INTRODUCTION
Mating systems and patterns of social organization have been shown to be functions of ecological factors such as resource distribution and breeding density (Lin et al. Reference LIN, YOU and LIN2009, Macdonald et al. Reference MACDONALD, ATKINSON and BLANCHARD1997). Recently, it has become evident that social and genetic mating systems differ in many taxa, particularly in birds (Griffith et al. Reference GRIFFITH, OWENS and THUMAN2002, Westneat & Sherman Reference WESTNEAT and SHERMAN1997). Molecular studies of avian breeding systems have revealed that many socially monogamous species are not genetically monogamous, with extra-pair paternity (EPP) occurring in the majority of broods in some populations (Westneat & Sherman Reference WESTNEAT and SHERMAN1997).
Although differences in life-history strategies and ecological factors have generally been proposed as the main factor in the variation of EPPs among species, there has been little evidence explaining this variation (Griffith et al. Reference GRIFFITH, OWENS and THUMAN2002). Phylogenetic analysis has shown that more than 50% of interspecific variation in EPP can be explained by phylogeny (Arnold & Owens Reference ARNOLD and OWENS2002). In raptors, EPP is reported to occur in low frequency in socially monogamous species (Alcaide et al. Reference ALCAIDE, NEGRO, SERRANO, TELLA and RODRIGUEZ2005, Korpimäki et al. Reference KORPIMÄKI, KATRIINA, MAY, PARKIN, POWELL, TOLONEN and WETTON1996, Rutz Reference RUTZ2005).
Stutchbury & Morton (Reference STUTCHBURY and MORTON1995) proposed the breeding synchrony hypothesis to explain low EPP in tropical regions. In the tropics, the majority of species tend to breed asynchronously (thus fewer females are fertile at the same time) resulting in fewer opportunities for EPP. The limited number of tropical bird species for which mating system studies have been carried out show relatively low levels of EPP (Douglas et al. Reference DOUGLAS, HEATH and MENNILL2012, Krueger et al. Reference KRUEGER, WILLIAMS and SEARCY2008, Stutchbury & Morton Reference STUTCHBURY and MORTON2001).
Griffith (Reference GRIFFITH2000) found lower rates of EPP for species on islands compared with mainland counterparts. The author mentions several non-mutually exclusive hypotheses to explain lower EPP on islands including: reduced genetic variability in island species, reduced breeding densities, lower food abundance, phenotypic plasticity to harsher environments, and the cost of mate abandonment on islands.
The level of male parental care also appears to be a predictor of EPP level (Neudorf Reference NEUDORF2004). Males involved in parenting activities may be limited in obtaining extra-pair matings because of constraints imposed by caring for young. In taxa that display male parental care, such as Strigiformes and Falconiformes (Müller et al. Reference MÜLLER, EPPLEN and LUBJUHN2001), EPP occurs infrequently or is sometimes entirely absent.
The social mating system of diurnal raptors can vary, and cooperative breeding has been reported in up to 14% of species (Kimball et al. Reference KIMBALL, PARKER and BEDNARZ2003). Certain populations of the Galapagos hawk Buteo galapagoensis are polyandrous and produce broods with multiple paternity (Faaborg et al. Reference FAABORG, PARKER, DELAY, DE VRIES, BEDNARZ, MARIA PAZ, NARANJO and WAITE1995), but the majority of species in the genus Buteo are reported to be socially monogamous (Ferguson-Lees & Christie Reference FERGUSON-LEES and CHRISTIE2001). The genetic mating system is documented for only one other socially monogamous Buteo species, Swainson's hawk Buteo swainsoni (Briggs & Collopy Reference BRIGGS and COLLOPY2012) which has a very low percentage (5%) of broods with extra-pair young.
Ridgway's hawk is reported to exhibit a social mating system of monogamy (Wiley & Wiley Reference WILEY and WILEY1981). To corroborate this, we examined the detailed spatial organization of breeding adults over five breeding seasons. Further, considering Ridgway's hawk is a tropical island raptor with attributes of parental care, we hypothesized the mating system to be genetically monogamous. To test our hypothesis, we used microsatellite profiles and examined the level of EPP via paternity exclusion analysis and by determining within-brood nestling relatedness. We predicted the frequency of broods with EPP would be very low or absent.
METHODS
Study area
The island of Hispaniola (19°0’N, 71°0’W) is located in the Caribbean, and consists of the nations of Haiti and the Dominican Republic (Figure 1). Less than 1.5% of Haiti's original forest is left, most of which is in the inaccessible uplands of the island and is highly degraded (Rimmer et al. Reference RIMMER, TOWNSEND, TOWNSEND, FERNANDEZ and ALMONTE2005). The Dominican Republic has not fared much better with only 10% of its original forest cover remaining and under threat to further loss from unregulated logging, slash-and-burn agriculture and charcoal production (Latta et al. Reference LATTA, RIMMER, KEITH, WILEY, RAFFAELE, MCFARLAND and FERNANDEZ2006). We conducted our study in Los Haitises National Park (19ºN, 70ºW) which ranges from 0–380 m asl in altitude and is located on the north-east coast of the Dominican Republic (Figure 1). It is a platform karst (eroded limestone) formation, with dense clusters of steep conical hills, or mogotes, of nearly uniform height (200–300 m) separated by sinkhole valleys. The Los Haitises region consists of thousands of such mogotes.

Figure 1. Map showing relative location of Hispaniola in the Caribbean, the nations of Haiti and Dominican Republic and their respective capital cities: Port-au-Prince (1) and Santo Domingo (2); and the study area of Los Haitises National Park (3).
Study species
Ridgway's hawk (Buteo ridgwayi) is a forest raptor endemic to the island of Hispaniola in the Caribbean. The species is limited to moist low-elevation rain-forest habitats that host high reptile abundance, as reptiles constitute ~80% of its diet (Woolaver et al. Reference WOOLAVER, NICHOLS, MORTON and STUTCHBURY2013a). The species was locally common in areas of Haiti and the Dominican Republic at the turn of the century (Cory Reference CORY1885, Wetmore & Lincoln Reference WETMORE and LINCOLN1934), but is now listed as Critically Endangered (BirdLife International 2011). The current global population size is estimated at <110 pairs, limited to an area of 1600 km2 of rain forest in Los Haitises National Park on the north-east coast of the Dominican Republic (Woolaver Reference WOOLAVER2011).
Nest monitoring
Breeding pairs of Buteo ridgwayi were studied over five breeding seasons, 2005–2009. Early-season observations for breeding pairs were made from vantage points on hillsides overlooking valleys to identify nest locations. Due to the topography of the study area, nesting sites were easy to distinguish for each pair as nests were always located in separate valleys. Once found, nests were visited every 1–3 d for easily accessible nests, or every 1–2 wk for sites that were more difficult to access. During each visit, the following behavioural observations were recorded during standardized 4-h sessions: nest-building activities, brooding and incubation behaviour and duration, copulations, territorial defence or displays, pair interactions. Nest observations were carried out with binoculars and a spotting scope from a covered vantage point 10–25 m away.
Trapping, measuring and ringing Ridgway's hawk
Nestlings were accessed at the nest when 15–40 d old. Nestlings were placed in cotton bags and lowered to the ground below the nest, where they were measured, ringed and their blood sampled for DNA. Handling time of nestlings did not exceed 20 min per individual. Adults were captured using bal-chatri noose traps baited with white domestic mice Mus musculus (Thorstrom Reference THORSTROM1996). Adults were not trapped when the pair was incubating eggs. Ridgway's hawks were ringed with colour anodized Acraft© aluminium rivet bands. The bands were coloured and individually numbered so that different colour combinations could be used to identify individual birds. No more than one band was ever placed on a leg.
DNA collection and extraction
Whole blood was collected from 146 Ridgway's Hawks within the study period from 2005–2008: 34 adults and 112 nestlings. Approximately 0.2 ml of blood was drawn via capillary tube from a patagial vein puncture, half of which was stored in 1.6 ml of Queen's lysis buffer (Seutin et al. Reference SEUTIN, WHITE and BOAG1991). The other 0.1 ml of blood was stored in 1.8 ml of 95% ethanol. All samples were stored at ambient temperature until delivered to laboratory facilities where they were preserved at −20 °C.
Total cell DNA was isolated by blood cell lysis, followed by DNA precipitation using ammonium acetate and isopropanol (L. de Sousa, B. Woolfenden and S. Tarof, unpublished protocol). This involved the addition of 50 μl of blood/Queen's lysis buffer to 600 μl of cell lysis buffer and 5 μl of ice-cold Proteinase K (40 ng μl−1). This solution was then incubated at 55–60 °C for 5 h and then at 37 °C overnight. Ice cold ammonium acetate (200 μl) was then added, mixed gently, and centrifuged to precipitate protein. The aqueous phase, including the dissolved genomic DNA was removed and placed in a fresh tube. Ice-cold isopropanol (600 μl) was added and the solution inverted until DNA was visible as a white floating string or flake. This solution was then centrifuged to collect genomic DNA as a pellet at the bottom of the tube. The supernatant was removed and the DNA pellet washed with ice-cold 70% ethanol. This solution was then centrifuged and the ethanol then removed. This ethanol wash was repeated a second time. The tube was then left open and inverted overnight to allow the DNA pellet to dry completely. The DNA pellet was then suspended in 100–200 μl of TE buffer (10 mM Tris-HCl, 1 mM EDTA) at 37 °C for 24 h. DNA was stored at 4 °C while in use, and at −20 °C for longer-term storage.
DNA was visualized under ultraviolet radiation on a 1% agarose test gel, pre-stained with ethidium bromide. Samples were visualized next to a MassRuler high range DNA ladder mix (Fermentas O’GeneRuler™).
Microsatellite genotyping
Eleven microsatellite loci isolated from common buzzard Buteo buteo (Johnson et al. Reference JOHNSON, FOWLIE and AMOS2005) and Swainson's hawk Buteo swainsoni (Hull et al. Reference HULL, TUFTS, TOPINKA, MAY and ERNEST2007) were tested for examining allelic variation in B. ridgwayi (Woolaver et al. Reference WOOLAVER, NICHOLS, MORTON and STUTCHBURY2013b). Polymerase Chain Reaction (PCR) protocols for each set of primers were optimized for B. ridgwayi using blood from 24 individuals (6 adult females, 6 adult males and 12 nestlings) sampled in 2005–2007. Non-radioactive, fluorescently labelled (Black, Blue and Green) microsatellite primers were provided by Integrated DNA Technologies (IDT™). Optimal reagent volumes and annealing temperatures varied by primer sets. In general, genomic DNA was amplified for each individual in 10 μl reactions containing 5.3–6.4 μl distilled water, 1.0 μl of PCR reaction buffer (10× TSG), 0.6–1.4 μl of 20mM MgSO4, 0.4 μl of 10mM dNTPs, 0.2 μl of fluorescently dyed 10 μM forward and reverse primers, 0.2 μl of Taq DNA polymerase (TSG), and 1 μl of DNA template (c. 15 ng DNA in TE buffer). PCR reactions were carried out in an Eppendorf MasterCycler™ thermal cycler.
For the Bbu primer pairs: an initial 2-min denaturing step at 94 °C was followed by 12 cycles of 45 s at 94 °C, 45 s at the primer specific annealing temperature, and a 50 s extension step at 72 °C. This was followed by a further 22 cycles of 30 s at 89 °C, 45 s at the primer specific annealing temperature, and a 50-s extension step at 72 °C. The PCR reaction finished with a final 5-min extension step at 72 °C, and samples were then held at 4 °C until taken from the thermal cycler. Primer specific annealing temperatures were as follows: Bbu51 50 °C, Bbu17 and Bbu34 53 °C, Bbu46 54 °C, Bbu42 55 °C, Bbu03 56 °C, Bbu33 58 °C and Bbu59 59 °C. For the Bsw primer pairs (Bsw107, 122, 207, 234, 310 and 324): An initial 2-min denaturing step at 94 °C was followed by 30 cycles of 30 s at 94 °C, 45 s at 58 °C and a 45 s extension step at 72 °C. This reaction finished with a 30-min extension step at 72 °C and PCR products were then held at 15 °C until removed from the thermal cycler.
Each locus was amplified separately but since primers had been fluorescently labelled, loci were pooled post-PCR in Poolplex reactions. PCR products were visualized using a CEQ 8000™ DNA sequencer, and allele sizes were assigned using the Beckman Coulter CEQ 8000 Genetic Analysis System™ software.
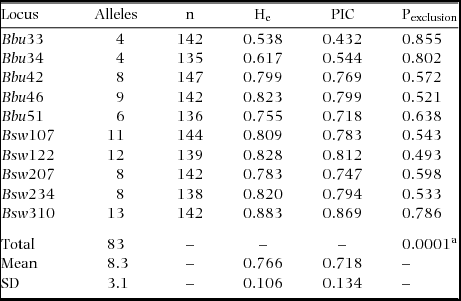
The genetic mating system of Ridgway's hawk was examined using two complementary analyses of microsatellite profiles: the first was a likelihood ratio method to identify and assign putative fathers, and the second was a method for estimating genetic relatedness among nestlings in a brood. Paternity and nestling relatedness were examined using 10 polymorphic loci (Table 1).
Table 1. Characteristics of microsatellite loci of Ridgway's hawk used for assigning paternity and estimating relatedness. Individuals were sampled within Los Haitises National Park, Hispaniola, between 2005 and 2008. Parameters include sample size (n), expected heterozygosity (He), Polymorphic Information Content (PIC) and probability of exclusion (Pexclusion).

aTotal probability of exclusion using all loci.
Data analysis
Paternity exclusion
Paternity assignment was performed using the microsatellite profiles and the likelihood-based method in the computer program CERVUS 3.0 (Kalinowski et al. Reference KALINOWSKI, TAPER and MARSHALL2007, Reference KALINOWSKI, TAPER and MARSHALL2010). To identify the putative father and to assess the statistical confidence of this identification, CERVUS calculates an LOD score (natural logarithm of the likelihood-odds ratio) for each candidate male (Kalinowski et al. Reference KALINOWSKI, TAPER and MARSHALL2007, Reference KALINOWSKI, TAPER and MARSHALL2010). A positive LOD indicates that a candidate is more likely to be the father than one randomly drawn from the population. The male with the highest score is assigned as the putative father (Kalinowski et al. Reference KALINOWSKI, TAPER and MARSHALL2007). The statistical confidence of this estimate is determined by measuring the difference between the LOD scores (Delta, Δ) of the males with the two highest scores (Kalinowski et al. Reference KALINOWSKI, TAPER and MARSHALL2007). The larger the difference, the more confidence that the male with the highest LOD score is the actual father. Critical values for Δ are generated in CERVUS by bootstrapping from the experimental population (Kalinowski et al. Reference KALINOWSKI, TAPER and MARSHALL2007). CERVUS takes into account genotyping errors, and limits the number of extra-pair paternities according to the estimated proportion of potential fathers that have been sampled (Kalinowski et al. Reference KALINOWSKI, TAPER and MARSHALL2007).
Parameters used in CERVUS for maximum likelihood paternity assignment included 100000 simulation cycles with relaxed and strict confidence levels set at 80% and 95%, respectively. The proportion of candidate parents sampled was 0.20. The proportion of loci typed and loci mistyped were 0.90 and 0.01, respectively. The minimum number of typed loci used for the analysis was seven.
Relatedness
Relatedness among nestlings in a brood was carried out using the genetic software program KINGROUP v2 (Konovalov et al. Reference KONOVALOV, MANNING and HENSHAW2004). Relatedness estimators using co-dominant markers are unbiased even when allele frequencies are estimated from relatively small samples (Queller & Goodnight Reference QUELLER and GOODNIGHT1989), making them the best available markers for estimating relatedness (Blouin Reference BLOUIN2003, Blouin et al. Reference BLOUIN, PARSONS, LACAILLE and LOTZ1996, DeWoody Reference DEWOODY2005). They have been shown to be a valuable tool for inferring relationships among individuals with unknown ancestry, in the absence of pedigree information (Russello & Amato Reference RUSSELLO and AMATO2004).
Relatedness was determined using pairwise relatedness coefficients (r), which can detect relationships between significantly related individuals including that of parent–offspring, full siblings and half-siblings. This method was chosen for its proven accuracy in detecting relatedness among individuals (Gautschi et al. Reference GAUTSCHI, GWENAËL, NEGRO, GODOY, MÜLLER and SCHMID2003). The pairwise relatedness coefficients were calculated based on Queller & Goodnight (1989). This relatedness estimator ranges from −1 to +1. A positive value indicates that two individuals share more alleles by descent than expected by chance. First-order relatives such as full siblings should have an r value of approximately 0.5, and half-siblings an r of 0.25. Pairwise relatedness coefficients within broods were compared to theoretical values using t-tests. A genetic relationship between two nestlings was considered significant (P < 0.05) by KINGROUP (Konovalov et al. Reference KONOVALOV, MANNING and HENSHAW2004), if the pairwise relatedness coefficient was ≥0.25, similar to that of half-sibling relatedness or greater.
RESULTS
Social mating system
During all observation periods during the 5-y study (>2000 observation hours) no more than two adults were ever observed interacting non-aggressively within a territory. Aggressive interactions between adjacent individuals or pairs were observed during aerial territorial displays, but these sightings were rare (n = 15). Whenever individual birds were identified from band combinations (23 of 35 adults re-sighted over the study period), identification confirmed the attending female and male at nests to be the social pair within the territory. No non-paired males were observed provisioning food or seen near the resident female in an active territory and extra-pair copulation attempts were not observed.
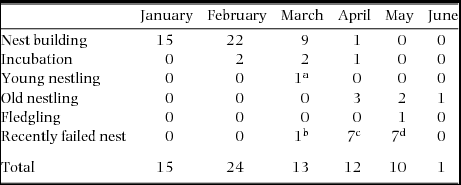
Copulations were observed during all stages of the breeding episode over a 6-mo period (n = 74, Table 2). Copulations were observed from January to June during nest building (n = 47), incubation stage (n = 5), nestling stage (n = 7), fledgling of young (n = 1) and after recent nest failures (n = 15). Peaks in copulation frequencies occurred just prior to egg-laying (January–March) and to a lesser extent after a nest had failed (April–May). Copulation behaviour between two individuals may be indicative of whether copulations are within-pair or extra-pair. Female solicitation followed by male mounting without interruption by the female can be suggestive of a within-pair copulation. Of 62 copulation attempts observed to completion, 60 were carried out without any interruption by the female. On two occasions, a male mounted the female but the female did not lift her tail to allow the copulation to continue. In both instances the male dismounted and remained perched next to the female and was identified as the social male.
Table 2. Number of copulations of Ridgway's hawk breeding pairs recorded by month and breeding stage in Los Haitises National Park, Hispaniola, from 2005–2009.
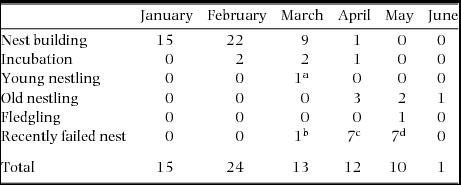
aNest with 6 d-old nestlings.
bFailed during incubation.
cThree failed with young chicks, others failed at unknown stage.
dFive failed with nestlings.
Microsatellite genotyping
A total of 34 adults and 112 nestlings were used for the microsatellite genotype profiling. Of these samples, 19 adults and 86 nestlings from 48 broods were used for the paternity exclusion and brood relatedness analysis (samples with only one individual per family unit were not applicable). We were able to catch and sample seven nests with full family units (social male, social female, + all nestlings), 12 nests with social male + nestlings, eight nests with social female + nestlings, and 19 nests where only the nestlings could be sampled.
Eighty-three different alleles were found from 10 loci, with an observed heterozygosity of 0.766 ± 0.106 (Table 1). The mean polymorphic information content of all loci was 0.718 ± 0.134 (Table 1). The combined probability of non-exclusion for the marker set was 0.001 (Table 1).
Genetic mating system
Paternity assignment
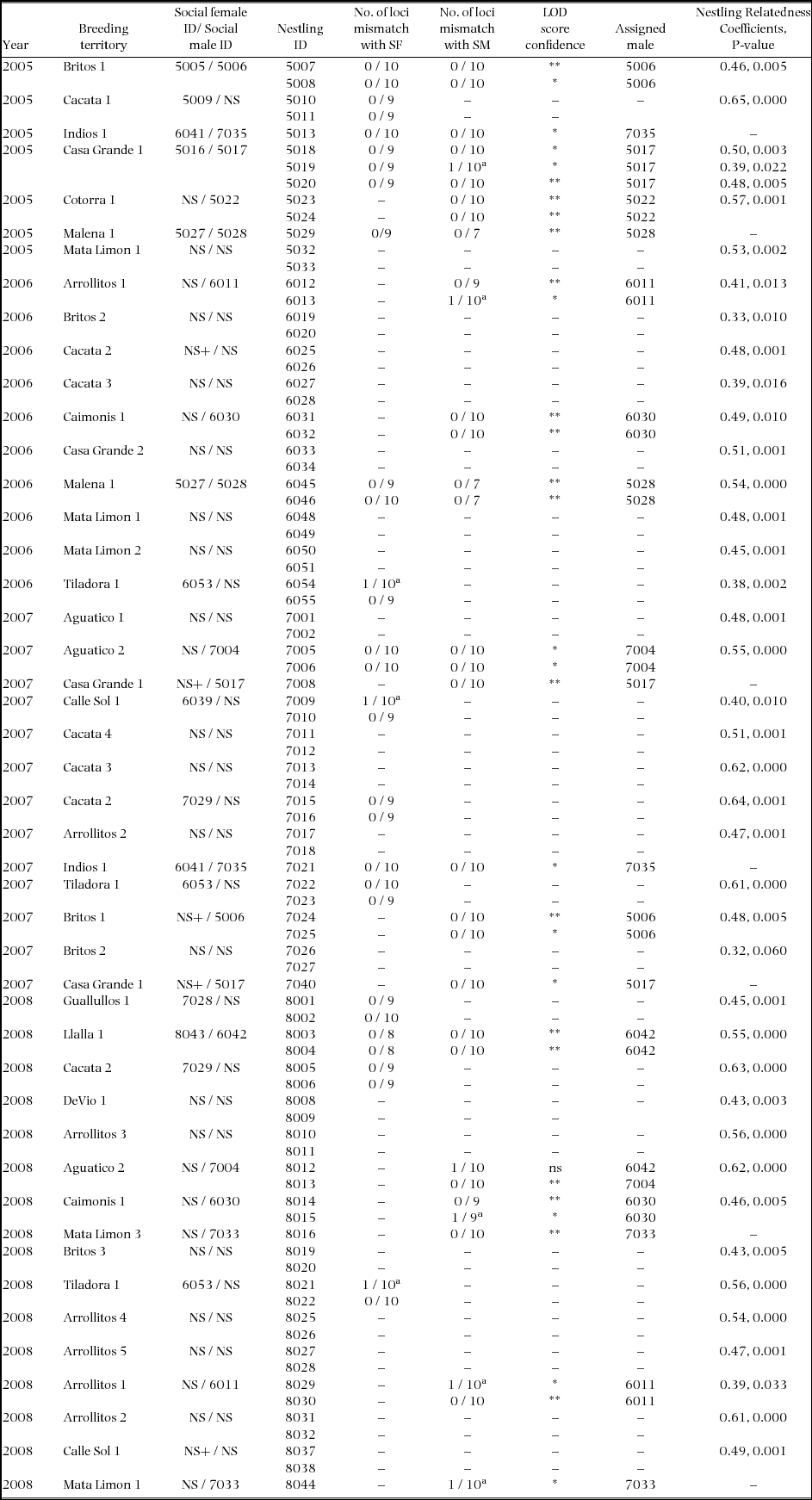
The attending male was assigned as the putative father for 29 out of 30 nestlings from 19 broods (Appendix 1). From 2005–2007, none of the broods showed evidence of extra-pair young. In 2008 there was one instance of an attending male not being assigned as the actual father to one of two nestlings. However, the confidence level of the assignment was not significant (Delta Value of 0), with both the attending male and the assigned male having similar genotypes, and found to be closely related similar to that of a parent-offspring or full sibling (relatedness coefficient r = 0.52, P < 0.00). Thus it is probable that this one case was not an instance of extra-pair paternity but an incorrect assignment of parentage due to the similar genotypes.
Brood relatedness
Brood relatedness values were used to assess potential EPP in cases where one or both parents were not genotyped. The relatedness coefficient among nestlings in the 41 broods sampled over the study period ranged from 0.32 to 0.65 and averaged 0.48 ± 0.08 (Appendix 1). These values did not significantly differ from the expected 0.50 (t1,40 = −0.35, P = 0.73) and indicated full-sibling relatedness on average for all broods. For comparison, relatedness coefficients were obtained from known half-siblings (based on social pairings); nestlings from nests of different years where one parent was identified from the previous year and the other parent was a new mate. Half-sibling relatedness coefficients ranged from 0.16 to 0.29 and averaged 0.23 ± 0.04 (n = 12). Relatedness coefficients of half-siblings did not differ significantly from the expected 0.25 (t1,11 = −1.58, P = 0.09) and were significantly lower compared with relatedness coefficients of nestlings within the same brood (F1,40 = −11.1, P < 0.001). This suggests that all 41 broods sampled over the study period consisted of full siblings with no cases of extra-pair young.
DISCUSSION
Nest site and territory observations verified a socially monogamous mating system for Ridgway's hawk, as originally noted by Wiley & Wiley (1981). The majority of Buteo species appear to hold socially monogamous pair bonds (Newton Reference NEWTON1979) however cooperative breeding systems are not uncommon in diurnal raptor species (Kimball et al. Reference KIMBALL, PARKER and BEDNARZ2003). The Galapagos hawk, which consists of several island subpopulations that range from social monogamy to polyandry (Bollmer et al. Reference BOLLMER, SANCHEZ, CANNON, SANCHEZ, CANNON, BEDNARZ, DE VRIES, STRUVE and PARKER2003, Faaborg et al. Reference FAABORG, PARKER, DELAY, DE VRIES, BEDNARZ, MARIA PAZ, NARANJO and WAITE1995), is the only documented Buteo species to have a clear cooperative breeding system. Polyandry has been suggested for the Puna subspecies of Red-backed hawk Buteo polyosoma (Kimball et al. Reference KIMBALL, PARKER and BEDNARZ2003), but this has not been confirmed.
For the closest taxonomic relative to Ridgway's hawk, the red-shouldered hawk B. lineatus (Amaral et al. Reference AMARAL, SHELDON, GAMAUF, HARING, RIESING, SILVEIRA and WAJNTAL2009), there is a single record of three adults attending a nest in Florida (http://bna.birds.cornell.edu/BNA/species/107). For the geographically closest forest Buteo species, the Puerto Rican broad-winged hawk B. platypterus brunnescens, there is one report of a nesting female accepting food from an intruding male in the absence of the attending male (Delannoy & Cruz Reference DELANNOY and CRUZ1988). Similar documented cases have been reported for B. buteo (Barrientos & López-Darias Reference BARRIENTOS and LÓPEZ-DARIAS2006), B. swainsoni (Cash Reference CASH1989) and B. jamaicensis (Santana et al. Reference SANTANA, KNIGHTS and TEMPLE1986) but these anecdotal sightings are rare and not suggestive of a cooperative social breeding system.
We found no evidence of extra-paternity in 41 broods, with nestlings showing a genetic similarity level congruent with those expected among full siblings, implying that the nestlings were produced by the social parents. Since there was one instance of a social male not being assigned paternity (even though the confidence level of the assignment was very low), it is not possible to rule out the possibility of EPP in the species. However, our results indicate the species is effectively genetically monogamous.
Ridgway's hawk is a socially monogamous species, exhibiting male parental care (Woolaver Reference WOOLAVER2011). These traits are assumed to be associated with low EPP levels. Male Ridgway's hawks were observed incubating albeit less often than females, and were the main food provider to the nestlings, and therefore would have reduced time to pursue EPCs. In addition, the valley landscape of each territory would allow for easy detection of intruding males, making it easy for social males to limit EPCs within their territory. Within avian taxa, socially monogamous males appear to have evolved two strategies to ensure paternity: mate guarding (Birkhead Reference BIRKHEAD1979) and/or frequent within-pair copulations (Birkhead et al. Reference BIRKHEAD, ATKIN and MØLLER1987). Ridgway's hawks seem to have employed the latter strategy. During the study period, copulations were observed between breeding pairs during all stages of the breeding episode, and were frequent during late nest-building and early incubation stages. Males were often away from their nests for extended periods of time to hunt and provide food to the female and nestlings, and would not be able to effectively guard their mates.
Other contemporary ecological factors, such as breeding synchrony, have also been suggested to be correlated to EPP levels (Griffith et al. Reference GRIFFITH, OWENS and THUMAN2002). Species that breed over a short period of time with most females in breeding condition at the same time, allow for EPC opportunity; whereas species that breed over a longer period with females in breeding condition at different times would support much fewer opportunities. Tropical species may have fewer cases of EPP because most species tend to breed asynchronously, with fewer females fertile at the same time (as few as 8%), resulting in less opportunity for EPC (Stutchbury & Morton Reference STUTCHBURY and MORTON1995). During the present study, Ridgway's hawk females were observed breeding over a 6-mo period with a large temporal variation in copulation activity and egg-laying, from January until late June (Woolaver Reference WOOLAVER2011).
The challenge in applying hypotheses to a given taxon is that often certain life-history traits co-occur (Westneat & Stewart Reference WESTNEAT and STEWART2003) and it is difficult to determine to what extent each trait may contribute to EPP rates. For example, longevity and male parental care are both life-history traits for several taxa including seabirds (Arnold & Owens Reference ARNOLD and OWENS2002), owls (Arsenault et al. Reference ARSENAULT, STACEY and HOELZER2002, Saladin et al. Reference SALADIN, RITSCHARD, ROULIN, BIZE and RICHNER2007), parrots (Masello et al. Reference MASELLO, SRAMKOVA, QUILLFELDT, EPPLEN and LUBJUHN2002) and vultures (Decker et al. Reference DECKER, PARKER, MINCHELLA and RABENOLD1993). The phylogenetic component also adds to the challenge of trying to explain or predict EPP. As a general rule, EPP tends to be higher in passerines and lower in raptors and seabirds (Westneat & Sherman Reference WESTNEAT and SHERMAN1997). However recent studies have reported species which appear to be exceptions to the rule. Genetic monogamy or very low EPP has been reported in several passerines (Kleven et al. Reference KLEVEN, BJERKE and LIFJELD2008, Maguire & Mulder Reference MAGUIRE and MULDER2008, Taylor et al. Reference TAYLOR, BOESSENKOOL and JAMIESON2008), and high levels of EPP have been found in albatross (Huyvaert et al. Reference HUYVAERT, ANDERSON, JONES, DUAN and PARKER2000). At the same time, many species appear to be conforming to original predictions, including low EPP in raptors (Arsenault et al. Reference ARSENAULT, STACEY and HOELZER2002, Hogan & Cooke Reference HOGAN and COOKE2010, Saladin et al. Reference SALADIN, RITSCHARD, ROULIN, BIZE and RICHNER2007, this study), seabirds (Anker-Nilssen et al. Reference ANKER-NILSSEN, KLEVEN, AARVAK and LIFJELD2008, Reference ANKER-NILSSEN, KLEVEN, AARVAK and LIFJELD2010; Wojczulanis-Jakubas et al. Reference WOJCZULANIS-JAKUBAS, JAKUBAS, OIGARDEN and LIFJELD2009) and higher EPP in passerines (Stewart et al. Reference STEWART, WESTNEAT and RITCHISON2010). It is not surprising that factors contributing to the frequency of EP mating are challenging to resolve since just a few ecological or biological differences between populations of closely related species can result in very large variation in EPP (Kingma et al. Reference KINGMA, HALL, SEGELBACHER and PETERS2009, Labarbera et al. Reference LABARBERA, LOVETTE and LLAMBIAS2012, Stutchbury et al. Reference STUTCHBURY, MORTON and WOOLFENDEN2007).
Overall, our genetic results, along with our observations of the species spatial organization, support our hypothesis that the social and genetic mating system for Ridgway's hawk is monogamy. However, there are other species that do not follow these general predictions (Huyvaert et al. Reference HUYVAERT, ANDERSON, JONES, DUAN and PARKER2000, Macedo et al. Reference MACEDO, KARUBIAN and WEBSTER2008, Taylor et al. Reference TAYLOR, BOESSENKOOL and JAMIESON2008, Townsend et al. Reference TOWNSEND, BOWMAN, FITZPATRICK, DENT and LOVETTE2011), thus more molecular studies of tropical species are needed for a better understanding, not only of general genetic mating systems, but also of the contributing evolutionary and ecological factors that determine EPP frequency (Stutchbury & Morton Reference STUTCHBURY and MORTON2008).
ACKNOWLEDGEMENTS
We would like to thank the following people for their advice during the study: B. Woolfenden, S. Tarof, N. Collar, J. Wiley, R. Thorstrom, S. Latta, D. Wege, J. Shore, L. Packer and A. Zayed. A number of people provided significant contributions to the field research including J. Vetter, J. Sinclair, T. Nichols, J. Brocca, J. Almonte, J. Cespedes, N. Corona, S. Balbuena de la Rosa, T. Bueno Hernandez, Pastor de Leon Franco and H. Jorge Polanco. The Sociedad Ornitólogica Hispaniola provided much appreciated logistical support. All research was carried out with the permission of Subsecretaría de Estado de Areas Protegidas y Biodiversidad, and the Secretaría de Estado de Medio Ambiente y Recursos Naturales, Republica Dominicana. The research was funded by Wildlife Preservation Canada, the Natural Sciences and Engineering Research Council of Canada (NSERC) and the James Bond Fund of the Smithsonian Institution. An anonymous reviewer provided comments that improved this manuscript. We would particularly like to thank E. Williams and K. Wallace for their support throughout the study.
Appendix 1. Results of genetic mating system analysis of Ridgway's hawks (breeding pairs and nestlings) sampled within their breeding territories in Los Haitises National Park, Hispaniola, between 2005 and 2008. Microsatellite profiles were used from 10 loci to estimate genetic relatedness among nestlings in a brood (KINGROUP Nestling Relatedness Coefficients) and assignment of putative fathers (CERVUS paternity assignment and LOD Score). Social male (SM) and female (SF) were the adults observed attending the nest. Assigned male refers to the male identified by the CERVUS program as the most likely candidate to be the genetic father. NS refers to not sampled, + denotes a new mate, and LOD Score is reported with strict confidence ** (95%) and relaxed confidence * (80%).