I. INTRODUCTION
The original X-ray diffraction (XRD) detector was silver halide-based photographic emulsions coated on glass. With the onset of World War I, glass was replaced with various cellulose ester films. At that time, cellulose nitrate (CN) became the de facto polymer film base in the photographic industry. However, because of stability concerns, including possible spontaneous combustion, CN was eventually replaced by cellulose triacetate (CTA) (referred to as safety film).
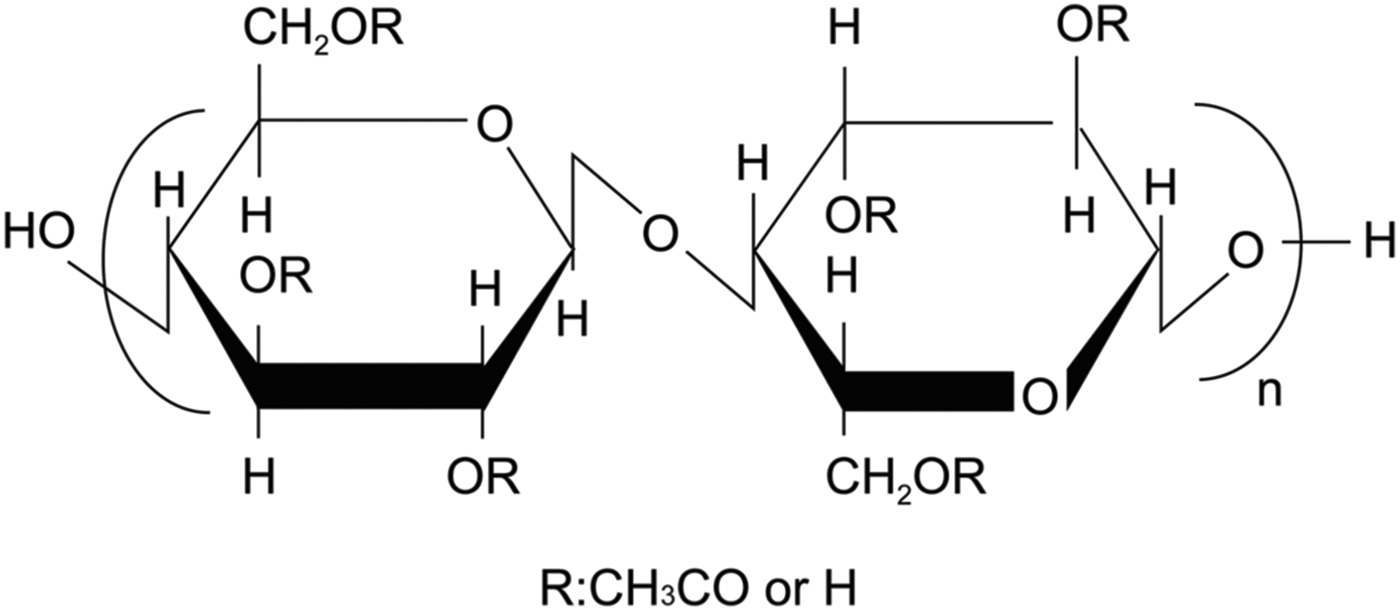
CTA is sometimes mistakenly referred to as cellulose acetate (CA). The degree of substitution (DS) of cellulose hydroxyl groups by acetyl groups can range from 0 (cellulose) to 3 (completely substituted CTA) in CA polymers (Figure 1). If CA materials have a DS greater than 2.6, they should be referred to as CTA.

Figure 1. Base molecular structure for cellulosic materials. Cellulose when R = H, cellulose acetate when R = CH3CO (monoacetate DS = 1, diacetate DS = 2, and triacetate DS = 3).
Eventually poly(ethylene terephthalate) (PET) became the polymer of choice for all X-ray and motion picture photographic products because of its thermal and dimensional stability during and after the photographic film development processing.
In 1865, French Chemist Paul Schutzenberger reacted cellulose with acetic anhydride to make the first CA (Plastics Historical Society, 2011). When CTA was introduced in 1938 to the still and motion picture photographic industries, it was thought that acetate base was less vulnerable to long-term decomposition than nitrate base (Ram et al., Reference Ram, Kopperl, Sehlin, Masaryk-Morris, Vincent and Miller1994). However, within a decade CTA-based photographic films archived in hot, humid conditions were observed to be degrading, characterized by the emission of acetic acid from storage containers (referred to as the vinegar syndrome, Eastman Kodak Company, 2013), producing a film that overtime becomes brittle and shrinks resulting in a distorted shape, and in some cases turns to powder. It was not until the 1980s that scientists began to understand reactions surrounding CTA deterioration (Edge et al., Reference Edge, Allen, Jewitt and Horie1989).
Commercially produced CTA is the product primarily of a chemical reaction between cellulose and acetic acid. Vinegar syndrome is the reverse reaction resulting in the breakdown of the CTA. This process has been labeled “deacetylation” and results from hydrolysis; the acetate ion reacts with moisture to form acetic acid
 $$\eqalign{& \left[{{\rm C}_6 {\rm H}_7 {\rm O}_2 \left({{\rm OCOCH}_3 } \right)_3 } \right]_n+\ n\left({3 - s} \right){\rm H}_2 {\rm O} \cr & \to \left[{{\rm C}_6 {\rm H}_7 {\rm O}_2 \left({{\rm OCOCH}_3 } \right)_{\rm s} \left({{\rm OH}} \right)_{3 - {\rm s}} } \right]_n+\ n\left({3 - s} \right){\rm CH}_3 {\rm COOH}}$$
$$\eqalign{& \left[{{\rm C}_6 {\rm H}_7 {\rm O}_2 \left({{\rm OCOCH}_3 } \right)_3 } \right]_n+\ n\left({3 - s} \right){\rm H}_2 {\rm O} \cr & \to \left[{{\rm C}_6 {\rm H}_7 {\rm O}_2 \left({{\rm OCOCH}_3 } \right)_{\rm s} \left({{\rm OH}} \right)_{3 - {\rm s}} } \right]_n+\ n\left({3 - s} \right){\rm CH}_3 {\rm COOH}}$$where n is the degree of polymerization and s is the DS.
It is this acid that produces the characteristic vinegar odor. A fully deacetylated 1000 ft (305 m) roll of CTA-based photographic film can generate about 6.2 ml of acetic acid (Reilly, Reference Reilly1993). Major factors affecting CTA deacetylation include humidity, pH, temperature, storage conditions, photographic film-processing conditions (particularly how well the film was washed after processing), and environmental surroundings (Allen et al., Reference Allen, Edge, Appleyard, Jewitt, Horie and Francis1987). Considering storage conditions, iron has been shown to act as a catalyst for the degradation reaction, with potential sources being steel film canisters and steel spools that were used for motion picture film storage, and even paper clips used to hold paper on film strips or sheets. Once started, the chemical reaction (1) shown above produces more acid, becoming “autocatalytic”. Although it cannot be stopped it is possible to significantly slow down deacetylation of CTA by storing films in low humidity, in aerated nonmetal containers, at temperatures below 5 °C.
In this study, XRD was used to analyze a Debye–Scherrer film that had been stored for several years in an office environment file cabinet. This film was observed to be shriveled and distorted in appearance (Figure 2). The phases identified from the XRD results were used to help explain the degradation of this X-ray film.

Figure 2. (a) Processed Debye–Scherrer X-ray film strip (PET base) – normal. (b) Processed Debye–Scherrer film strip analyzed in this study – distorted. (Color online)
II. EXPERIMENTAL
A. Sample preparation
Distorted Debye–Scherrer film – a region of the film strip that had minimal curl was cut to generate a small piece of film used as the analyzed specimen that was placed on a zero-background quartz disk.
Cellulose Type II Powder – into a glass flask was added 180 g deionized H2O, 180 g NH4OH (NH3 assay 29.8%, J.T Baker), and 45 g CA (Eastman Kodak, semicrystalline Type II triacetate). Using a magnetic stirrer/hot plate, the mixture was continuously stirred at room temperature for 45 h, then over a period of 1.5 h while heating to 50 °C, and then held at 50 °C for 3 h. The flask was removed from the hot plate, allowed to cool to 35 °C, stirred for 1 min, and then filtered (Millipore Type LS) using a vacuum filtration apparatus. The collected solids were washed with 250 ml of 40% (v/v) acetic acid/H2O, followed by two washes with 200 ml glacial acetic acid, and four washes with 250 ml methanol. All solids were transferred to a glass-drying dish and dried at 40 °C for 14 h. A powder specimen was packed into a 50 µm deep quartz zero-background cell. For structure determination, a portion of the powder sample was blended with ~5 wt.% NIST SRM 640b silicon (Si) as an internal standard.
B. X-ray diffraction
XRD data collection on the distorted Debye–Scherrer film and cellulose Type II powder was carried out using a Rigaku D2000 Bragg–Brentano diffractometer equipped with a copper-rotating anode, diffracted beam monochromator tuned to CuKα radiation, and scintillation detector. Specimens were analyzed in reflection mode geometry. Analysis of the XRD data was carried out using JADE 9.0 (MDI, 2011) and phase identification was confirmed using the ICDD Powder Diffraction File (PDF) (ICDD, Reference Kabekkodu2012). The X-ray powder pattern for structure elucidation was measured on a Bruker D2 Phaser diffractometer, CuKα radiation, and a LynxEye position-sensitive detector using the central 96/192 channels. Details of data collection, structure analysis, and results have been described previously (Kaduk and Blanton, Reference Kaduk and Blanton2013).
III. RESULTS AND DISCUSSION
The XRD pattern for the distorted Debye–Scherrer film strip is shown in Figure 3. Using the PDF, three phases were identified: cellulose, silver [Ag(0)], and silver chloride (AgCl). The presence of cellulose in the Debye–Scherrer film was unexpected, as the initial assumption was that the polymer support was PET, and is an indication that the original base was CTA. Silver is the result of developed Ag(0) after the X-ray exposure and chemical processing. AgCl was the original silver halide salt used for image capture, and was also a surprise as most X-ray films use silver bromide (AgBr) or silver iodobromide (AgBr1−xIx) because of their higher X-ray absorption properties compared to AgCl (Corney et al., Reference Corney, Erickson, Splettstosser and James1977). The presence of silver halide is an indication that the film was not completely fixed (i.e. soak in sodium thiosulfate aqueous solution) to remove undeveloped AgX during the chemical processing, and likely is an indication that the developed Debye–Scherrer film strip was not properly washed after photo processing.

Figure 3. XRD pattern for the specimen collected from the distorted processed Debye–Scherrer film and analyzed in this study. (Color online)
Although deacetylation of CA polymers is known to result in making cellulose, this study was able to confirm the presence of the Type II form of cellulose. The change in appearance of the converted cellulose II Debye–Scherrer film strip is a result of stress created with the loss of acetyl groups from the original CTA-based film strip. The molecular volume (MV) (unit-cell volume/number of molecules per unit cell) is 375.06 for CTA and 170.88 for cellulose II (ICDD, Reference Kabekkodu2012). This significant decrease in MV results in a compressive stress in the polymer film bringing about the shriveled, distorted appearance as observed in Figure 2(b).
When comparing the cellulose II XRD pattern in Figure 3 to entries in the PDF, it was observed that the interplanar d-spacings matched reasonably well; however, there were some differences of note in the diffraction peak intensities. The NH4OH chemical treatment of CTA described in the experimental section was used to obtain a phase pure reference powder sample of cellulose II. The XRD patterns for the neat CTA and resulting cellulose II powders are shown in Figure 4.

Figure 4. XRD patterns for powder samples of (a) neat CTA (semicrystalline Type II) and (b) cellulose II after NH4OH treatment. The inset in Figure 4(b) is a scanning electron micrograph (SEM) of the cellulose II powder. (Color online)
Mixing the cellulose II powder with a Si internal standard allowed for a more precise determination of the lattice parameters by the Rietveld method. Cellulose II is monoclinic with space group P1121, refined unit-cell parameters a = 8.076(13) Å, b = 9.144(10) Å, c = 10.3868(20) Å, γ = 117.00(8)°, and V = 683.5(18) Å3. A density functional geometry optimization using these fixed lattice parameters resulted in an improved structural model for cellulose II with R wp = 0.0589 and χ 2 = 4.704 (Kaduk and Blanton, Reference Kaduk and Blanton2013). The refined cellulose profile X coefficient of 202(3) corresponds to an average crystallite size of 39(1) Å (Figure 5).

Figure 5. Observed (red+), calculated (green−), and difference (purple−) patterns from the Rietveld refinement used to determine the lattice parameters of cellulose II. The inset shows the crystal structure of cellulose II, viewed down the c-axis. (Color online)
The improved crystal structure for cellulose II obtained as part of this study along with raw and calculated powder patterns have been submitted to the International Centre for Diffraction Data (ICDD) for inclusion in future releases of the PDF. These data allow for multiple analysis options as shown in Figure 6. Users of the PDF can compare their raw data to the experimental powder XRD pattern or a simulated pattern where the user can define the peak profile and crystallite size as well as account for preferred orientation. Electron diffraction, electron backscatter, and transmission Laue ring patterns can also be simulated using the PDF database software. Details of the three-dimensional structure including bond lengths and angles are available as well as the atomic coordinates.

Figure 6. ICDD PDF4+ Release 2013 cellulose II diffraction data for phase identification and display options available, allowing for detailed materials characterization. (Color online)
IV. SUMMARY
XRD has been used to characterize a processed Debye–Scherrer film that was observed to be physically distorted after years of storage. Identification of the phases present indicates that the original polymer film base was CTA and that the CTA had converted to cellulose II due to deacetylation. The presence of AgCl was an indication that the photo processing of the Debye–Scherrer film was incomplete. Storage of the Debye–Scherrer film in a cabinet, incomplete processing, and likely incomplete washing all are potential contributors to the initiation of deacetylation that continued over a period of many years. Beyond the chemical and materials properties discussed in this study, the results highlight the uncertainty that can exist in any archival storage medium.
Extending the study to include chemically converting CTA to cellulose II led to an improved structural model for cellulose II that has been added to the PDF allowing for enhanced analysis of cellulosic materials.