INTRODUCTION
Neurocysticercosis, the disease caused by the larval stage of the cestode Taenia solium, is an important cause of morbidity, and in some cases, mortality (Fleury et al. Reference Fleury, Flisser, Flores-Rivera, Corona, Smith, Brogden and Fratamico2009). It affects many developing countries where people eat pork meat. Knowledge of the epidemiology and risk factors has been gained through many studies (Flisser and Gyorkos, Reference Flisser and Gyorkos2007). The key finding is that the main risk factor for acquiring human and swine cysticercosis is the presence of a tapeworm carrier in the household (Schantz et al. Reference Schantz, Moore, Muñoz, Hartman, Schaefer, Aron, Persaud, Sarti, Wilson and Flisser1992). Humans acquire the intestinal infection after consuming pork meat that is insufficiently cooked containing live cysticerci; the adult flatworm develops in the small intestine; the last proglottids, which are gravid, are shed with faeces. Some segments can be damaged within the intestinal lumen, thus parasite eggs may also be liberated within faeces. Free-roaming pigs that live where open latrines or airborne faecalism exist, ingest human faeces and, if contaminated with gravid proglottids or free eggs, cysticerci develop in their muscles and brain. The life cycle is repeated when an infected pig is slaughtered without inspection and a person eats insufficiently cooked meat (Flisser, Reference Flisser1988; Garcia et al. Reference Garcia, Gonzalez, Evans and Gilman2003a).
Establishing laboratory models of the adult T. solium infection is important for the following reasons: 1. Human beings are the only natural hosts for the adult stage of T. solium, therefore hampering the study of the life cycle stage of the parasite that causes taeniosis. 2. Tapeworm carriers in the household are the main risk factor for acquiring cysticercosis. 3. Taeniosis is generally a silent disease, which, in spite of the likelihood of self-diagnosis, is difficult to identify. 4. The frequency of taeniosis in autopsy studies is very low as compared to that of neurocysticercosis. 5. Sensitive and accessible molecular assays, such as ELISA for coproantigen detection, have been standardized to identify tapeworm carriers. 6. Drugs against T. solium are usually chosen based on treatment efficacy against T. saginata, a different species that also causes intestinal infection in humans, but does not cause human cysticercosis. 7. Humoral and cellular immune responses can be studied in the intestinal mucosa, mesenteric lymph nodes, spleen, serum, saliva and faeces of experimental hosts. 8. Vaccines against human taeniosis can be evaluated. 9. Immune regulation by helminths in the intestinal mucosa is now an emerging area of immunological research.
In this review information that has been generated with experimental models of taeniosis due to T. solium is discussed.
ELISA FOR HUMAN COPROANTIGEN DETECTION
The initial goal of developing an experimental model for taeniosis was to obtain T. solium antigenic material in order to develop an immunological assay to detect antigens in human faeces, so that the sensitivity of diagnosis by coprological analysis could be increased. Based on the description given by Anne Verster (Reference Verster1971, Reference Verster1974) for the successful establishment of T. solium in hamsters, this model was reproduced using immunodepressed outbred Syrian golden hamsters that were infected orally with T. solium cysticerci recovered from naturally infected pigs. Faeces were collected from infected and control hamsters and the intestines examined post-mortem for the presence of worms. The coproantigen (CpAg) ELISA is based on polyclonal rabbit antibodies directed against a whole saline extract of adult worms or excretion/secretion products in order to bind tapeworm antigens from faeces and the reaction visualized using different enzymatic systems (Allan et al. Reference Allan, Avila, García-Noval, Flisser and Craig1990; Maravilla et al. Reference Maravilla, Avila, Cabrera, Aguilar and Flisser1998; Avila et al. Reference Avila, Benitez, Aguilar and Flisser2003). The main conclusions are: (1) Antigen detection has a sensitivity greater than 90% (Allan et al. Reference Allan, Velasquez-Tohom, Torres-Alvarez, Yurrita and García-Noval1996) and is genus specific since faeces obtained from T. solium and T. saginata carriers are positive and no cross-reaction is found with faeces from other parasitic infections including Hymenolepis; (2) CpAg are stable for days in unfixed faecal samples, or for long periods in frozen samples or in samples fixed in formalin and kept at room temperature; (3) Sensitivity is dependent on the assay format and the quality of the rabbit sera used, a high titre offers a higher sensitivity, but since rabbit anti-Taenia serum is not commercially available, antibody titres and affinity vary; (4) CpAg detection becomes positive prior to patency in human samples and infected hamsters become positive 10 days after infection; positivity is independent of the presence or number of eggs in faeces and the assay becomes negative within a week of treatment (Allan et al. Reference Allan, Avila, García-Noval, Flisser and Craig1990, Reference Allan, Wilkins, Tsang and Craig2003; Garcia et al. Reference Garcia, Gonzalez, Evans and Gilman2003a; Flisser and Gyorkos, Reference Flisser and Gyorkos2007; Guezala et al. Reference Guezala, Rodriguez, Zamora, Garcia, Gonzalez, Tembo, Allan and Craig2009). These conclusions indicate that CpAg ELISA is useful for diagnosis of human tapeworm carriers and may thus be applied in field studies.
USE OF CpAg ELISA IN FIELD INTERVENTION STUDIES
CpAg ELISA has been successfully used in several community-based studies. One such study performed in Mexico allowed detecting changes in the rate of human taeniosis before and after an educational campaign directed especially towards generating changes in KAP (knowledge, attitudes and practices) regarding cysticercosis and taeniosis. The high efficacy of the research project was demonstrated because no new pigs with cysticercosis were found 6 months after the intervention; it also proved that education prompted people to seek cestocidal treatment. In addition, this study confirmed that egg detection does not reflect the real frequency of taeniosis in field conditions since of 1404 individuals analyzed before intervention, 0·8% were positive by CpAg ELISA, but only 0·1% by egg detection; after the campaign 0·5% of 792 individuals were positive for CpAg but no changes in the frequency of people releasing Taenia eggs was found (Sarti et al. Reference Sarti, Flisser, Schantz, Gleizer, Loya, Plancarte, Avila, Allan, Craig, Bronfman and Wijeyaratne1997). Similar conclusions were obtained in another field study performed in Peru, where CpAg provided 2·8% positivity in 1620 samples; the 45 CpAg positive samples were submitted to coproparasitoscopical studies and only 38% were positive, confirming the higher sensitivity of CpAg ELISA (Garcia et al. Reference Garcia, Gilman, Gonzalez, Verastegui, Rodriguez, Gaviria, Tsang, Falcon, Lescano, Moulton, Bernal and Tovar2003b). When mass drug treatment against the adult tapeworm was administered in another community, the use of CpAg ELISA demonstrated that the dosage used (praziquantel at 5 mg/kg) was insufficient since prior to treatment 1·1% positive individuals (of 1865 samples) were detected, while after 6 months 0·5% of the 1311 individuals were positive, showing a reduction in taeniosis of 55% instead of 95% as reported with 10 mg/kg (Sarti et al. Reference Sarti, Schantz, Avila, Ambrosio, Medina-Santillán and Flisser2000). The decrease in human CpAg positivity and in swine cysticercosis was still evident 42 months after health education (Flisser, unpublished results) or drug treatment (Sarti et al. Reference Sarti, Schantz, Avila, Ambrosio, Medina-Santillán and Flisser2000).
EXPERIMENTAL MODELS
Several mammal species were evaluated as T. solium tapeworm carriers. Unsuccessful results were obtained with rhesus monkeys, pigs, dogs, cats, white rabbits, white rats, guinea pigs and white mice in which, depending on the species, between 2 and 20 immunodepressed and non-immunodepressed animals were used. Only one cat and one dog had incipient tapeworms that remained for a few days in the small intestine (Varma and Ahluwalia, Reference Varma and Ahluwalia1992; Maravilla et al. Reference Maravilla, Avila, Cabrera, Aguilar and Flisser1998, Avila et al., unpublished results). In a different study, one gibbon without immunosuppression, was experimentally infected and a gravid tapeworm was recovered (Cadigan et al. Reference Cadigan, Santon, Tanticharoenyus and Chaicumpa1967); no further studies have been performed in primates. Studies carried out with different adult cestode species indicate that only rodents from Cricetidae, Heteromyidae and Chinchillidae are susceptible as experimental models.
Morphological characteristics of the scolex of worms developed in hamsters were identical to those of T. solium obtained from humans (Avila et al. Reference Avila, Teran, Aguilar, Maravilla, Mata and Flisser2006). Electron microscopy of attached parasites to the intestinal mucosa of hamsters showed that the 4 suckers were anchored and tegumentary microtriches were well preserved (Merchant et al. Reference Merchant, Aguilar, Avila, Robert, Flisser and Willms1998). The brush-border seen in an epithelial cell appeared intact in a 3 day infection, whereas host tissue in 10 and 40 day-old infections exhibited loss of cellular, nuclear, and mitochondrial membranes, resulting in a necrotic intestinal epithelium (Merchant et al. Reference Merchant, Aguilar, Avila, Robert, Flisser and Willms1998). Tapeworm maturation is related to the size of the strobila. Longer parasites develop pre-gravid proglottids, while in worms less than 2 cm long, only the scolex and neck could be identified (Avila et al. Reference Avila, Teran, Aguilar, Maravilla, Mata and Flisser2006). Fig. 1 shows the scolex, immature proglottids and one pre-gravid segment with a central uterus and some initial lateral branches. When mature segments were present, many reproductive structures could be seen, such as the cirrus (Fig. 2).
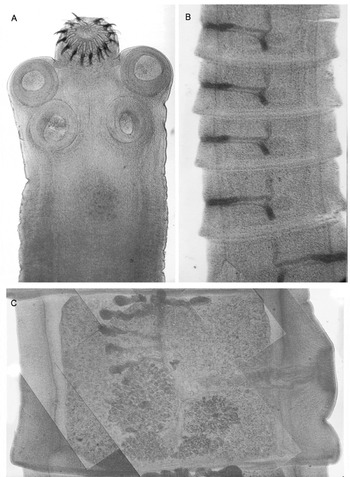
Fig. 1. Photographic images showing histological characteristics of the structures that constitute a tapeworm: the scolex and neck (A) were obtained from an infected hamster as was the ribbon of mature proglottids (B), while the pregravid proglottid was obtained from an infected gerbil (C).

Fig. 2. The ejected cirrus in a mature proglottid recovered from a sample of sieved hamster faeces can be seen slightly curved towards the vagina.
Steroids administered to rodents increase worm recovery rate and parasites have a greater sexual development and survive longer periods (Gnezdilov, Reference Gnezdilov1957; Verster, Reference Verster1971, Reference Verster1974; Andersen, Reference Andersen1978, Kamiya and Sato, Reference Kamiya and Sato1990a, Reference Kamiya and Satob; Allan et al. Reference Allan, García-Domínguez, Craig, Rogan, Lowe and Flisser1991; Maravilla et al. Reference Maravilla, Avila, Cabrera, Aguilar and Flisser1998; Avila et al. Reference Avila, Aguilar, Benitez, Yepez-Mulia, Lavenat and Flisser2002; Reference Avila, Benitez, Aguilar and Flisser2003; Reference Avila, Teran, Aguilar, Maravilla, Mata and Flisser2006; Conchedda et al. Reference Conchedda, Gabriele and Bortoletti2006). Regarding adult infections of T. solium, golden hamsters (Mesocricetus auratus) and Mongolian gerbils (Meriones unguiculatus) are particularly interesting because in both Cricetidae the host-parasite relationship is different. Establishment of tapeworms was associated with the concentration of methyl prednisolone acetate (MPA). Gerbils which were not suppressed or received 2 mg of MPA showed 5% tapeworm recovery, when treated with 4 mg and 8 mg, recovery rate increased to 18% and 45%, respectively. A similar picture was observed when tapeworm development was analyzed. Immature worms were obtained from non-depressed gerbils, while pre-gravid ones were recovered from gerbils which received the higher dose of MPA (Maravilla et al. Reference Maravilla, Avila, Cabrera, Aguilar and Flisser1998). Golden hamsters are more permissive hosts that gerbils. When hosts are not immunodepressed infections last up to 46 days in hamsters and up to 25 days in gerbils, and worms measure up to 40 cm in hamsters but only 1–2 cm in gerbils (Avila et al. Reference Avila, Aguilar, Benitez, Yepez-Mulia, Lavenat and Flisser2002), also showing the need of immunodepression to maintain the infection in the latter. Tapeworm recovery rates were 41% in hamsters compared to 10% in gerbils. Cp-Ag ELISA was useful to follow infections, in immunodepressed hamsters, parasite antigens could be detected up to 18 weeks post infection, while in gerbils positivity remained up to 8 weeks only in animals that were treated with 4 or 8 mg of MPA, corresponding with tapeworm recovery at necropsy (Maravilla et al. Reference Maravilla, Avila, Cabrera, Aguilar and Flisser1998; Avila et al. Reference Avila, Teran, Aguilar, Maravilla, Mata and Flisser2006). In contrast to the higher Taenia solium recovery found in gerbils that received higher doses of MPA, gerbils infected with T. crassiceps and depressed with 3 or 5 mg of butyl-acetate of prednisolone, had 25% and 22% tapeworms, respectively. This study also showed a high correlation between worm weight, length and total proglottid number (Sato and Kamiya, Reference Sato and Kamiya1990).
Different immunosuppressant drugs have been assayed to try to obtain gravid parasites in the rodent models. Immunodepression of infected hamsters with hydrocortisone allowed recovery of tapeworms (Pathak and Gaur, Reference Pathak and Gaur1985; Varma and Ahluwalia, Reference Varma and Ahluwalia1992) as well as prednisolone or mepyramine maleate but not cyclophosphamide and triamcinolone acetonide. The rate of worm development was faster in treated groups and the mean length of worms on day 25 was 10 mm compared to 1 to 2 mm in controls. The worms recovered from hamsters given prednisolone had developed to sexual maturity but were unable to produce eggs. The development of worms was best seen in hamsters treated with mepramine maleate or prednisolone (Pathak and Gaur, Reference Pathak and Gaur1985). methotrexate (MTX) and mycophenolate mofetil (MMF), non-steroid drugs widely used in autoimmunity, transplant and oncology medicine, were also assayed. No tapeworms were found at necropsy when MTX (2 mg/100 gr) or MMF (200 mg/100 gr) were used, while those treated with MPA had tapeworms but also had alopecia and weight loss (Jimenez-Gonzalez, unpublished results). An important outcome of this study was the use of guidelines for the evaluation of the effect of different immunodepressant drugs in rodents (Buckwell, Reference Buckwell and Dolan1999). Table 1 shows the waste parameters defined by Buckwell, based mainly on physical observation of the animal. These factors were analyzed in the experiment performed with MTX, MMF and MPA along 6 weeks of weekly drug administration. The wasting and suffering index increased along the study, with statistical significance only in the last week in the MPA group, but never reached the 12 points required to stop the experiment and sacrifice the animal (Jimenez-Gonzalez, unpublished results). These data indicate that the wasting effect should be evaluated when experimental rodents are submitted to steroid or other potentially damaging treatments.
Table 1. Waste parameters defined by Buckwell (Reference Buckwell and Dolan1999), based mainly on physical observation of the experimental rodent

* When the sum of all characteristics reaches 12 points the hamster is humanely euthanized
Experimental hamster infections are also useful to demonstrate the effect of cobalt irradiation for pork meat sterilization. Higher doses inhibited evagination of cysticerci, while lower doses only avoided infection of hamsters (Aluja et al. Reference Aluja, Nuñez and Villalobos1993). Additional experiments showed apoptosis in cysticerci inhibiting their normal development into the adult worm. Gamma irradiation of food is considered a plausible approach to control food-borne diseases, including cysticercosis (Flores-Perez et al. Reference Flores-Perez, Fragoso-Gonzalez, Sciutto and de Aluja2003).
Chinchillas (Chinchilla laniger) are currently the only host species, with the exception of one gibbon, that supported completion of the life cycle of T. solium. Gravid tapeworms were recovered from MPA-treated chinchillas and infective eggs were used to successfully infect pigs which developed cysticercosis. Three experiments were performed, each with 11 chinchillas that were immunodepressed with MPA and orally infected with cysticerci. CpAg ELISA was performed and was positive starting at week 4 post infection; gravid proglottids containing infective eggs were found in faeces at 7 weeks post-infection and tapeworms were recovered at necropsy (Fig. 3). Recovery rate was 36%, lower than in hamsters (67%) but tapeworms had gravid proglottids, which were used to infect 6 pigs, all of which developed cysticerci (Maravilla et al. Reference Maravilla, Avila, Cabrera, Aguilar and Flisser1998, Avila et al. Reference Avila, Teran, Aguilar, Maravilla, Mata and Flisser2006; Garza-Rodriguez, A., unpublished results; Gomez-Diaz, B., unpublished results). Therefore the experimental model of taeniosis due to T. solium in Chinchilla laniger could be a good alternative for analyzing the development and growth of gravid tapeworms in the host and for providing eggs and adult parasites to be used in different types of experiments, including vaccine trials. Nevertheless more studies have to be performed in order to improve this model since wasting due to steroid treatment was evident (see above).

Fig. 3. Small fraction of a chinchilla intestine after necropsy showing several attached tapeworms, with well developed strobila.
TAPEWORM SURVIVAL
An intriguing question that was answered using the hamster model was whether tapeworms can survive the death of the host. An apparent discrepancy arises from a study performed in 20,206 human necropsies that reports 0·05% prevalence of intestinal taeniosis compared to 2·4% brain cysticercosis (Villagran and Olvera, Reference Villagran-Uribe, Olvera-Rabiela, Flisser and Malagón1989), and most reports on human necropsies performed in Mexico do not identify intestinal tapeworms. Two alternatives may explain the absence of taeniosis in necropsies performed in Mexico: either the tapeworm is easily destroyed after the host dies or it has a short life span in humans and therefore flatworms are rarely present in post-mortem studies. The experimental model in hamsters allowed comparison with the human intestine since as stated by Spady et al. (Reference Spady, Cuthbert, Willard and Meidell1996), the hamster closely resembles humans with respect to basal rates of cholesterol and bile salt synthesis, composition of the bile salt pool, various aspects of lipoprotein transport, and response to dietary and pharmacologic interventions. In fact, some of these characteristics must be important in allowing development of T. solium in both hosts. Worms could be identified even at 96 hours post-mortem although damage increased over time. The results obtained indicate that the absence of tapeworms in the human intestine during necropsy is not due to post-mortem digestion, and supports the view that the existing assumptions about the longevity of T. solium may be incorrect (Garza-Rodriguez et al. Reference Garza-Rodriguez, Maravilla, Mendlovic, Mata-Miranda, Robert and Flisser2007).
HUMORAL IMMUNE RESPONSE
Hamsters are also useful to study antibody kinetics against excretory/secretory (E/S) antigens in faeces and in circulation. All infected hamsters in both groups (with or without MPA) developed an intense systemic humoral immune response indicating that MPA had a partial effect on antibody production. Specific IgG was detected in both faecal and serum samples at 2 weeks post infection and throughout infection until necropsy. On the other hand, local IgG production (faecal antibodies) was successfully inhibited by MPA. Only when this treatment was suspended due to wasting effect, faecal IgG peaked and tapeworm expulsion in this group occurred (Avila et al. Reference Avila, Aguilar, Benitez, Yepez-Mulia, Lavenat and Flisser2002). Serum antibodies can also be detected by western blot in people harbouring a tapeworm, and no cross-reaction is found with patients having cysticercosis or with T. saginata carriers. The assay has 95% sensitivity and 100% specificity with E/S obtained from hamster tapeworms (Wilkins et al. Reference Wilkins, Allan, Verastegui, Acosta, Eason, Garcia, Gonzalez, Gilman and Tsang1999). Intriguing is the finding that when E/S was obtained from T. solium oncospheres, although the same degree of sensitivity was found, 20% of cross-reactivity was detected in patients with active neurocysticercosis (Verastegui et al. Reference Verastegui, Gilman, Garcia, Gonzalez, Arana, Jeri, Tuero, Gavidia, Levine and Tsang2003).
INFLAMMATORY RESPONSE AND CYTOKINES IN THE INTESTINAL MUCOSA
Monroy-Ostria et al. (Reference Monroy-Ostria, Monroy-Ostria, Gomez and Hernandez1993) showed that in non-immunodepressed hamsters the inflammatory reaction, induced by T. solium, was located in the mucosa surrounding the scolices. Macrophages, epithelioid cells and some plasma cells, with little alteration of the epithelium were observed at 2 weeks post infection, while at 6 and 8 weeks the epithelium was damaged and necrotized, and around five months after infection the lesions started to resolve. Tapeworms started to be expelled at 4 weeks post-infection and by 14 weeks no parasites could be detected. Avila et al. (Reference Avila, Aguilar, Benitez, Yepez-Mulia, Lavenat and Flisser2002, Reference Avila, Teran, Aguilar, Maravilla, Mata and Flisser2006) analyzed the type of cells present in the small intestine of infected rodents. Both hamsters and gerbils were studied since, as mentioned above, they differ in their permissiveness to T. solium. The normal shape of the mucosa was lost in infected animals; oedema and loss of the villous structure, stunted and deformed villi were observed. Interestingly, in both hosts the inflammatory reaction developed only around the attachment site of the scolices, with scarce macrophages, a slight increase of plasma cells, lymphocytes and fibroblasts, a moderate increase of eosinophils and neutrophils, and high numbers of goblet cells, which were small and round during the first 6 days post infection (dpi), later on, they increased in size and hypersecretion of mucus between villi and in the lumen of the small intestine was observed (Fig. 4). The highest increase in these cells was seen at 13 dpi in gerbils but until 18 dpi in hamsters. Still more interesting was the finding that only gerbils developed an intense mast cell response at the attachment site and histamine concentration was elevated in the intestinal fluid with a maximum detection at the same time that the peak of mast cells (19 dpi). The recovery of tapeworms was inversely related to the number of both cell types, which finally decreased when tapeworms were eliminated. These data confirm that golden hamsters are more permissive hosts for T. solium adults than gerbils and that most probably mast cells are main players in the expulsion of parasites (Avila et al. Reference Avila, Aguilar, Benitez, Yepez-Mulia, Lavenat and Flisser2002, Reference Avila, Teran, Aguilar, Maravilla, Mata and Flisser2006).

Fig. 4. Histological section of the small intestinal mucosa of an infected gerbil, stained with periodic acid/Schiff. A tapeworm is attached to the mucosa close to a Peyer's patch; one sucker has host tissue within. The mucosa and the tapeworm show intense staining of mucin and, in the mucosa, goblet cells are seen as darker dots.
Since cytokines play a crucial role in determining the outcome of parasitic infections (Anthony et al. Reference Anthony, Rutitzky, Urban, Stadecker and Gause2007), cytokine expression was evaluated in infected hamsters by the detection of mRNA at the site of tapeworm attachment at different times post-infection (Avila et al. Reference Avila, Aguilar, Romero-Valdovinos, Garcia-Vasquez and Flisser2008). mRNA for IFN-γ and IL-13 were the first detected at 2 and 4 dpi, while IL-4 was detected at 6 dpi and IL-5 was the last one detected at 8 dpi. The number of positive animals for each cytokine increased with time of infection, except for IFN-γ at 16 dpi and a predominance of mRNA for Th2 cytokines was observed; mRNA for IL-13 increased mostly at 8 dpi with respect to initial values. Results indicate that T. solium induces a mixed Th1/Th2 response in the intestinal mucosa of hamsters, with a predominance of a Th2 response after two weeks post-infection, when the tapeworms began to be expelled (Avila et al. Reference Avila, Aguilar, Romero-Valdovinos, Garcia-Vasquez and Flisser2008). Mixed Th1 and Th2 response have been reported in brain granulomas from neurocysticercosis patients. The inflammatory reaction was constituted by abundant plasma cells, B and T lymphocytes, macrophages and mast cells. Th1 cytokines were prevalent and included IFN-γ, IL-18 and TGF-β, but Th2 cytokines, such as IL-4, IL-13 and IL-10, were also present (Restrepo et al. Reference Restrepo, Alvarez, Castaño, Arias, Restrepo, Trujillo, Colegial and Teale2001).
PROTECTION INDUCED BY TAENIA SOLIUM CALRETICULIN
Calreticulin is a highly conserved, multifunctional protein, with an important role in housekeeping and in host-parasite relationships (Michalak et al. Reference Michalak, Corbett, Mesaeli, Nakamura and Opas1999; Mendlovic et al. Reference Mendlovic, Ostoa-Saloma, Solis, Martínez Ocaña, Flisser and Laclette2004). Taenia solium calreticulin (TsCRT) is expressed in cysticerci and adult tapeworms and is preferentially localized in tegumentary and muscle cytons of the suckers and rostellum; interestingly it is spatially and temporally regulated during the development of T. solium, especially during germ cell maturation and embryogenesis (Fig. 5). Expression was demonstrated by immunolocalization using mouse antibodies elicited with recombinant TsCRT (Mendlovic et al. Reference Mendlovic, Carrillo-Farga, Torres, Laclette and Flisser2006). Calreticulin interacts with the immune system of the host; anti-CRT antibodies have been detected in patients with infections caused by trypanosomes, schistosomes and Onchocerca (Rokeach et al. Reference Rokeach, Zimmerman and Unnasch1994; Marcelain et al. Reference Marcelain, Colombo, Molina, Ferreira, Lorca, Aguillon and Ferreira2000) and it has been detected as an excretion/secretion product in some nematode and trematode parasites (Suchitra et al.; Reference Suchitra and Joshi2005; Guillou et al. Reference Guillou, Roger, Mone, Rognon, Grunau, Theron, Mitta, Coustau and Gourbal2007). Calreticulin is over-expressed in worms during chronic intestinal parasitic diseases (Morgan et al. Reference Morgan, LaCourse, Rushbrook, Greetham, Hamilton, Barrett, Bailey and Brophy2006); it stimulates B and T cells, induces IL-4 production and interacts with C1q (El Gengehi et al. Reference El Gengehi, El Ridi, Tawab, El Demellawy and Mangold2000; Kasper et al. Reference Kasper, Brown, Eberl, Vallar, Kieffer, Berry, Girdwood, Eggleton, Quinnell and Pritchard2001; Rzepecka et al. Reference Rzepecka, Rausch, Klotz, Schnöller, Kornprobst, Hagen, Ignatius, Lucius and Hartmann2009). Moreover, it has been identified as a vaccine candidate for hookworm infection resulting in 43–49% reduction in worm burden (Winter et al. Reference Winter, Davies, Brown, Garnett, Stolnik and Pritchard2005). This information indicates that TsCRT may be involved in the modulation of the immune system during infection and might be a good vaccine candidate.
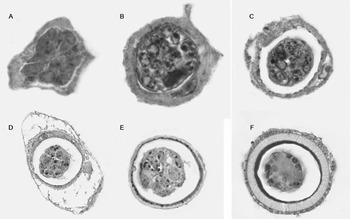
Fig. 5. Fertilized eggs from gravid proglottids at different stages of maturation show differential expression of TsCRT. In the morula stage (A), and early differentiating embryos (B, C), TsCRT expression is evident in most oncosphere cells. In later stages, (D, E) oncosphere cells show diminished expression of the protein. No TsCRT expression is observed in the mature oncosphere with a fully formed embryophore (F). TsCRT was visualized with specific anti-TsCRT antibodies and a secondary antibody coupled to horseradish peroxidase. The reaction was evident as dark precipitates.
Only few examples of vaccination trials against adult tapeworms have been published. A vaccine with E/S antigens obtained from Echinoccoccus granulosus adults showed 64% reduction in the number of worms found in vaccinated dogs and 90% suppression of egg production (Herd et al. Reference Herd, Chappel and Biddell1975). In a recent study, an oral vaccine using two recombinant proteins of the same parasite, induced in dogs 80% decrease in parasite burden and a slower development rate in all remaining worms (Petavy et al. Reference Petavy, Hormaeche, Lahmar, Ouhelli, Chabalgoity, Marchal, Azzouz, Schreiber, Alvite, Sarciron, Maskell, Esteves and Bosquet2008). Although Petavy's results are promising, the study was performed with few control dogs and, due to the normal distribution of parasite numbers, the efficacy in the vaccinated group was less evident than the authors claimed (Torgerson, Reference Torgerson2009). The hamster model of taeniosis was used to assess vaccination with S3Pvac, a synthetic vaccine developed against cysticercosis (Huerta et al. Reference Huerta, de Aluja, Fragoso, Toledo, Villalobos, Hernandez, Gevorkian, Acero, Díaz, Alvarez, Avila, Beltran, Garcia, Martinez, Larralde and Sciutto2001) in six golden hamsters. Subcutaneous vaccination significantly reduced the number of tapeworms suggesting the possibility of a protective immune response against the adult tapeworm (Cruz-Revilla et al. Reference Cruz-Revilla, Toledo, Rosas, Huerta, Flores-Perez, Peña, Morales, Cisneros-Quiñones, Meneses, Díaz-Orea, Anciart, Goldbaum, Aluja, Larralde, Fragoso and Sciutto2006). A recombinant TsCRT and cholera toxin (CT) as adjuvant was consequently employed for the evaluation of oral vaccination in the hamster model of taeniosis in several trials. Protection (between 33 and 100%) was associated with the type of cysticerci and the age of the hamsters used. Furthermore, tapeworms found in immunized hosts were smaller and were attached further from the pylorus as compared to those found in control hamsters (Leon-Cabrera et al. Reference Leon-Cabrera, Cruz-Rivera, Mendlovic, Avila-Ramírez, Carrero, Laclette and Flisser2009). These data agree with findings of other authors that suggest that size reduction, fecundity and expulsion rates in helminths are a consequence of protective immunity (Onah and Nawa, Reference Onah and Nawa2000) and that local immune responses affect the capacity of attachment and nutrition of the parasite, creating a hostile environment in the gastrointestinal tract (Anthony et al. Reference Anthony, Rutitzky, Urban, Stadecker and Gause2007).
CONCLUSIONS
The use of animal models of taeniosis has been critical for understanding the host-parasite relationship during Taenia solium infection. Development of the CpAg ELISA is a result of the use of experimental models of taeniosis and has proven more sensitive than the existing coproparasitological assays. After nearly 40 years of use of golden hamsters and, more recently, gerbils and chinchillas as experimental hosts, the results obtained exhibit a significant impact upon future research focused on applied aspects such as vaccination and drug evaluation, as well as basic research directed towards the parasite's biology, the host's immune response and novel knowledge on the host-parasite relationship.
ETHICAL AND FINANCIAL SUPPORT
All protocols were submitted to the Ethics and Research Committees of the Hospital General “Dr. Manuel Gea González”, Ministry of Health or of the Faculty of Medicine, National Autonomous University of Mexico. Studies were commenced only after approval.
The National Autonomous University of Mexico (UNAM) provided grants: DGAPA/PAPIIT IN203900, IN206703, IN206908, IN209994, IN220007 and support from the University Program for Research in Health (PUIS). The Mexican National Council of Science and Technology (CONACYT) made available grant 28094-B; contract CI1-CT940081 from the Commonwealth of European Communities was used for CpAg ELISA development and the International Development Research Centre of Canada (IDRC) supported the community based intervention study from 1990 to 1993.








