INTRODUCTION
The continental shelf and shelf-slope waters are the most productive areas in terms of world fisheries catches and hold some of the highest levels of marine biodiversity (Pauly et al., Reference Pauly, Christensen, Dalsgaard, Froese and Torres1998, Reference Pauly, Christensen, Guénette, Pitcher, Sumaila, Walters, Watson and Zeller2002, Reference Pauly, Alder, Bennett, Christensen, Tyedmers and Watson2003). However, within these environments, the rocky areas are difficult to sample using bottom trawling or other types of nets, which can be easily destroyed and lost. Under this context, natural predators can be used as biological samplers to characterize the biodiversity and the trophic structure of the ecosystems (Clarke, Reference Clarke2006). Indeed, they may identify potential ocean resources that need to be preserved and managed.
The European conger eel Conger conger (Linnaeus, 1758) is a common fish in the north-east Atlantic, Mediterranean and western Black Sea (Bauchot & Saldanha, Reference Bauchot, Saldanha, Whitehead, Bauchot, Hureau, Nielsen and Tortonese1986), and is commercially important in the central and eastern Atlantic, including Portuguese waters (Tregenza et al., Reference Tregenza, Berrow, Hammond and Leaper1997; Morato et al., Reference Morato, Sola, Gros and Menezes1999; Machado et al., Reference Machado, Gordo and Figueiredo2004). There, the conger eel is one of the most abundant predators that inhabit the continental shelf and the rocky shelf-slope areas. This species is a large opportunistic predator and, although its diet is poorly studied, it is thought to feed on a wide range of taxa, including crustaceans, fish and cephalopods (Cau & Manconi, Reference Cau and Manconi1984; Morato et al., Reference Morato, Sola, Gros and Menezes1999; O'sullivan et al., Reference O'sullivan, Moriarty and Davenport2004). Moreover, conger eels live in a wide depth range (Mytilineou et al., Reference Mytilineou, Politou, Papaconstantinou, Kavadas, D'Onghia and Sion2005) and, due to its ecology (i.e. living and foraging close to rocky areas, where they have a degree of fidelity to obtain refuge in rocks), the prey items come from the area where the fish are collected. Despite their commercial importance, information on the feeding ecology of conger eels in the north-east Atlantic is scarce.
In the marine waters of the Algarve (southern Portugal), little is known about the local biodiversity and marine trophic interactions (Neiva et al., Reference Neiva, Coelho and Erzini2006) despite those waters supporting major fisheries (Borges et al., Reference Borges, Erzini, Bentes, Costa, Gonçalves, Lino, Pais and Ribeiro2001). As the feeding patterns of marine species (in particular generalist predators) may provide valuable information from an ecological and fisheries conservation point of view, the main goal of the present study is to assess the feeding ecology of conger eels and collect further information on the marine communities and biodiversity inhabiting the rocky areas of the continental shelf and shelf-slope waters of the Algarve (southern Portugal). The study focused on the feeding habits of conger eels in order to: (1) identify trophic links in rocky shelf/shelf-slope areas; (2) provide new insights into feeding and foraging strategies of C. conger in comparison with previous diet studies; and (3) discuss potential competition between natural predators and local fisheries for prey.
MATERIALS AND METHODS
Fieldwork was carried out in southern Portugal (Algarve region), off Sagres (37°01′N 8°58′W) on board a commercial bottom longliner, between May 2005 and August 2006. The fishery operates with daily trips, and sampling took place on 3–6 successive trips every three months. A total of 14 trips was conducted. The fishing gear was deployed up to 3 hours before dawn (up to 1500 hooks) and the gear haul always started at dawn, through the morning. This procedure lasts 3–5 hours.
While onboard, all individuals of Conger conger with commercial value in the catch, defined by their size, were measured and dissected immediately. The stomachs were placed in labelled bags and preserved on ice until the return to the laboratory. All smaller individuals (<90 cm total length) were also separated from the catch and retained, but because they had no commercial value, they could be preserved whole for later analysis in the laboratory.
The stomach samples were weighed and the overall mass was recorded. All components were then sorted into categories (cephalopods, crustaceans and fish) and weighed. The cephalopod beaks were identified according to Clarke (Reference Clarke1986) and the reference collections held at the Centre of Marine Sciences, Portugal and at the Centre d'Etudes Biologiques de Chizé (CEBC–CNRS), France. The lower rostral length (LRL, mm) for squid and lower hood length (LHL, mm) for octopods were measured with Vernier callipers to the nearest 0.1 mm. Allometric equations, of mantle length (ML, mm) and estimated mass (M, g), for cephalopods were taken from the literature (Clarke, Reference Clarke1986; Lu & Ickeringill, Reference Lu and Ickeringill2002) or from relationships based on individuals caught in Algarve waters:




The fish (when in good condition) and fish otoliths were identified using Härkönen (Reference Härkönen1986), Whitehead et al. (Reference Whitehead, Bauchot, Hureau, Nielsen and Tortonese1986), Hecht (Reference Hecht1987), Smale et al. (Reference Smale, Watson and Hecht1995), Assis (Reference Assis2004), Campana (Reference Campana2004) and the reference collections held at the Centre of Marine Sciences, Portugal and at the Faculty of Sciences of the University of Lisbon, Portugal. The fish size/otolith size relationships used were those of Assis (Reference Assis2000), where OL = otolith length (mm), SL = standard length (mm) and M = body mass (g) of the fish:










The mass of prey fish species for which no regression equations between otolith size and fish size were available was estimated from equations established for closely related species/genus/families. The number of fish prey was estimated from the number of intact crania (containing both sagittae otoliths) and from loose otoliths. These loose sagittae otoliths were compared with each other (i.e. right otolith compared with left otolith by size and level of erosion) and paired if possible, following the procedure in Xavier et al. (Reference Xavier, Croxall and Reid2003).
The bait used by longliners was mostly the sardine Sardina pilchardus (Walbaum, 1792) but the chub mackerel Scomber japonicus and the Atlantic mackerel Scomber scombrus (Linnaeus, 1758) were also used. Therefore, any food items that, based on the best evidence, could be identified as bait, were not included in the diet analysis. Differentiation between bait and non-bait was through: (i) comparison of species and sizes of bait used on the day of fishing with individuals found in the stomachs; (ii) by the process of baiting—fishermen take the bait tails off; and (iii) the level of digestion. Similarly, evidence of double-catch (i.e. fish found in the stomachs of conger eels that had already been caught by the longline; conger eels frequently attacked fish already caught in the longline and were themselves consequently caught. When analysing the stomach of the conger eel, this fish would be found with a hook in its mouth) was not included. The crustaceans were identified using Falciai & Minervini (Reference Falciai and Minervini1995) and Zariquiey-Alvarez (Reference Zariquiey-Alvarez1968).
The indices used to characterize diet of conger eels were frequency of occurrence (number of stomach samples with a certain prey present divided by the number of stomach samples containing food analysed), the proportion by number of prey (number of prey of a certain species divided by the total number of prey in sampled stomachs) and by estimated mass (g, both for all taxa—mass of all individuals of a certain prey species divided by the total estimated mass of all prey; and per component—mass of all individuals of a certain prey species within a diet component, such as cephalopoda, fish or crustaceans, divided by the total estimated mass for all prey within that component), following Xavier et al. (Reference Xavier, Rodhouse, Purves, Daw, Arata and Pilling2002). Stomach fullness index (SFI) was calculated following Okach & Dadzie (Reference Okach and Dadzie1988):
The composite index of relative importance (IRI) for each prey was calculated according to Cortés (Reference Cortés1999):

Diet diversity was quantified using the Shannon–Wiener index (H′) (Shannon & Weaver, Reference Shannon and Weaver1949), using the numeric proportion of prey and the estimated mass. Values of H′ range between 0, meaning that predator feeds only on one prey species, and 1.45 (log (1/number of taxa present in the diet of predator), meaning that predator has no preference on prey species (i.e. prey species are evenly represented in the diet).
The trophic level of C. conger was determined according to Cortés (Reference Cortés1999) and Pauly & Christensen (Reference Pauly and Christensen1995):

Minitab statistical software (Sowers Printing Company, PA, USA) was used for statistical analysis (i.e. correlations and analysis of variance statistical tests). Test assumptions were verified before applying parametric tests. Values are given as means±standard error (SE) unless stated otherwise.
RESULTS
Diet of conger eels off the Algarve
A total of 342 individuals of Conger conger (size range: 550–1900 mm; 1011±16 mm total length) were caught in rocky shelf/shelf-slope areas off the Algarve (depth range: 90–580 m; 358±9 m) and analysed. Of these, 27.8% of the individuals examined contained food. No signs of prey regurgitation by conger eels were found. Double-catch occurred in 3.2% of the stomachs of conger eels studied (i.e. N = 11 prey individuals were clearly caught by the longline and conger eels were caught as a result of ingesting these prey), of which ten of these individual prey were of the species Helicolenus dactylopterus dactylopterus and the remaining individual of the species Beryx decadactylus. The overall mass of the stomach contents (with food) ranged from 0.3 g to 165 g (24±4 g), with a low stomach fullness (SFI), ranging between 0.01 and 12% of the total mass of the fish (1.5±0.3%). The Shannon–Wiener index values applied to the conger eels' diet were relatively high (Table 1), showing that these fish feed on a wide range of prey species; a total of 29 prey taxa were identified (Table 1). However, the values of the IRI were coincident with the contribution in terms of mass, emphasizing the importance of two species: Scomber japonicus (% IRI = 24.3%) and C. conger (% IRI = 16.0%), which together contributed 32.3% of the mass (Table 1). The estimated trophic level of C. conger was 3.6.
Table 1. The diet of European conger eel Conger conger off the Algarve (southern Portugal). Frequency of occurrence and number of individuals of each prey item found in the non empty stomachs (*, benthic; +, benthopelagic; #, pelagic).
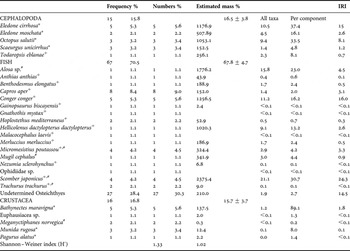
IRI, index of relative importance.
Fish, cephalopod and crustacean components of the diet
Conger eels fed mainly on fish (67.8±4.7% by mass), followed by cephalopods (16.5±3.8%) and crustaceans (15.6±3.7%) (Table 1) and each individual had fed on up to two prey individuals (1.1±0.03 items per stomach when not empty) at the time of capture. Fish were also the most frequent prey component in the diet of conger eels, occurring in 70.5% of the stomachs that contained food (Table 1). The most numerous (identifiable) fish was Capros aper, occurring in 8.4% of the stomachs that contained food. Due to its small size, this species only represented 1.4% of the diet of the mass. The second most important prey by number were the octopod Eledone cirrhosa, the crustacean Bathynectes maravigna (Prestandrea, 1839) and small C. conger (5.6% in numbers), showing that conger eels are cannibalistic. The benthopelagic fish Micromesistius poutassou and S. japonicus were present in 4.2% of the stomachs, with the latter being the most important fish in mass (21.1%; Table 1).
Cephalopods occurred in 15.8% of the stomach contents. Among them, the octopodids dominated the cephalopod component of the diet of conger eels, with E. cirrhosa as the most important cephalopod in numbers (5.6%) and in mass (10.5%) (Table 1). The only squid species found in the diet was the squid Todaropsis eblanae (1.1% in numbers and 2.3% in mass).
The crustacean B. maravigna dominated the crustacean component of the diet of conger eels, which is present in 5.3% of the samples, but representing only 1.2% in mass.
Size relationships between conger eels and their prey
The size of C. conger individuals were larger (1016±48 mm total length) than cephalopods (range: 71–157 mm ML; 100±10 mm) and fish (range: 11–772 mm SL; 206±44 mm) present in the diets, with no signs of scavenging prey (Table 2). Indeed, a positive relationship was found between the body mass of conger eels and the standard length of fish (r 2 = 0.51, F1,20 = 7.06, P = 0.02) and fish mass (r 2 = 0.75, F1,20 = 25.67, P < 0.01; Figure 1). However, no significant differences were found between the body mass of conger eels and the mantle length (r 2 = 0.52, F1,6 = 2.2, P = 0.19) and mass of cephalopods (r 2 = 0.21, F1,6 = 1.61, P = 0.25; Figure 2).
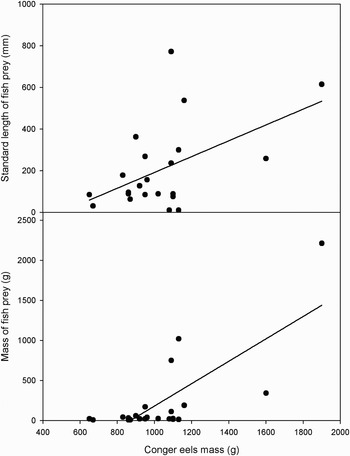
Fig. 1. Relationship between conger eels' sizes and their fish prey in north-eastern Atlantic waters (the Algarve) using prey standard length (conger mass = 0.68 × prey standard length+895.7) and mass (conger mass = 0.40 × prey mass+943.3).

Fig. 2. Relationship between conger eels' sizes and their cephalopod prey in north-eastern Atlantic waters (the Algarve) using prey mantle length (conger mass = 4.61 × prey mantle length+545.1) and mass (conger mass = 1.29 × prey mass+779.8).
Table 2. Measurements of lower rostral length (LRL, mm) and lower hood length (LHL, mm) of cephalopods and otolith length (OL, mm) of prey present in the diet of conger eels.

Catches of conger eels in the fishery
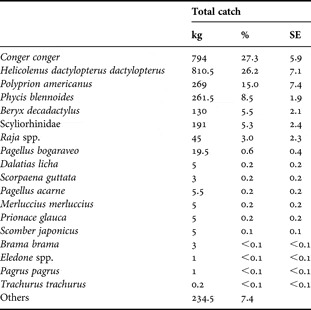
During the study, a total of 14 trips (i.e. 14 days) were carried out. Out of those, on 12 trips the total catch (by mass) of all species by the fishing vessel were collected (Table 3). More than 18 taxa were caught (Table 3). Conger conger was the main fished species, corresponding to 27.3±5.9% of the total catch in terms of mass (Table 3). Along with the blackbelly rosefish Helicolenus dactylopterus dactylopterus (26.2±7.1% of the total catch), corresponded to more than half of the catches (Table 3). Of the 18 taxa present in the overall catches, 33% of the species were also present in the diet of C. conger (Tables 1 & 3), although not all items from the fishery and from the diet could be identified to species level. The most important species in the diet of C. conger (i.e. M. poutassou and Capros aper) were not caught by the longlining fishery.
Table 3. Total catch of the commercial fishery for 12 days of fishing off the Algarve between May 2005 and August 2006. The part of the catches named ‘others’ include commercial species with no legal size (e.g. Conger conger) and fish with no commercial value (e.g. deep-sea sharks Etmopterus spp.).
DISCUSSION
Advantages and limitations of using conger eels to assess trophic interactions
Bottom longlining is a passive fishing method relying on attraction to baited hooks. Consequently, longlining might select for individuals that are still actively foraging (i.e. individuals not satiated) which could explain why the proportion of stomachs with food (28%) and the fullness of stomachs (i.e. only up to 12% of the total mass of the fish) was low. Despite this, numerous prey were found in very good condition in the stomachs, which gives a good insight into what has been recently consumed and allows a description of trophic interactions in the study area.
Could the diet of fish (such as conger eels) differ according to the fishing gear used? A recent study comparing the diets of deep-sea sharks Etmopterus pusillus (Lowe, 1839) (C. Antunes, personal communication) caught with bottom longlining and bottom trawling from the same study area showed that no differences in the diet existed between fishing gear. Indeed, these shark species live at the same depth as conger eels, have similar foraging behaviour and a similar diet, with fish dominating. Therefore, despite fishing gear having particular sampling problems, it is likely that the diet of conger eels is well characterized using longlining. The present study on the feeding ecology of Conger conger is a first step towards the understanding of the marine food web (centred on C. conger) in the area.
Feeding and foraging strategies of conger eels
The present study shows that conger eels are useful predator indicators of some of the trophic interactions in rocky shelf/shelf-slope areas in Algarve waters. Of the total 29 prey taxa found in the diet, 21 taxa are actively swimming species and correspond to 46% of the prey individuals (73% in mass) (Table 1). The majority of the fish prey taxa have a demersal or benthopelagic behaviour, except for Mugil cephalus and Alosa sp., which are pelagic fish (Whitehead et al., Reference Whitehead, Bauchot, Hureau, Nielsen and Tortonese1986), suggesting that C. conger forage mostly in the water column (Table 1). Accordingly, O'sullivan et al. (Reference O'sullivan, Moriarty and Davenport2004) indicated that conger eels are not primarily dependent on benthic organisms but take fish from the water column as well. However, the presence of eight benthic octopods and crustaceans, representing 29% in numbers of prey and 32% in mass (Table 1), also shows that benthic prey are a good energy source for conger eels in Algarve waters.
Conger eels consume a wide range of prey, including cephalopods, crustaceans and fish. They are also cannibalistic (Table 1). The fact that no prey species clearly dominates in terms of numbers (Table 1), suggests that conger eels are opportunistic in Algarve waters. Elsewhere, in Irish waters for example, conger eels may be considered as specialist feeders, as they tend to feed mostly on 2–3 fish species (O'sullivan et al., Reference O'sullivan, Moriarty and Davenport2004). Such contrasting diets show the adaptability of this fish species which might partially explain its wide distribution in the north-east Atlantic.
Diet composition of conger eels in relation to other studies and locations
To our knowledge, this is the first study to report, to a species level, the diet of conger eels in Algarve waters, which complements the diet data available from elsewhere in the north-east Atlantic (Table 4). The only comparable study in the Algarve (Santos & Borges, Reference Santos and Borges2001), on the diet of C. conger, characterized the prey components only (i.e. as crustaceans, fish or cephalopods and not to species level), and showed that C. conger fed mostly on crustaceans (54% in mass) and fish (40% in mass) (Table 4). In contrast, the results of the present study, and of previous diet studies (from the Mediterranean, Irish waters and from the Azores), show that fish are the most important component in the diet of conger eels in those regions (range: 68–96% in mass; Tables 1 & 4).
Table 4. Composition by mass of the main prey components of the diet of conger eels in comparison with other studies.

Of the prey consumed by conger eels in Portuguese waters (the Algarve and the islands of the Azores), Capros aper, Helicolenus dactylopterus and Scomber japonicus occurred in both studies (Morato et al., Reference Morato, Sola, Gros and Menezes1999; present study). Also in both these studies, C. aper was the most important species numerically (17% in Algarve and 41% in the Azores) (Morato et al., Reference Morato, Sola, Gros and Menezes1999; present study, respectively), suggesting that C. aper is an abundant prey species to conger eels in Portuguese waters.
Understanding food web interactions in the north-east Atlantic using conger eels
The present study shows that octopodids can play a more important role in the diet of conger eels than previously thought. A total of four species of octopodids occurred in the diet of conger eels off the Algarve, which corresponded to 93% of the cephalopods (Table 1). Could this reflect the availability (and relative abundance) of octopodids in Algarve waters or a specific feeding strategy of conger eels? In Algarve waters, conger eels are opportunistic feeders, which suggest that conger eels feed on what is available to them at a given time. The occurrence of the octopod species found in the diet of conger eels agrees with the high frequency of occurrence of those octopod species in fisheries in Algarve waters (Borges et al., Reference Borges, Erzini, Bentes, Costa, Gonçalves, Lino, Pais and Ribeiro2001; Table 1). Overall, the occurrence of octopodids in the diet of conger eels is most likely to be attributable to a high occurrence of Eledone cirrhosa, E. moschata, Scaeurgus unicirrhus (Orbigny, 1840) and O. salutii in Algarve waters.
The fish species that occurred in higher numbers in the diet of conger eels were C. aper, Conger conger (i.e. being cannibalistic), Micromesistius poutassou and S. japonicus. This is expected, as Capros aper, M. poutassou and S. japonicus are some of the most abundant species in Portuguese waters, including the Algarve (Borges et al., Reference Borges, Erzini, Bentes, Costa, Gonçalves, Lino, Pais and Ribeiro2001; Lopes et al., Reference Lopes, Murta and Cabral2006). Moreover, they live in overlapping distribution with conger eels: C. aper lives close to the bottom, mainly at 100–400 m over rock or coral, M. poutassou can live near the bottom at 180–400 m and S. japonicus in mid-waters and in pelagic and demersal waters in depths between 250 and 300 m (Collette & Nauen, Reference Collette and Nauen1983; Whitehead et al., Reference Whitehead, Bauchot, Hureau, Nielsen and Tortonese1986). These species play an important role in the marine trophic ecosystem in the region, as they are commonly found in the diet of a range of north-east Atlantic predators, including mammals (e.g. dolphins Delphinus delphis Linnaeus, 1758 (Silva, Reference Silva1999b)), fish (e.g. blue shark Prionace glauca (Linnaeus, 1758) (Clarke et al., Reference Clarke, Clarke, Martins and Silva1996), tope shark Galeorhinus galeus (Linnaeus, 1758) (Morato et al., Reference Morato, Solá, Grós and Menezes2003), velvet belly lanternshark Etmopterus spinax (Linnaeus, 1758) (Neiva et al., Reference Neiva, Coelho and Erzini2006), swordfish Xiphias gladius Linnaeus, 1758 (Moreira, Reference Moreira1990; Clarke et al., Reference Clarke, Clarke, Martins and Silva1995), almaco jack Seriola rivoliana Valenciennes, 1833 (Barreiros et al., Reference Barreiros, Morato, Santos and Borba2003), Merluccius merluccius, Phycis phycis (Linnaeus, 1766) (Morato et al., Reference Morato, Sola, Gros and Menezes1999), thornback ray Raja clavata (Linnaeus, 1758) (Morato et al., Reference Morato, Solá, Grós and Menezes2003), Trachurus trachurus (Cabral & Murta, Reference Cabral and Murta2002) and John Dory Zeus faber Linnaeus, 1758 (Silva, Reference Silva1999a) and seabirds (e.g. Cory's shearwaters Calonectris diomedea (Scopoli, 1769) (Granadeiro et al., Reference Granadeiro, Monteiro and Furness1998)).
Potential competition between conger eels and local fisheries for prey?
One of the most important issues for fisheries management is to understand if marine organisms compete for prey with local fisheries. This is particularly important as conger eels are abundant in European waters (Borges et al., Reference Borges, Erzini, Bentes, Costa, Gonçalves, Lino, Pais and Ribeiro2001) and have commercial importance (Machado et al., Reference Monteiro, Araujo, Erzini and Castro2004). Of the species preyed upon by conger eels in Algarve waters, six species (21% of the total prey taxa) are caught commercially: T. trachurus, S. japonicus, Micromesistius poutassou, H. dactylopterus and Conger conger (Whitehead et al., Reference Whitehead, Bauchot, Hureau, Nielsen and Tortonese1986; Table 1). These represent 18% of the numbers of prey, and nearly half (44%) in mass, of all prey species found in the diet of conger eels (Table 1). Also, a total of 33% of taxa caught by the fishery vessel and the taxa found in the diet of C. conger were the same (Tables 1 & 3). Therefore, C. conger might be competing with commercial fishing for prey, particularly those that are commercially exploited.
As conger eels can also take advantage of discarded material released by fisheries, through scavenging (Castro et al., Reference Castro, Araújo and Monteiro2005), are the prey found in the diet caught naturally, by active predation, or obtained from discarded material? If scavenging plays a significant role in the conger eels' feeding ecology, it would be expected that their diet would reflect the frequency of the species discarded by the local fisheries. In Algarve waters, there are two main trawl fisheries that produce high levels of discards. In the crustacean trawl fishery (the closest fishery that overlaps with the depth range where conger eels were caught in this study, which operates at depths bellow 150 m, and up to 700 m), the most discarded species, in terms of mass, were M. poutassou (34%), silvery cod Gadiculus argenteus Guichenot, 1850 (10%), Hoplostethus mediterraneus (8%) and roughtip Nezumia sclerorhynchus (Valenciennes, 1838) (8%) (Monteiro et al., Reference Monteiro, Araujo, Erzini and Castro2001). In trawling fishery for fish (operating in shallower waters, between 100 and 200 m) torpedo Torpedo nobiliana Bonaparte, 1835 (15%), small spotted catshark Scyliorhinus canicula (Linnaeus, 1758) (15%), C. conger (10%) and Capros aper (6%) were the most discarded (Borges et al., Reference Borges, Erzini, Bentes, Costa, Gonçalves, Lino, Pais and Ribeiro2001). As the most important identifiable prey taxa in mass in the diet of conger eels was S. japonicus (but not as bait), Alosa sp. and E. cirrhosa (Table 1), it is suggested that most prey were caught actively in that study region. This is probably attributed to the foraging habits of the Conger conger in the Algarve, that are limited to their rocky areas that allows them to hide in rocks (and hence are potentially away from trawling fishing grounds) and hence feeding from discards might be minimal. In other populations of C. conger, the foraging ecology and behaviour is likely to take advantage of this food resource.
ACKNOWLEDGEMENTS
The authors extend their warmest thanks to all the fishermen, and the Captains Francisco Diogo and Ilídio Diogo of the fishing vessel ‘Branca de Sagres’. Dr Simeon Hill (British Antarctic Survey) and Graham Pilling (Cefas) for discussions and comments on an earlier draft. This research was supported by the Foundation for Science and Technology, Portugal.








