Introduction
Traumatic brain injury (TBI) is a common cause of childhood disability that typically involves disruption to rapidly developing, anatomically distributed neural networks implicated in social cognition; a multidimensional construct that refers to mental processes used to perceive and process social cues, stimuli and the environment (Adolphs, Reference Adolphs2009; Beauchamp & Anderson, Reference Beauchamp and Anderson2010). While impairments in social cognition may be among the most disabling consequences of TBI and confer secondary risk for elevated aggression and conduct problems (Ryan et al. Reference Ryan, Anderson, Godfrey, Eren, Rosema, Taylor and Catroppa2013b ; Robinson et al. Reference Robinson, Fountain-Zaragoza, Dennis, Taylor, Bigler, Rubin, Vannatta, Gerhardt, Stancin and Yeates2014), neuroanatomical risk factors for persisting social difficulties are poorly understood.
Social cognitive skills undergo protracted development through late childhood and adolescence, corresponding with extended structural and functional maturation of connectivity between medial prefrontal, lateral temporo-parietal and cerebellar hub regions of the ‘social brain’ network (Blakemore, Reference Blakemore2008; Adolphs, Reference Adolphs2009; Burnett & Blakemore, Reference Burnett and Blakemore2009). Given the well-documented vulnerability of these regions to the acceleration–deceleration forces of paediatric TBI (Tasker et al. Reference Tasker, Salmond, Westland, Pena, Gillard, Sahakian and Pickard2005; Wilde et al. Reference Wilde, Hunter, Newsome, Scheibel, Bigler, Johnson, Fearing, Cleavinger, Li, Swank, Pedroza, Roberson, Bachevalier and Levin2005; Bigler et al. Reference Bigler, Abildskov, Petrie, Farrer, Dennis, Simic, Taylor, Rubin, Vannatta, Gerhardt, Stancin and Owen Yeates2013), one hypothesis suggests that TBI elevates risk for social cognitive impairment via traumatic axonal injury that may disrupt the integration properties of networks supporting social cognitive processes (Ryan et al. Reference Ryan, Anderson, Godfrey, Beauchamp, Coleman, Eren, Rosema, Taylor and Catroppa2013a ; Hayes et al. Reference Hayes, Bigler and Verfaellie2016).
Consistent with the vulnerability of the ‘social brain’ to disruption from TBI, social cognitive impairments are commonly documented in children with TBI, and include difficulty recognizing emotions from facial expressions and prosody (Tlustos et al. Reference Tlustos, Chiu, Walz, Taylor, Yeates and Wade2011; Ryan et al. Reference Ryan, Anderson, Godfrey, Beauchamp, Coleman, Eren, Rosema, Taylor and Catroppa2013a ), using language to meet social constraints (McDonald et al. Reference McDonald, English, Randall, Longman, Togher and Tate2013; Ryan et al. Reference Ryan, Anderson, Godfrey, Eren, Rosema, Taylor and Catroppa2013b ), and taking the perspective of others (Dennis et al. Reference Dennis, Simic, Gerry Taylor, Bigler, Rubin, Vannatta, Gerhardt, Stancin, Roncadin and Yeates2012, Reference Dennis, Agostino, Taylor, Bigler, Rubin, Vannatta, Gerhardt, Stancin and Yeates2013a ; Bellerose et al. Reference Bellerose, Bernier, Beaudoin, Gravel and Beauchamp2015). Despite a well-documented association between paediatric TBI and social cognitive deficits, there is likely substantial variability in social outcome that is not explained by clinical indicators of injury severity (Hayes et al. Reference Hayes, Bigler and Verfaellie2016). For instance, while the severity of traumatic axonal injury is often linked to the duration of loss of consciousness and other clinical markers of severity (Hayes et al. Reference Hayes, Bigler and Verfaellie2016; Wilde et al. Reference Wilde, Li, Hunter, Narayana, Hasan, Biekman, Swank, Robertson, Miller and McCauley2016), findings regarding the relationship between social cognitive outcomes and injury severity have been mixed. Research is needed to evaluate the utility of newer and more sensitive imaging techniques to characterize the distribution and extent of white matter injury, and identify neuroanatomical correlates of social cognitive outcomes (Ashwal et al. Reference Ashwal, Tong, Ghosh, Bartnik-Olson and Holshouser2014; Roberts et al. Reference Roberts, Mathias and Rose2016).
Diffusion tensor imaging (DTI) measures the magnitude and directionality of water diffusion in tissue, notably of white matter tracts, and represents a sensitive biomarker of diffuse white matter injury in TBI via detection of traumatic axonal injury (Wilde et al. Reference Wilde, Ayoub, Bigler, Chu, Hunter, Wu, McCauley and Levin2012; Dennis et al. Reference Dennis, Jin, Villalon-Reina, Zhan, Kernan, Babikian, Mink, Babbitt, Johnson, Giza, Thompson and Asarnow2015). DTI is used to index the microstructural organization of white matter using commonly derived metrics, including fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD) and radial diffusivity (RD) (Huisman et al. Reference Huisman, Schwamm, Schaefer, Koroshetz, Shetty-Alva, Ozsunar, Wu and Sorensen2004; Dennis et al. Reference Dennis, Jin, Villalon-Reina, Zhan, Kernan, Babikian, Mink, Babbitt, Johnson, Giza, Thompson and Asarnow2015). Compared with typically-developing and orthopaedic injury controls, children and adolescents with chronic moderate-severe TBI show lowered FA and/or higher MD in numerous white matter fibre bundles, including the corpus callosum (Wilde et al. Reference Wilde, Chu, Bigler, Hunter, Fearing, Hanten, Newsome, Scheibel, Li and Levin2006, Reference Wilde, Ayoub, Bigler, Chu, Hunter, Wu, McCauley and Levin2012; Wu et al. Reference Wu, Wilde, Bigler, Li, Merkley, Yallampalli, McCauley, Schnelle, Vasquez and Chu2010), inferior and superior frontal white matter (Wozniak & Lim, Reference Wozniak and Lim2006; Oni et al. Reference Oni, Wilde, Bigler, McCauley, Wu, Yallampalli, Chu, Li, Hunter and Vasquez2010), internal capsule (Yuan et al. Reference Yuan, Holland, Schmithorst, Walz, Cecil, Jones, Karunanayaka, Michaud and Wade2007), superior cerebellar peduncle (SCP) (Caeyenberghs et al. Reference Caeyenberghs, Leemans, Geurts, Taymans, Linden, Smits-Engelsman, Sunaert and Swinnen2010, Reference Caeyenberghs, Leemans, Geurts, Vander Linden, Smits-Engelsman, Sunaert and Swinnen2011), orbitofrontal white matter, cingulum and uncinate fasciculus (UF) (Roberts et al. Reference Roberts, Mathias and Rose2014).
Despite evidence for widespread white matter disruption to neural networks supporting social cognition (Levin et al. Reference Levin, Wilde, Hanten, Li, Chu, Vasquez, Cook, Yallampalli and Hunter2011; Roberts et al. Reference Roberts, Mathias and Rose2014), DTI correlates of these skills have received limited investigation in survivors of paediatric TBI. Preliminary cross-sectional findings from Levin et al. (Reference Levin, Wilde, Hanten, Li, Chu, Vasquez, Cook, Yallampalli and Hunter2011) showed that reduced FA in left medial prefrontal white matter and the cingulum bundle were associated with reduced accuracy on a mental state attribution task. To date, however, prospective studies of these brain–behaviour relationships are lacking, and thus the prognostic value of acute and/or sub-acute DTI for long-term social cognitive outcomes is unexplored.
In the present study, we aimed to evaluate social cognitive outcomes at 6- and 24 months after mild complex-severe paediatric TBI, and quantify sub-acute white matter microstructural differences using a Tract-Based Spatial Statistics (TBSS) in TBI and typically developing control (TDC) groups. From the TBSS WM skeleton of comparison across subjects, we aimed to identify significant regional differences in the sub-acute period and relate microstructural white matter differences with social cognitive outcomes, both at 6- and 24-months post-injury, in children with TBI. Consistent with recent meta-analytic evidence (Roberts et al. Reference Roberts, Mathias and Rose2016), we predicted a widespread pattern of DTI abnormalities in fronto-temporal white matter (e.g. UF, inferior occipito-frontal fasciculus), limbic (e.g. cingulum, fornix), commissural (e.g. splenium) and cerebro-cerebellar white matter regions (e.g. middle and superior cerebellar penduncles). Given that fronto-temporal, limbic, commissural and cerebro-cerebellar tracts are a key locus of pathology in paediatric TBI and play a critical role in large-scale networks supporting social cognition (Carrington & Bailey, Reference Carrington and Bailey2009), we expected that abnormal white matter organization in these regions would be prospectively associated with poorer social cognitive outcomes at 6- and 24-months post-injury.
Methods
Participants
This study included 95 children: 52 survivors of TBI (30 males) and 43 typically developing (TD) children (24 males), group-matched for age and sex. Children were recruited to participate in a larger longitudinal study, which aimed to investigate the psychosocial consequences of TBI (Anderson et al. Reference Anderson, Beauchamp, Yeates, Crossley, Hearps and Catroppa2013; Catroppa et al. Reference Catroppa, Crossley, Hearps, Yeates, Beauchamp, Rogers and Anderson2015). Children with TBI were recruited at time of injury, and represented consecutive admissions to The Royal Children's Hospital (RCH), Melbourne, Australia. TD children were recruited from the community, through local schools chosen to provide a range of socio-economic backgrounds. All participants were aged between 8.3 and 15.4 years at time of recruitment.
For the TBI group, inclusion criteria were: (i) documented evidence of closed head injury, including a period of altered consciousness or presence of at least two post-concussive symptoms; (ii) medical records sufficiently detailed to determine injury severity, including the Glasgow Coma Scale (GCS) (Teasdale & Jennett, Reference Teasdale and Jennett1974), and neurological and radiological findings; and (iii) child and at least one parent fluent in English. Using emergency department (ED) records and information obtained from an in-house, non-standardized parent interview administered upon study enrolment, the following exclusion criteria were applied: (i) non-accidental head injuries; (ii) diagnosed congenital, neurological, developmental, or psychiatric condition (e.g. mood/anxiety disorder); (iii) prior intervention for social impairment; and (iv) previous TBI based on parent report.
Participants with TBI were classified as: (i) mild-complex TBI (n = 14): GCS 13–15, evidence of mass lesion on CT or clinical magnetic resonance imaging (MRI); (ii) moderate TBI (n = 25): GCS 9–12, and/or mass lesion or other evidence of specific injury on CT/MRI, and/or neurological impairment; (iii) severe TBI (n = 13): GCS 3–8, and/or mass lesion or other evidence of specific injury on CT/MRI, and/or neurological impairment.
Measures
Pre-injury functioning
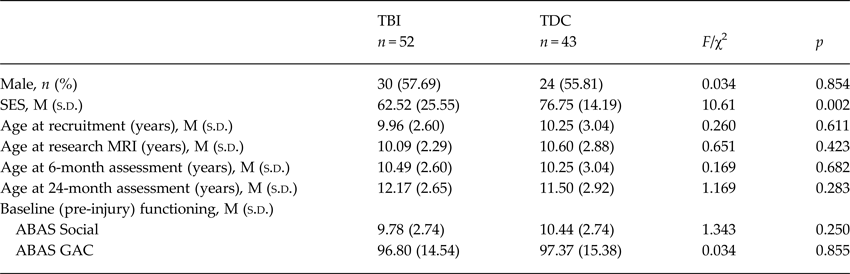
At the time of injury, parents provided retrospective ratings of their child's pre-injury adaptive and social skills in the weeks preceding injury using the Adaptive Behavior Assessment System-II (ABAS-II) (Harrison & Oakland, Reference Harrison and Oakland2003), which is a parent questionnaire that examines functional skills necessary for daily living. The Global Adaptive Composite (M = 100, s.d. = 15) and Social Composite are reported in Table 1. No significant group differences were identified when estimates of pre-injury functioning in the TBI group were compared with ratings provided by TDC parents who completed the same questionnaire about their child at initial recruitment.
Table 1. Demographic characteristics of sample

TDC, typically developing control; SES, socioeconomic status; ABAS, Adaptive Behavior Asessment System; GAC, Global Adaptive Composite.
Social cognition: 6- and 24-months post-injury
As described in the sub-sections that follow (i–iii), children were administered a standardized test of pragmatic language, alongside two experimental measures of ToM, which have been previously validated in the child TBI population (Dennis et al. Reference Dennis, Simic, Gerry Taylor, Bigler, Rubin, Vannatta, Gerhardt, Stancin, Roncadin and Yeates2012, Reference Dennis, Simic, Bigler, Abildskov, Agostino, Taylor, Rubin, Vannatta, Gerhardt and Stancin2013b ; Robinson et al. Reference Robinson, Fountain-Zaragoza, Dennis, Taylor, Bigler, Rubin, Vannatta, Gerhardt, Stancin and Yeates2014). Due to the experimental nature of the two ToM tasks, performance of the TBI groups was compared with the age- and gender-matched TDC group.
-
(i) Cognitive ToM
The Jack and Jill Task (Dennis et al. Reference Dennis, Simic, Gerry Taylor, Bigler, Rubin, Vannatta, Gerhardt, Stancin, Roncadin and Yeates2012) was administered to assess cognitive ToM, reflected in children's understanding of false belief. Participants are shown three consecutive frames on a computer screen. Each frame includes a character (Jack and/or Jill), two hats (red and blue) and a ball. In Frame A of each sequence, Jack is preparing to drop a ball into either a blue or red hat while Jill watches. In Frame B, Jack either moves the ball further into the blue hat (unswitched trials) or switches the ball to the red hat (switched trials). Jill is present in half of Frame B trials (witnessed trials) and absent in the other half (unwitnessed trials). In Frame C, participants decide whether Jill's belief about the location of the ball is correct or incorrect. Jill's judgement depends on what she believes about the ball's location, not its actual location: she will choose the original (Frame A) hat if she did not witness the switch. The ToM trials involve an unwitnessed switch of hat colour; control trials are those in which the switch was witnessed. Percentage of correct responses for switched, unwitnessed trials was the primary measure of cognitive ToM.
-
(ii) Affective ToM
The Emotional and Emotive Faces Task (EFFT) (Dennis et al. Reference Dennis, Agostino, Taylor, Bigler, Rubin, Vannatta, Gerhardt, Stancin and Yeates2013a ) was administered to assess affective ToM, or the child's understanding of the difference between emotional expression (a character's inner emotion) and emotive communication (the emotion a character coveys socially, which may be different from the inner emotion). Participants were presented with 10 narratives that described a character in situations that were designed to evoke one of five basic emotions: happiness, disgust, fear, sadness and anger. In each vignette, a discrepancy existed between the emotion felt ‘inside’ and the character’s facial expression. In keeping with the interpretative guidelines provided by the test developers (Dennis et al. Reference Dennis, Agostino, Taylor, Bigler, Rubin, Vannatta, Gerhardt, Stancin and Yeates2013a ), each vignette involves (i) affective ToM trials, which ask the child how the character looked on his/her face (‘look on face’ condition) and (ii) otherwise identical control trials, which merely require the child to select the facial emotion display that matches the in-text description of how the protagonist was feeling (‘feel inside’ condition). ‘Feel inside’ control trials are considered distinct from affective ToM trials since they simply require the child to select the facial emotion display that matches the explicit in-text description of the protagonist’s emotional state (Dennis et al. Reference Dennis, Agostino, Taylor, Bigler, Rubin, Vannatta, Gerhardt, Stancin and Yeates2013a ). Percentage accuracy for emotive communication trials (i.e. ‘look on face’) was the primary measure of affective ToM.
-
(iii) Pragmatic language
The Making Inferences subtest from the Test of Language Competence-Expanded Edition (TLC-E) (Wiig & Secord, Reference Wiig and Secord1989) evaluates the ability to make permissible inferences on the basis of existing causal relationships or chains on short paragraphs. The task requires the ability to interpret propositions, recognise and generate underlying social scripts, and to make logical inferences based on knowledge of possible causal chains in an evoked script. TLC results, in combination with error pattern analyses and behavioural observations, can: (a) differentiate patients with traumatic closed head injury who have reached a high level of recovery from patients at lower levels of recovery; (b) identify error patterns that provide suggestions for the locus and level of pragmatic problems; and (c) suggest a focus and objectives for language intervention (Vogel, Reference Vogel1992). Age-adjusted standard scores were calculated and employed for statistical analyses (M = 10; s.d. = 3).
MR acquisition
Children underwent a MRI research scan at 5 weeks post-injury (M = 5.55, s.d. = 3.05 weeks) using imaging specifications reported previously (Genc et al. Reference Genc, Anderson, Ryan, Malpas, Catroppa, Beauchamp and Silk2016). In brief, transverse 2D single-shot echo-planar images were acquired at 3.0 T (Siemens Tim Trio, Erlangen, Germany): Repetition-time/Echo-time (TR/TE): 9300/104 ms, voxel size 2.0 mm isotropic, with 60 diffusion-encoding gradient directions (b = 2000 s/mm2) and 10 images acquired with no diffusion weighting (b = 0 s/mm2). A susceptibility-weighted imaging (SWI) sequence was also acquired using a standardized imaging protocol previously reported elsewhere (Beauchamp et al. Reference Beauchamp, Ditchfield, Babl, Kean, Catroppa, Yeates and Anderson2011). TDCs also underwent the same MR imaging protocol at initial recruitment.
Image processing
Diffusion-weighted images were visually inspected for motion artefact, signal loss and slice drop-out. The diffusion data were then pre-processed using the FSL toolbox (Smith et al. Reference Smith, Jenkinson, Woolrich, Beckmann, Behrens, Johansen-Berg, Bannister, De Luca, Drobnjak and Flitney2004) by correcting for eddy current distortions and subject motion, skull-stripping and diffusion tensor fitting with weighted-least squares.
A whole brain voxel-wise statistical analysis was carried out using TBSS implemented in FSL (Smith et al. Reference Smith, Jenkinson, Johansen-Berg, Rueckert, Nichols, Mackay, Watkins, Ciccarelli, Cader and Matthews2006). The mean FA image was created and skeletonized (excluding voxels with an FA ⩽0.3), and projected onto each participant's FA map. These steps were repeated for MD, AD and RD maps.
To assess the potential impact of focal lesions on our processing, we visually inspected SWI images overlaid with the white matter skeleton from TBSS, and observed minimal overlap between the skeleton and the visible lesions.
Statistical analysis
All analyses (except for voxel-wise analyses performed with FSL) were conducted using SPSS Version 22.00 (IBM Corporation). All variables were screened for violations of normality. Normality plots indicated that all primary outcome measures were normally distributed, and preliminary analyses indicated no violation of statistical assumptions across analyses (including ANCOVAs) unless otherwise stated. Multicollinearity was explored, with age and injury and age at testing being highly correlated, consistent with the longitudinal design of the project.
Demographic and social cognitive outcomes
Analysis of variance (ANOVA) or χ2 was conducted to investigate group differences for demographic and clinical variables. Analysis of covariance (ANCOVA) was used to examine potential differences between TBI and TD groups for social cognitive outcomes, covarying for age at assessment and sex.
Imaging analysis
A whole brain voxel-wise approach was used to identify regional white-matter differences between the TBI and TD groups. The mean value across the whole white-matter skeleton was extracted and subjected to further analysis. For the voxel-wise analysis, a general linear model (GLM) was generated using the FSL software, with age and sex added as nuisance variables. Each diffusion metric was analysed using the permutation-based method ‘Randomise’ in FSL with 10 000 permutations. Multiple comparisons across voxels were corrected using the threshold-free cluster enhancement (TFCE) method at p < 0.05, with a cluster size of >100 voxels (Smith & Nichols, Reference Smith and Nichols2009). TFCE avoids making an arbitrary choice of the cluster-forming threshold, while preserving the sensitivity benefits of cluster-wise correction (Miyata et al. Reference Miyata, Yamada, Namiki, Hirao, Saze, Fujiwara, Shimizu, Kawada, Fukuyama and Sawamoto2010). From a raw statistical image, TFCE produces an output image in which the voxel value represents a weighted sum of the local clustered signal.
Brain structure–function relationships in the TBI sample
Multivariate linear regression models were implemented in SPSS 22.0 (IBM Corporation), and used to examine relationships between sub-acute WM microstructure and social cognition measures at 6- and 24 months post-injury in the TBI group.
The results of randomize analysis in FSL were used to inform the selection of atlas-defined regions of interest (ROIs) for subsequent analyses of brain–behaviour relationships. In order to select these atlas-defined ROIs the JHU ICBM-DTI-81 DTI white matter atlas was registered to the study-specific mean images (Wakana et al. Reference Wakana, Caprihan, Panzenboeck, Fallon, Perry, Gollub, Hua, Zhang, Jiang and Dubey2007). The peak co-ordinates from each cluster were located using the atlas to determine if they lay within the boundary of a particular white matter tract; if the peak co-ordinates were ‘unclassifiable’, then the co-ordinates were inspected visually to assign an approximate location of the cluster within a particular brain region. The goal of these analyses was to find WM regions, which act as predictors of social cognitive performance in children with TBI; hence, only regions showing between-group differences in the overlapping map were included in the brain–behaviour analyses (Veeramuthu et al. Reference Veeramuthu, Narayanan, Kuo, Delano-Wood, Chinna, Bondi, Waran, Ganesan and Ramli2015; Liu et al. Reference Liu, Wang, Liu, Yu, Yang, Jin, Sun, Yang, Qin and Calhoun2016). WM regions of the overlapping map were defined as those atlas-defined regions exhibiting TBSS group-wise differences across all MD, AD and RD maps. For brain structure–function analyses, the significance criterion was set at a p value of 0.05 applying false discovery rate (FDR) correction for multiple comparisons.
For all analyses involving the primary outcome measures, we acknowledged factors previously shown to influence social cognitive outcomes after TBI, including injury severity (Dennis et al. Reference Dennis, Simic, Gerry Taylor, Bigler, Rubin, Vannatta, Gerhardt, Stancin, Roncadin and Yeates2012), age and sex (Zupan et al. Reference Zupan, Babbage, Neumann and Willer2016). Since measures of social cognition in the TBI sample were significantly related to age and sex (p < 0.05) but not injury severity (all p > 0.10), multivariate models controlled for age at testing and sex. This approach enabled us to determine if brain structure measures are unique and therefore useful markers of post-injury social cognitive outcomes in children with TBI.
Results
Demographic variables
Group comparisons identified no significant group differences in sex, age at testing or family structure. Similarly, baseline child adaptive behaviour was comparable across groups. A significant group difference was present for socioeconomic status (SES) (see Table 1), and therefore SES was included as a covariate in group analyses of the primary outcome measures.
Social cognition at 6-months post-TBI
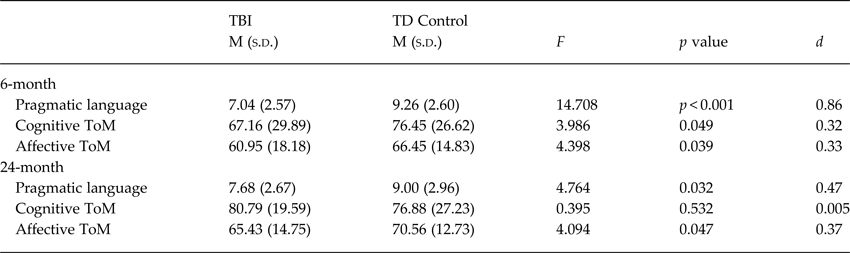
As shown in Table 2, ANCOVAs controlling for SES, sex and age revealed that children with TBI performed significantly worse than TD controls on measures of pragmatic language (p < 0.001), cognitive ToM (p = 0.049) and affective ToM (p = 0.039).
Table 2. Performance on social cognition measures at 6- and 24-months post-injury

Social cognition at 24-months post-TBI
Eighty-one of the original 95 participants enrolled in the study (41 TBI and 40 TDC) completed measures of social cognition at 24-months post-injury. A sensitivity analysis was carried out between children with and without 24-month data, and revealed no clinical or significant differences on any pre-injury, demographic or injury-related variable.
For 24-month outcomes (see Table 2), ANCOVAs controlling for SES, sex and age revealed that children with TBI performed significantly worse than TD controls on measures of pragmatic language and affective ToM (p = 0.032 and 0.047, respectively). Groups showed comparable cognitive ToM.
Relation between social cognition and injury severity
Multivariate regression analyses revealed that GCS was not significantly associated with pragmatic language (β = 0.191; p = 0.174), cognitive ToM (β = 0.193; p = 0.174) or affective ToM (β = 0.007; p = 0.960) at 6-months post-injury. Similarly, injury severity was not a significant predictor of pragmatic language (β = 0.126; p = 0.432), cognitive ToM (β = 0.263; p = 0.097) or affective ToM (β = −0.169; p = 0.291) at 24-months post-injury.
Exploring prospective relationships between 6- and 24-month social cognitive outcomes
As a basis of comparison to analyses of the relation between DTI and longitudinal social cognitive outcomes, we used multivariate regression models to examine the predictive utility of baseline social cognitive outcomes (i.e. 6 months) for social cognitive outcomes at 24-months post-injury.
Pragmatic language
Analyses revealed that 24-month pragmatic language outcome was not significantly associated with baseline pragmatic language (p = 0.064), cognitive ToM (p = 0.718) or affective ToM (p = 0.167).
Affective ToM
Regression analyses revealed that 24-month affective ToM was significantly associated with baseline affective ToM (p = 0.004). Affective ToM at 24-months post-injury was not significantly associated with baseline cognitive ToM (p = 0.684) or pragmatic language (p = 0.185).
Cognitive ToM
Analyses revealed that 24-month cognitive ToM was significantly associated with baseline cognitive ToM (p = 0.042) and pragmatic language (p = 0.031); however, these results did not survive FDR correction for multiple comparisons. Twenty-four-month cognitive ToM was not significantly associated with baseline affective ToM (p = 0.069).
Evaluating the impact of TBI on white matter microstructure
DTI data for 65 participants (35 TBI, 30 TD control) were of sufficient quality for TBSS analyses. A sensitivity analysis was carried out between children with and without complete DTI data, and revealed no statistically significant differences on any pre-injury, demographic or injury-related variable.
To determine whether TBI had a sub-acute effect on white matter, we performed a whole-brain voxel-wise analysis on diffusion metrics across the TBI and TDC groups. Regions of significant group difference are bilateral unless reported otherwise.
The voxel-wise analysis revealed significantly higher MD, AD and RD in the sub-acute TBI group compared with TDC, when controlling for age and sex. There was no significant difference in FA. Areas of MD difference were identified in the splenium of the corpus callosum (sCC), fornix, middle cerebellar peduncle (MCP), superior cerebellar peduncle (SCP), internal capsule, corona radiata, sagittal stratum (SS), dorsal cingulum (DC) and uncinate fasciculus (UF). Regions of AD and RD differences were found in the external capsule, SCP, DC, SS, sCC, MCP, SCP and UF.
Prospective brain–behaviour relationships in the TBI group
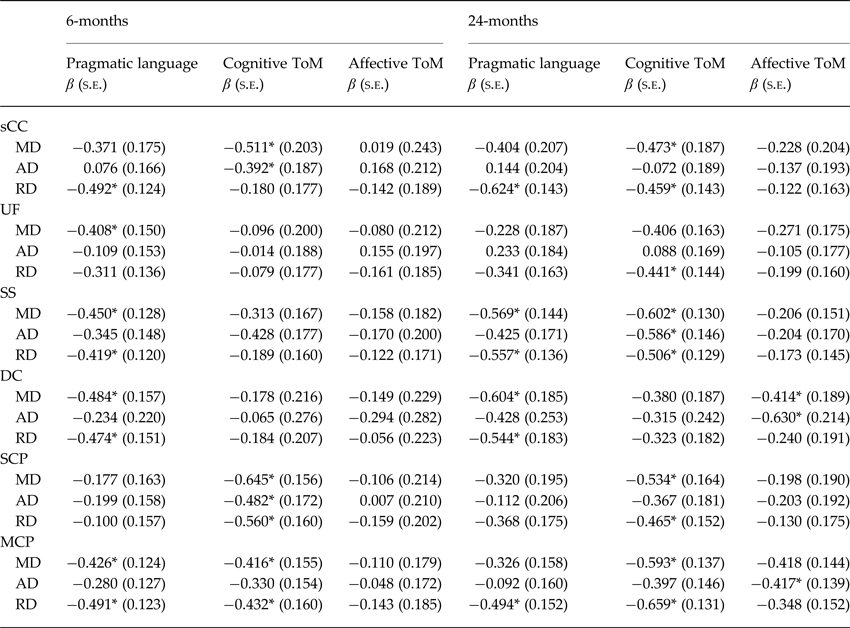
Multivariate linear regression models were used to examine relationships between social cognition and WM regions showing between-group differences in the overlapping map. The overlapping WM regions exhibiting TBSS group-wise differences across all MD, AD and RD maps included the SS, sCC, UF, DC, MCP and SCP.
Mean diffusivity (MD)
After applying FDR correction, MD was negatively associated with cognitive ToM and pragmatic language at 6- and 24-months post-injury. As shown in Table 3, higher MD in the sCC, SS, SCP and MCP predicted poorer cognitive ToM. In addition, higher MD in the UF, SS, DC and MCP was associated with poorer pragmatic language.
Table 3. Adjusted associations between sub-acute white matter microstructure and social cognitive outcomes at 6- and 24-months post-injury

sCC, Splenium of the corpus callosum; UF, Uncinate Fasciculus; SS, Sagittal Stratum; DC, Dorsal Cingulum; SCP, Superior Cerebellar Peduncle; MCP, Middle Cerebellar peduncle.
* Significant after false discovery rate correction. FDR adjusted p = 0.011.
Axial diffusivity (AD)
Similarly, after applying FDR correction AD was negatively related to cognitive and affective ToM. More specifically, higher AD in the sCC, SCP and SS predicted poorer cognitive ToM. Moreover, higher AD in the DC and MCP predicted poorer affective ToM at 24-months post-injury only (Table 3).
Radial diffusivity (RD)
Finally, RD was negatively associated with cognitive ToM and pragmatic language. As shown in Table 3, higher RD in the sCC, SS, DC and MCP predicted poorer pragmatic language. Moreover, higher RD in the sCC, SCP, MCP, UF and SS were associated with lower cognitive ToM.
Average beta coefficients were derived from the correlation matrix (Table 3), and were used to assess whether the strength of the brain–behaviour relationships varied as a function of assessment time point (i.e. 6- v. 24-months post-injury). Correlations between DTI indices and social cognitive outcomes became marginally stronger with increasing time since injury [i.e. average β = −0.47 (6-months post-injury) v. average β = −0.54 (24-months post-injury].
When brain–behaviour analyses were repeated in the TDC group, no significant predictive ability of sub-acute DTI was found for pragmatic language, cognitive ToM or affective ToM at either the 6- or 24-month assessment points (all p > 0.10).
Discussion
To the best of our knowledge, this was the first study to investigate prospective relationships between sub-acute WM microstructure and longitudinal social cognitive outcomes in the child TBI population. Compared with TDCs, children with sub-acute TBI show widespread increases in mean, radial and axial diffusivity observed mainly in the sCC, UF, SS, DC, MCP and SCP. Relative to TDCs, children with TBI showed poorer cognitive ToM, affective ToM and pragmatic language at 6-months post-insult, and those deficits were related to abnormal diffusivity of the sCC, UF, SS, DC, MCP and SCP. Moreover, children with TBI showed poorer affective ToM and pragmatic language at 24 months post-injury, and those difficulties were predicted by altered diffusivity of the DC and MCP.
Characterizing the sub-acute impact of TBI on white matter microstructure
Comparison of TBI and TDC children using TBSS revealed microstructural differences in children with TBI, including increases in MD, AD and RD. Interestingly, and in contrast to previous studies of chronic phase paediatric TBI (Wozniak et al. Reference Wozniak, Krach, Ward, Mueller, Muetzel, Schnoebelen, Kiragu and Lim2007; Ewing-Cobbs et al. Reference Ewing-Cobbs, Prasad, Swank, Kramer, Cox, Fletcher, Barnes, Zhang and Hasan2008), there was no evidence for group differences in FA, which may reflect across-study variation in the timing of DTI acquisition (Roberts et al. Reference Roberts, Mathias and Rose2014). For instance, there is evidence to suggest that findings may differ depending on whether the DTI is acquired in the acute phase of paediatric TBI – when axonal swelling or cytotoxic oedema may be associated with temporary increases in FA – or the chronic phase when these effects have reversed and, consequently, FA decreases (Wozniak et al. Reference Wozniak, Krach, Ward, Mueller, Muetzel, Schnoebelen, Kiragu and Lim2007; Ewing-Cobbs et al. Reference Ewing-Cobbs, Prasad, Swank, Kramer, Cox, Fletcher, Barnes, Zhang and Hasan2008; Bigler & Bazarian, Reference Bigler and Bazarian2010; Roberts et al. Reference Roberts, Mathias and Rose2014).
Overall, the pattern of regional sub-acute white matter abnormalities in our TBI sample is in keeping with recent meta-analytic evidence pointing to the vulnerability of fronto-temporal, limbic and projection fibres to the acceleration–deceleration forces of moderate-to-severe TBI (Roberts et al. Reference Roberts, Mathias and Rose2014). Despite considerable overlap with findings from previous TBI research, with few exceptions (Genc et al. Reference Genc, Anderson, Ryan, Malpas, Catroppa, Beauchamp and Silk2016), previous studies of moderate-severe paediatric TBI have focused primarily on microstructural abnormalities detected in the medium to long-term post-injury (Roberts et al. Reference Roberts, Mathias and Rose2014). The current findings therefore add to a small body of existing research in the sub-acute phase by suggesting that DTI provides a sensitive index of the distribution and extent of white matter injury in mild-complex-severe TBI (Dennis et al. Reference Dennis, Jin, Villalon-Reina, Zhan, Kernan, Babikian, Mink, Babbitt, Johnson, Giza, Thompson and Asarnow2015).
Extending previous TBI research, which has seldom quantified clinically useful radial and axial diffusivities in the sub-acute phase, we show that TBI is associated with whole-brain increases in RD and AD, in addition to regional microstructural abnormalities in several association, projection and commissural fibre pathways considered common loci of pathology in paediatric TBI (Roberts et al. Reference Roberts, Mathias and Rose2014). While MD may index several variables, including fibre density, myelination and expansion of extracellular space (Aung et al. Reference Aung, Mar and Benzinger2013), AD and RD are believed to assess the status of axonal morphology and the myelin sheath, respectively (Sun et al. Reference Sun, Liang, Trinkaus, Cross, Armstrong and Song2006; Aung et al. Reference Aung, Mar and Benzinger2013). Although the complex relationships among the DTI variables precludes categorical inferences about underlying biological causes of alterations in brain microstructure, higher AD and RD in the TBI sample may be suggestive of de-myelination and axonal shearing that results from acceleration–deceleration forces of paediatric TBI (Adamson et al. Reference Adamson, Yuan, Babcock, Leach, Seal, Holland and Wade2013). These findings are broadly consistent with studies of adult TBI reporting higher AD and RD in voxel-wise analyses, with higher AD in areas of focal injury in the splenium believed to reflect axotomy (Kraus et al. Reference Kraus, Susmaras, Caughlin, Walker, Sweeney and Little2007; Newcombe et al. Reference Newcombe, Williams, Nortje, Bradley, Harding, Smielewski, Coles, Maiya, Gillard and Hutchinson2009). Overall, while our findings suggest that mild-complex-severe TBI is associated with widespread neural changes affecting tissue organization, myelin and axonal integrity (Ewing-Cobbs et al. Reference Ewing-Cobbs, Prasad, Swank, Kramer, Cox, Fletcher, Barnes, Zhang and Hasan2008), the prognostic value of sub-acute microstructural abnormalities for post-acute and chronic social outcomes has been unexplored in previous paediatric TBI research.
Prospective structure–function relationships
Cerebro-cerebellar and commissural pathways
The most robust white matter correlates of cognitive ToM were identified within cerebro-cerebellar and commissural pathways previously implicated in social cognitive neural networks (Carrington & Bailey, Reference Carrington and Bailey2009; Van Overwalle et al. Reference Van Overwalle, D'aes and Mariën2015; Van Overwalle & Mariën, Reference Van Overwalle and Mariën2016). The finding that poorer cognitive ToM and pragmatic language were associated with higher diffusivity of the splenium is consistent with previous paediatric TBI research linking impaired social cognition and pragmatic communication to structural abnormalities of the posterior corpus callosum (Beauchamp et al. Reference Beauchamp, Anderson, Catroppa, Maller, Godfrey, Rosenfeld and Kean2009; Ewing-Cobbs et al. Reference Ewing-Cobbs, Prasad, Swank, Kramer, Mendez, Treble, Payne and Bachevalier2012; Ryan et al. Reference Ryan, Anderson, Godfrey, Beauchamp, Coleman, Eren, Rosema, Taylor and Catroppa2013a ), which consists of larger-diameter fibres that carry connections to superior temporal and posterior parietal regions recruited for social and emotion processing (Schmahmann & Pandya, Reference Schmahmann and Pandya2009; Gilliam et al. Reference Gilliam, Stockman, Malek, Sharp, Greenstein, Lalonde, Clasen, Giedd, Rapoport and Shaw2011). Moreover, the strong prospective relation between cognitive ToM and the MCP and SCP aligns with recent evidence linking cognitive ToM to regions of a distributed cerebro-cerebellar mentalising network (Van Overwalle et al. Reference Van Overwalle, D'aes and Mariën2015; Van Overwalle & Mariën, Reference Van Overwalle and Mariën2016). For instance, a recent meta-analytic connectivity modelling (MACM) study found that cerebellar involvement in social cognitive processing reflects distinct social mentalising functionality, and that regions in lobules VI and Crus I show robust connectivity with the mentalising network in the cerebrum, including the dmPFC, TPJ and temporal pole (Van Overwalle et al. Reference Van Overwalle, D'aes and Mariën2015). Since the SCP represents a major efferent WM pathway from the cerebellum to the cerebral cortex (D'Mello & Stoodley, Reference D'Mello and Stoodley2015), our findings support the importance of cerebro-cerebellar connectivity for social cognitive function in the developing brain, and suggest that disruption to pathways exiting the cerebellum may be a risk factor for poor long-term social function after TBI.
Fronto-temporal and limbic pathways
Evidence for robust links between the UF and multiple measures of social function is consistent with previous evidence implicating frontal-limbic brain networks in social-affective processing (Sethi et al. Reference Sethi, Gregory, Dell'Acqua, Thomas, Simmons, Murphy, Hodgins, Blackwood and Craig2015). For instance, the UF is shown to play a key role in integrating linguistic and paralinguistic information coded in the superior temporal cortices with affective, motivational, evaluative and mentalising mechanisms in the inferior frontal regions (Von Der Heide et al. Reference Von Der Heide, Skipper, Klobusicky and Olson2013; Ameis & Catani, Reference Ameis and Catani2015). Consistent with previous studies linking disruption of the UF to abnormal social behaviour, impaired understanding of others’ affective states, and reduced empathy (Phan et al. Reference Phan, Orlichenko, Boyd, Angstadt, Coccaro, Liberzon and Arfanakis2009; Pugliese et al. Reference Pugliese, Catani, Ameis, Dell'Acqua, de Schotten, Murphy, Robertson, Deeley, Daly and Murphy2009; Tartaglia et al. Reference Tartaglia, Zhang, Racine, Laluz, Neuhaus, Chao, Kramer, Rosen, Miller and Weiner2012; Oishi et al. Reference Oishi, Faria, Hsu, Tippett, Mori and Hillis2015), our findings show that abnormal anterior frontal-temporal connectivity is associated with persisting social impairment in the TBI sample.
Finally, evidence linking both pragmatic language and affective ToM to abnormal diffusivity of the DC converges with previous research linking the dorsal subdivision of the default-mode network to introspection and social function, including evaluating self and others’ emotional states (Ochsner et al. Reference Ochsner, Knierim, Ludlow, Hanelin, Ramachandran, Glover and Mackey2004). More specifically, the DC connects the posterior cingulate cortex and medial prefrontal regions, which have been linked to both ToM (Amodio & Frith, Reference Amodio and Frith2006), and pragmatic language (Tesink et al. Reference Tesink, Buitelaar, Petersson, Van der Gaag, Kan, Tendolkar and Hagoort2009). Based on the robust link between abnormal diffusivity in the DC and poorer pragmatic language and affective ToM, our findings suggest that abnormal frontal-limbic connectivity may be a risk factor for persisting social impairments after paediatric TBI.
Cortico-subcortical fibres
The links between cognitive ToM, pragmatic language and the SS underline the distributed nature of brain networks that support social cognitive processes (Phan et al. Reference Phan, Orlichenko, Boyd, Angstadt, Coccaro, Liberzon and Arfanakis2009; Mahoney et al. Reference Mahoney, Rohrer, Omar, Rossor and Warren2011; Kennedy & Adolphs, Reference Kennedy and Adolphs2012; Chiong et al. Reference Chiong, Wilson, D'Esposito, Kayser, Grossman, Poorzand, Seeley, Miller and Rankin2013; Ameis & Catani, Reference Ameis and Catani2015). The SS is a large bundle of white matter fibres that serve to connect frontal, cingulate, temporal, parietal and occipital cortical regions to the thalamus and other deep structures (Davis et al. Reference Davis, Oishi, Faria, Hsu, Gomez, Mori and Hillis2016). Since integration of information between physically distant brain regions is essential for integrating social information from one's environment and responding appropriately (Jalbrzikowski et al. Reference Jalbrzikowski, Villalon-Reina, Karlsgodt, Senturk, Chow, Thompson and Bearden2014) this finding is perhaps not surprising, and is in keeping with previous reports linking SS damage to reduced emotion recognition (Philippi et al. Reference Philippi, Mehta, Grabowski, Adolphs and Rudrauf2009), and impaired sarcasm processing after stroke (Davis et al. Reference Davis, Oishi, Faria, Hsu, Gomez, Mori and Hillis2016).
Clinical implications
Group differences on the ToM and pragmatic language measures adds to an increasing body of literature documenting social cognitive sequelae that persist into the long-term post-paediatric TBI (Dennis et al. Reference Dennis, Simic, Gerry Taylor, Bigler, Rubin, Vannatta, Gerhardt, Stancin, Roncadin and Yeates2012, Reference Dennis, Agostino, Taylor, Bigler, Rubin, Vannatta, Gerhardt, Stancin and Yeates2013a ; Ryan et al. Reference Ryan, Catroppa, Cooper, Beare, Ditchfield, Coleman, Silk, Crossley, Beauchamp and Anderson2015). These findings thus underscore the importance of long-term follow-up of children presenting with early neuroanatomical and environmental risk factors. To the best of our knowledge, our study represents the first to show that sub-acute microstructural changes in limbic, projection and association pathways may hold prognostic significance for post-acute and chronic social cognitive outcomes after paediatric TBI.
Notably, abnormal brain microstructure was predictive of affective ToM at 24-months post-injury only. More specifically, affective ToM was related predominantly to altered diffusivity of the DC, which is a frontal-limbic connection that is considered one of the most immature structural links within the developing brain (Supekar et al. Reference Supekar, Uddin, Prater, Amin, Greicius and Menon2010) and continues to undergo myelination and structural organisation of axonal tracts well into adulthood (Gogtay et al. Reference Gogtay, Giedd, Lusk, Hayashi, Greenstein, Vaituzis, Nugent, Herman, Clasen and Toga2004; Thompson et al. Reference Thompson, Sowell, Gogtay, Giedd, Vidal, Hayashi, Leow, Nicolson, Rapoport and Toga2005). Since high-level social cognitive skills show extended development into mid-to-late adolescence (Choudhury et al. Reference Choudhury, Blakemore and Charman2006; Dumontheil et al. Reference Dumontheil, Apperly and Blakemore2010), these findings suggest that brain–behaviour relationships in the TBI group may not emerge until later in development when more complex aspects of ToM and associated fronto-temporal brain regions are undergoing rapid structural and functional maturation (Giedd et al. Reference Giedd, Blumenthal, Jeffries, Castellanos, Liu, Zijdenbos, Paus, Evans and Rapoport1999; Gogtay et al. Reference Gogtay, Giedd, Lusk, Hayashi, Greenstein, Vaituzis, Nugent, Herman, Clasen and Toga2004; Blakemore, Reference Blakemore2008).
Limitations
The relatively small size of the sample places constraints on our statistical analytic approach, with larger samples required to model potential mediational relationships between sub-acute DTI abnormalities, social cognition and broader indices of social outcome, including social adjustment (Robinson et al. Reference Robinson, Fountain-Zaragoza, Dennis, Taylor, Bigler, Rubin, Vannatta, Gerhardt, Stancin and Yeates2014). Moreover, several well-documented caveats apply to our TBSS approach (Bach et al. Reference Bach, Laun, Leemans, Tax, Biessels, Stieltjes and Maier-Hein2014). Although TBSS is the most widely used approach for characterizing microstructural changes in paediatric TBI (Roberts et al. Reference Roberts, Mathias and Rose2014), the tensor model is insufficient to model crossing fibre populations present in the majority of white matter (Tournier et al. Reference Tournier, Yeh, Calamante, Cho, Connelly and Lin2008; Wilde et al. Reference Wilde, Ayoub, Bigler, Chu, Hunter, Wu, McCauley and Levin2012). Accordingly, further studies are needed to model crossing fibres using a non-tensor based model to disentangle fibre-specific alterations as a result of TBI.
In addition, machine learning approaches likely represent a more sensitive method to improve the predictive value of DTI, particularly when making individual predictions. Future studies using larger paediatric samples could incorporate this type of technique, and should explicitly model a large number of clinical variables that are known to be important in explaining outcome (Hellyer et al. Reference Hellyer, Leech, Ham, Bonnelle and Sharp2013).
A further limitation of our study was the exclusive focus on social cognitive outcomes of the TBI sample. Given that neuroanatomical abnormalities were observed in distributed brain regions that are not uniquely associated with social cognition, further research is required to clarify whether these neuroanatomical abnormalities may be predictive of other possible co-occurring cognitive and behavioural difficulties in the TBI sample.
Conclusion
Consistent with previous reports highlighting the sensitivity of DTI to diffuse white matter injury in paediatric TBI (Dennis et al. Reference Dennis, Jin, Villalon-Reina, Zhan, Kernan, Babikian, Mink, Babbitt, Johnson, Giza, Thompson and Asarnow2015), our findings show that sub-acute TBI is characterised by widespread microstructural abnormalities in fronto-temporal, limbic, cerebro-cerebellar and cortico-subcortical fibres. The current study addresses a dearth of research examining potential relations between sub-acute DTI metrics and longitudinal social cognitive outcomes, and suggests that abnormal frontal-limbic and cerebro-cerebellar connectivity may be risk factors for persisting social impairment. These findings suggest that DTI has potential to unlock early predictive markers of post-acute and chronic social cognitive difficulties in paediatric TBI.
Acknowledgements
This work was supported by a grant from the Victoria Neurotrauma Initiative (CO6E1), Australia; the Victorian Government Operational Infrastructure Support Program; an Australian Postgraduate Award, MCRI Ph.D. scholarship, and NHMRC Moving Ahead Centre for Research Excellence in Brain Injury Recovery Seed Grant to NR; and an NHMRC Senior Practitioner Fellowship to VA. The funding bodies did not play a role in the design of the study, collection, analysis and interpretation of the data, or writing of the manuscript.
Declaration of Interest
None.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.