezing and thawing processes made these embryos of P. mesopotamicus unfeasible for hatching.
Acknowledgements
The authors are thank to the Estação de Hidrologia e Aqüicultura of the DUKE ENERGY INTERNATIONAL – Geração Paranapanema for providing the facilities where the present experiment was carried out, the Universidade Estadual de Maringá (UEM) and the Research Group PeixeGen for human resources, the CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) and CNPq (Conselho Nacional de Pesquisa) for economic support.