INTRODUCTION
Parkinson’s disease (PD) is the prototypical hypokinetic movement disorder, defined by its cardinal and diagnostic motor features of bradykinesia, rigidity, tremor, and postural instability. Increasingly, PD is also recognized as a syndrome with clinically distinct motor subtypes and phenotypic presentations. Based on the prevailing diagnostic motor feature, most classification systems emphasize several key motor subtypes; tremor dominant (TD), postural instability and gait disorder (PIGD), akinetic rigid (AR) and an indeterminate or mixed type where there is no predominant symptom (Alves, Larsen, Emre, Wentzel-Larsen, & Aarsland, Reference Alves, Larsen, Emre, Wentzel-Larsen and Aarsland2006; Jankovic et al., Reference Jankovic, McDermott, Carter, Gauthier, Goetz, Golbe and Shoulson1990; Paulus & Jellinger, Reference Paulus and Jellinger1991; Rajput, Voll, Rajput, Robinson, & Rajput, Reference Rajput, Voll, Rajput, Robinson and Rajput2009; Schiess, Zheng, Soukup, Bonnen, & Nauta, Reference Schiess, Zheng, Soukup, Bonnen and Nauta2000). PIGD and TD subtypes have been the most widely compared in the literature. They are not only distinguishable by the predominant motor symptom, but also demonstrate differential patterns of anatomical neurodegeneration and disease progression (Herb et al., Reference Herb, Rane, Isaacs, Van Wouwe, Roman, Landman and Claassen2016; Rajput et al., Reference Rajput, Voll, Rajput, Robinson and Rajput2009; Rosenberg-Katz et al., Reference Rosenberg-Katz, Herman, Jacob, Giladi, Hendler and Hausdorff2013; Verbaan et al., Reference Verbaan, Marinus, Visser, van Rooden, Stiggelbout, Middelkoop and van Hilten2007; Wu et al., Reference Wu, Guo, Wei, Ou, Song, Cao and Shang2016), with notable differences in the rate of cognitive decline and development of dementia (Alves et al., Reference Alves, Larsen, Emre, Wentzel-Larsen and Aarsland2006; Sollinger, Goldstein, Lah, Levey, & Factor, Reference Sollinger, Goldstein, Lah, Levey and Factor2010; Verbaan et al., Reference Verbaan, Marinus, Visser, van Rooden, Stiggelbout, Middelkoop and van Hilten2007).
Recent publications have also identified more subtle differences and deficits in cognitive and action control processes between PIGD and TD subtypes that emerge well before dementia onset and may have important clinical implications (Lyros, Messinis, & Papathanasopoulos, Reference Lyros, Messinis and Papathanasopoulos2008; Oh, Kim, Choi, Sohn, & Lee, Reference Oh, Kim, Choi, Sohn and Lee2009; Sollinger et al., Reference Sollinger, Goldstein, Lah, Levey and Factor2010). For example, we recently reported that patients with predominant PIGD are much more susceptible to acting on an initial motor impulse compared to patients with TD, which may have direct implications for fall or driving risk (Wylie et al., Reference Wylie, van den Wildenberg, Ridderinkhof, Claassen, Wooten and Manning2012). Here, we investigated how PIGD and TD subtypes differentially impact the ability to coordinate response initiation and response inhibition processes to meet the demands of the current required action.
As an example, consider how action initiation and inhibition processes are coordinated when navigating a crowd. The prospect of running into others or others running into you dictates a complex regulation of motor control that balances the initiation of movement (e.g., walking, side-stepping) with action control and inhibition processes (e.g., slowing ongoing actions, stopping abruptly) in response to the unpredictable and dynamic changes in the environment. Not surprisingly, navigating crowds or tight spaces is among the most feared circumstances reported by patients with PD, particularly in patients who have predominant PIGD symptoms (Błaszczyk, Orawiec, Duda-Kłodowska, & Opala, Reference Błaszczyk, Orawiec, Duda-Kłodowska and Opala2007; Giladi, Kao, & Fahn, Reference Giladi, Kao and Fahn1997; Kelly & Shumway-Cook, Reference Kelly and Shumway-Cook2014; Kerr et al., Reference Kerr, Worringham, Cole, Lacherez, Wood and Silburn2010; Nutt et al., Reference Nutt, Bloem, Giladi, Hallett, Horak and Nieuwboer2011).
The current study combined two experimental cognitive paradigms, a choice reaction task and the stop-signal task, to investigate the hypothesis that PIGD and TD subtypes involve dissociable effects on the coordination of action initiation and inhibition processes. This allowed us to compare situations in which action was initiated without concern about having to stop action versus situations in which actions were initiated but there existed the possibility that actions might have to be stopped abruptly and unpredictably. Individuals strategically slow their response initiation speed in situations where reactions might have to be stopped abruptly (Bissett & Logan, Reference Bissett and Logan2011, Reference Bissett and Logan2012). This proactive slowing is considered an act of executive motor control that adjusts and coordinates response initiation and inhibition processes to optimize performance to the situation (e.g., trading slower initiation speed to allow for better readiness to control or inhibit motor actions). The stop-signal task also permitted estimation of stopping (i.e., inhibition) latency, which is consistently slower among PD patients compared to age cohorts, but may vary by motor subtype (Bissett et al., Reference Bissett, Logan, van Wouwe, Tolleson, Phibbs, Claassen and Wylie2015; Gauggel, Rieger, & Feghoff, Reference Gauggel, Rieger and Feghoff2004; Obeso, Wilkinson, Casabona, et al., Reference Obeso, Wilkinson, Casabona, Bringas, Alvarez, Alvarez and Jahanshahi2011).
Prior work using the Simon conflict task indicated that even though PIGD patients show similar choice reaction times as TD patients, PIGD patients have much more difficulty controlling impulsive response tendencies (i.e., they make more fast impulsive reaction errors) (Wylie et al., Reference Wylie, van den Wildenberg, Ridderinkhof, Claassen, Wooten and Manning2012). This led us to hypothesize that PIGD patients might experience greater tradeoffs between the speed of initiating action and controlling action than TD patients. Consequently, in this study, we again expected PIGD patients to show similar response latencies to other PD patients in the choice reaction task without stop-signals. We believed the addition of stop signals would also force all patients to proactively slow their reactions and place greater priority on inhibitory control.
However, given prior experience with PIGD patients in the 2012 study (Wylie et al., Reference Wylie, van den Wildenberg, Ridderinkhof, Claassen, Wooten and Manning2012) and in PD patients with freezing of gait symptoms (a subset of PIGD patients) (Bissett et al., Reference Bissett, Logan, van Wouwe, Tolleson, Phibbs, Claassen and Wylie2015), we predicted that this tradeoff would be more difficult for PIGD patients, thus leading to greater slowing of reaction time to adapt to the increased demand on preparing to inhibit reactions, suggesting a fundamental deficit coordinating these two modes of action control. Because PD generally produces response inhibition deficits, we finally also expected TD and PIGD groups to show longer stopping (inhibition) latencies compared to healthy controls.
METHOD
Participants
We recruited three groups of participants: healthy controls (HCs), PIGD PD patients, and TD PD patients. All PD participants for this study were recruited from the Movement Disorder clinic at Vanderbilt University Medical Center, and an age-matched HC group was recruited from community advertisement. PD was diagnosed according to the UK brain bank criteria (Hughes, Daniel, Kilford, & Lees, Reference Hughes, Daniel, Kilford and Lees1992), response to dopaminergic medication, and expert clinical opinion. Participants were required to be older than age 40 at diagnosis. Both PD and HC subjects were excluded if they had a history of (i) a neurological condition besides PD; (ii) bipolar affective disorder, schizophrenia, or other psychiatric condition known to compromise executive cognitive functions; or (iii) a medical condition known to interfere with cognition (e.g., diabetes, pulmonary disease). They were required to score at a level of 25 or higher on the Mini-Mental Status Exam (MMSE) to rule out severe gross cognitive deficits.
All participants provided informed consent before participating in any study procedures in full compliance with the standards of ethical conduct in human investigation as regulated by our local Institutional Review Board.
Classification Into PIGD and TD subtypes
All subjects were classified into PIGD or TD groups based on the ratio between the mean PIGD and tremor subscores on the Unified Parkinson’s Disease Rating Scale (UPDRS) in the OFF state. These subscores were determined using criteria from accepted guidelines in prior published work (Jankovic et al., Reference Jankovic, McDermott, Carter, Gauthier, Goetz, Golbe and Shoulson1990; Stebbins et al., Reference Stebbins, Goetz, Burn, Jankovic, Khoo and Tilley2013). All scores were taken from a database cataloguing moderate PD patients receiving extensive preoperative assessments including neuropsychological screening for potential Deep Brain Stimulation surgery. Part I, II, and IV scores were calculated using the newer Movement Disorder Society UPDRS (MDS-UPDRS), whereas Part III scores were calculated using the older UPDRS. All Part III scores were calculated by the same independent rater before any experimental procedures.
Patients were classified as PIGD when the ratio of the mean tremor score (MDS-UPDRS item 2.10 and UPDRS items 20 and 21) divided by mean PIGD score (MDS-UPDRS items 2.12 and 2.13 and UPDRS items 29 and 30) was ≤0.9, whereas patients with a ratio of ≥1.15 were assigned to the TD group. When the ratio was >0.9 and <1.15, patients were classified as undetermined and excluded from analysis. The cutoffs for each subtype were chosen to follow the Stebbins 2013 classification cutoffs using parts 2 and 3 of the MDS-UPDRS (Stebbins et al., Reference Stebbins, Goetz, Burn, Jankovic, Khoo and Tilley2013).
Experimental Task and Procedures
Participants completed two reaction time tasks: a choice reaction task and a stop-signal task (Figure 1). Participants completed the study procedures using the same laptop computer in the same quiet room within the testing lab. PD participants completed the tasks in their best ON medication state.

Fig. 1 Illustration of trial process for (A) choice reaction time task and (B) stop-signal task.
In the choice reaction task, participants issued left or right thumb button presses to the direction of a series of “go” stimuli (i.e., dark grey arrows: ![]() =left button press;
=left button press; ![]() =right button press). Arrows were presented on a 15.5-inch digital display monitor placed at a distance of approximately 90 cm from the subject and positioned approximately at eye level. The arrow (i.e., the go stimulus) was a dark gray arrow shown at visual fixation against a white background. It consisted of a rectangular stem (0.95×0.88 cm) attached to a triangular arrowhead (1.10 cm height×1.90 cm base). Each block of trials began with the appearance of a small fixation square before a series of arrows began to appear one at a time. Left- and right-pointing arrows appeared randomly, but with equal probability, across a block of trials.
=right button press). Arrows were presented on a 15.5-inch digital display monitor placed at a distance of approximately 90 cm from the subject and positioned approximately at eye level. The arrow (i.e., the go stimulus) was a dark gray arrow shown at visual fixation against a white background. It consisted of a rectangular stem (0.95×0.88 cm) attached to a triangular arrowhead (1.10 cm height×1.90 cm base). Each block of trials began with the appearance of a small fixation square before a series of arrows began to appear one at a time. Left- and right-pointing arrows appeared randomly, but with equal probability, across a block of trials.
Following each participant’s response to an arrow (i.e., a left or right thumb press) or a period of 1250 ms elapsing without a response, an inter-stimulus interval ranging randomly from 750 to 1250 ms in increments of 50 ms occurred before the next trial arrow appeared. Responses were registered using a button located at the end of a grip held comfortably in each hand. Participants were instructed to respond quickly and accurately to each go arrow. In the choice reaction task, participants completed a practice session consisting of a block of 16 arrows, and then completed an experimental session consisting of two blocks of 84 arrows.
For the stop-signal task, participants made identical reactions to dark gray arrows as in the choice reaction task. However, on 30% of trials, identified as stop-signal trials, the arrow turned from dark gray to purple after a brief, variable delay. Whenever this color change occurred, participants were instructed to stop their button press response with the hand signaled by the direction of the initial dark gray arrow. For example, if a dark gray arrow pointing to the right appeared but then changed to purple, the participant was instructed to abruptly stop their right hand response. A staircase-tracking procedure was implemented that dynamically adjusted the interval between the onset of the go stimulus and the onset of the stop-signal (i.e., the stop-signal delay, or SSD) on the next stop-signal trial (Levitt, Reference Levitt1971). After a successful stop, the SSD was increased by 50 ms, making it more difficult to stop on the next stop trial. After a failed stop, the delay was decreased by 50 ms making it less difficult to stop on the next stop trial.
The tracking algorithms were designed to ensure that responses on stop trials were stopped successfully approximately 50% of the time, which is essential for estimating the speed of inhibiting a response (stop-signal reaction time, SSRT) (Band, van der Molen, & Logan, Reference Band, van der Molen and Logan2003). For the stop-signal task, participants completed a single block of 40 practice trials, and then completed an experimental session consisting of three blocks of 90 trials. Thus, the data acquisition blocks yielded 190 go trials and 80 stop-signal trials.
Data Analyses
Our first set of analyses compared go reaction times from the choice reaction task with go reaction times from the stop-signal task. A slowing of reaction time in the latter compared to the former provided a measure of proactive slowing in situations when the need to inhibit action may occur. We computed repeated-measures analyses of variance (ANOVAs) with Group (PIGD, TD, HC) as a between-subject factor and Go Context (Stop Trials Absent, Stop Trials Present) as a within-subjects factor. The second set of analyses used ANOVA to compare response inhibition latencies (i.e., stop-signal reaction time, or SSRT) as a function of Group (PIGD, TD, HC). SSRT was estimated using the well-established horse-race model and integration method (Logan, Reference Logan1994; Logan, Cowan, & Davis, Reference Logan, Cowan and Davis1984).
Briefly, the race model assumes that the go and stop processes operate independently. The beginning of the stop process is under experimental control by the duration of the SSD, whereas the finishing time of the stop process must be inferred by deriving the time point at which the internal response to the stop-signal occurs and subtracting the SSD from this point. That is, the time point in the go reaction time (GoRT) distribution corresponding to P (failure-to-stop) is assumed to equal (SSD + SSRT). Individual GoRTs were rank ordered and the nth GoRT was selected, where n is the point at which P (failure-to-stop). The mean SSD was then subtracted from this finishing time to obtain an estimate of SSRT. We first verified a few critical assumptions of the method requiring that the probability of stopping responses on stop-signal trials approximated 50% and that failed inhibition trials were associated with faster response latencies than average go trial response latencies (Band et al., Reference Band, van der Molen and Logan2003; Logan, Schachar, & Tannock, Reference Logan, Schachar and Tannock1997; Logan, Reference Logan1994).
Effect sizes were reported as eta squared for ANOVAs (ƞ2) and Cohen’s d for t tests (d). A final set of analyses used Pearson correlations (with p-value adjustments for multiple comparisons) to test associations between several clinical features of PD (e.g., dopamine dosage, UPDRS score in off state, disease duration, age) with both proactive slowing costs and SSRTs.
RESULTS
Analysis of Sample Demographics
Patient demographics were appropriately similar among groups (Table 1). HCs and PD subgroups had nonsignificant differences in age, education, and gender distribution (all p>.05). The PD subgroups were also similar in age, disease duration, education, gender distribution, mental status scores, depression ratings and UPDRS scores in the ON/OFF state (all p>.10). There was a significant difference in the Freezing of Gait (FOG) questionnaire score between subgroups with the PIGD group having a greater number of freezers (p=.013). This was expected given the nature of this motor subtype.
Table 1 Demographics and neuropsychological testing scores for PD subtypes and HC’s. MMSE, Mini Mental State Examination; UPDRS, Unified Parkinson’s Disease Rating Scale; LEDD, Levodopa equivalent daily dose; WTAR, Wechsler Test of Adult Reading; RBANS, Repeatable Battery for the Assessment of Neuropsychological Status; DKEFS, Delis Kaplan Executive Function System. Numerical values equal mean values with standard deviation (SD) in parentheses. Neuropsychological test scores (RBANS, WAIS, DKEFS) represent standard scores (mean=10; st.dev.=3) calculated from normative data provided by each test’s administration manual.

WTAR=Wechsler Test of Adult Reading; RBANS=Repeatable Battery for the Assessment of Neuropsychological Status; WAIS=Wechsler Adult Intelligence Scale; DKEFS=Delis–Kaplan Executive Function System; QUIP=Qualitative Impact Protocol.
There was also a significant difference between subgroups on the Qualitative Impact Protocol (QUIP) self-report (p=.02). The PIGD group reported higher impulsivity compared to the TD group.
Total levodopa equivalent daily dose (LEDD) and dopamine agonist LEDD was equivalent among subgroups (p=.85 and .43, respectively). Notably, one patient was not taking dopaminergic medications. Patients were receiving a combination of levodopa monotherapy (n=18), levodopa plus agonist dual therapy (n=32), or no medical therapy (n=1). Analysis of study endpoints with and without the patient receiving no medical therapy did not change the results or significance of outcomes.
A variety of neuropsychological tests were performed on the PD patients in this cohort as part of standard of care for the deep brain stimulation evaluation. Relevant tests were included in Table 1. There were no significant differences among PD subgroups.
Performance of PIGD and TD Subgroups Versus Healthy Controls
Go reactions and proactive slowing effects
Overall, mean reaction times (RTs) to go signals, irrespective of the presence or absence of stop signals, were approximately 50 ms slower among PD subgroups than healthy controls (Group: F(2,69)=5.39; p=.007; ƞ 2=.14; Bonferroni multiple comparisons: TD vs. HC; p=.043; d=.95; PIGD vs. HC; p=.002; d=1.2), but did not differ between the PD subgroups (TD vs. PIGD; p=1.00; d=.23) (Figure 2).

Fig. 2 Go reaction time (ms) separated by context (choice reaction task and stop-signal task) for Parkinson’s disease (PD) patients with postural-instability and gait disorder (PIGD), PD patients with tremor dominant (TD) symptoms, and healthy controls (HC).
Mean RTs to go stimuli were significantly slowed (average proactive slowing=140 ms) when stop-signals were embedded unpredictably in a block of trials compared to blocks of trials without stop-signals (Go Context: F(1,69)=190.70; p<.0001; ƞ 2=.73). Notably, the magnitude of proactive slowing varied by group (Group×Go context: F(2,69)=9.83; p<.0001; ƞ 2=.22). As Figure 3 depicts, the PIGD subgroup showed significantly greater proactive slowing than either HC (p=.004; d=.96) or TD (p<.0001; d=1.14) groups, the latter of which did not demonstrate differences in proactive slowing costs (HC vs. TD; p=1.00; d=.19).

Fig. 3 Proactive slowing costs (ms) for Parkinson’s disease (PD) subtypes and healthy controls (HC). PD patients with predominant postural-instability and gait disorder (PIGD) show exacerbated proactive slowing costs compared to tremor dominant (TD) patients and to HC. *statistically significant difference
The PIGD subgroup performed similar (489 ms) to HC (470 ms) on Go trials when stop signals were absent (t(46)=1.06; p=.30; d=.30) but faster than the TD group (527 ms; t(49)=2.4; p=.02; d=.67). In contrast, when stop signals were present, the PIGD group (690 ms) was significantly slower on Go trials than both HC (588 ms; t(46)=3.78; p<.0004; d =1.09) and TDs (629 ms, t(49)=2.38; p=.02; d=.67).
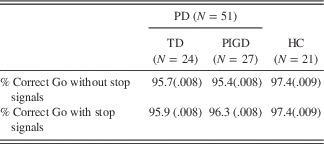
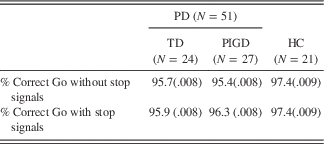
Mean accuracy rates to go stimuli did not differ across groups (Group: F(2,69)=1.91; p=.16; ƞ 2=.05). Accuracy rates to go stimuli also did not vary by whether go trials were embedded in blocks of trials without or with stop-signals (Go Context: F(1,69)=0.31; p=.58; ƞ 2=.004), a pattern that was also unaffected by group (Group×Go context: F(2,69)=0.15; p=.86; ƞ 2=.004). See Table 2 for accuracy rates by Go context and Group.
Table 2 Mean accuracy rates and standard errors by task and group
Stop-signal dynamics
The tracking algorithm for determining SSRT worked well and converged to successful stop percentages near 50% for all three groups (TD: 49%; PIGD: 47%; HC: 48%; F(2,69)=2.04; p=.137; ƞ 2=.06). Also consistent with a key assumption of the horse-race model, RT on failed stop (FS) trials (i.e., those that escaped inhibition) were shorter than the overall mean go RT (552 vs. 635 ms; F(1,69)=463.76; p<.001; ƞ 2=.87), and all groups showed this pattern (TD=82 ms; PIGD=102 ms; HC=65 ms). These patterns ensure the accuracy of the estimated SSRTs.
SSRTs, shown in Figure 4, differed among the groups (F(2,69)=3.63; p=.032; ƞ 2=.095). In line with prior studies, both PD subgroups showed slower SSRTs compared to HCs (TD vs HC: p=.018, d=.72; PIGD vs. HC: p=.024, d=.70). In line with race model predictions that going and stopping processes are independent, go RT did not correlate with SSRT (Overall: r=0.048; p=.687; TD: r=0.033; p=.879; PIGD: r=0.007; p=.974; HC: r=−0.253; p=.269), and the group effect on SSRT was preserved even with go RT included as a covariate (F(2,68)=3.61; p=.032; ƞ 2=.095).

Fig. 4 Stop-Signal Reaction Times (ms) separated into Parkinson’s disease (PD) subtypes and healthy controls (HC). PD patients with predominant postural-instability and gait disorder (PIGD) and tremor dominant (TD) motor subtypes show similar but exacerbated slowing of stop-signal reaction time (SSRT) compared to HC. *Statistically significant difference.
Association between proactive slowing, SSRT, and PD features
We explored potential relationships between proactive slowing costs, SSRTs, and general features of PD (age, disease duration, total LEDD, total UPDRS in ON/OFF medication states, QUIP self-report). None of the PD features correlated with proactive slowing costs or SSRTs (all p values>.10), even when analyzing PIGD and TD subgroups separately. Depression is common in PD. However, scores on the Beck Depression Inventory (BDI) did not correlate with proactive slowing (r=0.16; p=.259) and SSRT (r=−0.07; p=.633) in the overall PD group or with subgroups. Additionally, FOG questionnaire rating scores did not correlate with proactive slowing, (r=0.16; p=.292), or with SSRT (r=−0.09; p=.540).
DISCUSSION
The speeds to initiate and to inhibit responses are often studied in isolation but many action-oriented situations demand strategic tradeoffs between prioritizing the speed of going or initiating responses versus prioritizing the control or inhibition of responses. Studies of PD sometimes report slower response inhibition latencies but with intact response initiation latencies (Gauggel et al., Reference Gauggel, Rieger and Feghoff2004), but others report the opposite pattern where intact response inhibition latency is coupled with slower response initiation latency (Bissett et al., Reference Bissett, Logan, van Wouwe, Tolleson, Phibbs, Claassen and Wylie2015). This suggests that PD may involve exacerbated tradeoffs between these two modes of action control (van Wouwe et al., Reference van Wouwe, van den Wildenberg, Claassen, Kanoff, Bashore and Wylie2014; Wylie et al., Reference Wylie, van den Wildenberg, Ridderinkhof, Bashore, Powell, Manning and Wooten2009). Here, we extended this work by investigating the role of motor subtypes on response initiation, response inhibition, and their coordination to meet situational performance demands.
Overall, PD patients showed both slower response initiation latencies and longer response inhibition latencies compared to HCs. However, important differences emerged between the two motor subtype groups. In situations where there was no concern about having to suddenly stop responses (i.e., the choice reaction task), RTs to go signals among PIGD patients were similar to HCs but faster than TD patients. However, another critical group difference in response initiation speeds emerged when go trials were embedded in a situation that required the unpredictable and occasional need to inhibit responses abruptly. This situational demand induced an expected proactive slowing of response initiation speed across all groups, but was exacerbated among PIGD patients compared to HCs and TD patients (Bissett & Logan, Reference Bissett and Logan2011, Reference Bissett and Logan2012).
One immediate question is whether the pattern of slower RT in the choice reaction task among TD patients contributed to the proactive slowing differences between the groups. While this pattern is suggestive that TD patients have a fundamental response going deficit, we would have expected that the addition of stop signals would produce a degree of slowing proportional to the Go only reaction times as opposed to a much smaller magnitude of proactive slowing in PIGD patients. The preserved reaction GoRT in PIGD needs to be further studied to understand the underlying mechanism. TD patients and HCs showed a similar magnitude of proactive slowing, leading to response initiation latencies that were approximately 100 ms slower when stop signals could occur. In contrast, PIGD patients showed proactive slowing costs that were nearly twice the magnitude of TD patients and HCs. Thus, the addition of the need to unpredictably stop action led to an exacerbated tradeoff (i.e., cost) to response initiation latencies in the PIGD group.
Emerging linkages between proactive control, inhibitory control, and neural circuitries may provide clues about differential neural disruption in PIGD versus TD. For example, functional imaging studies have linked pre-supplementary motor area activity (preSMA) and the inferior frontal cortex (IFC) to action selection and inhibition mechanisms in response conflict tasks and the Stop-Signal task (Forstmann et al., Reference Forstmann, Dutilh, Brown, Neumann, von Cramon, Ridderinkhof and Wagenmakers2008; Kenemans, Reference Kenemans2015; Nachev, Wydell, O’Neill, Husain, & Kennard, Reference Nachev, Wydell, O’Neill, Husain and Kennard2007; Vink, Kaldewaij, Zandbelt, Pas, & du Plessis, Reference Vink, Kaldewaij, Zandbelt, Pas and du Plessis2015; Zandbelt and Vink, Reference Zandbelt and Vink2010). Similarly, our prior demonstration that PIGD patients showed a deficiency in proactively regulating a response threshold in the Simon task (Wylie et al., Reference Wylie, van den Wildenberg, Ridderinkhof, Claassen, Wooten and Manning2012) coincides with imaging studies showing that this vulnerability to impulsive actions errors is associated with diminished preSMA activity as well as to the integrity of white matter tracks between the preSMA (or IFC) and subthalamic nucleus (STN) (Frank, Reference Frank2006; Wylie et al., Reference Wylie, Ridderinkhof, Elias, Frysinger, Bashore, Downs and van den Wildenberg2010, Reference Wylie, van den Wildenberg, Ridderinkhof, Claassen, Wooten and Manning2012). Interestingly, atrophy in the preSMA appears to be more pronounced in PIGD patients compared to the TD subtype (Herb et al., Reference Herb, Rane, Isaacs, Van Wouwe, Roman, Landman and Claassen2016; Rosenberg-Katz et al., Reference Rosenberg-Katz, Herman, Jacob, Giladi, Hendler and Hausdorff2013).
These findings lead to the speculation and novel prediction that alterations in preSMA and its connectivity through the basal ganglia may represent a vulnerable pathway in PIGD that leads to deficits coordinating action initiation and inhibition processes. It should be noted that both preSMA and IFC have additionally been associated with reactive control in the Stop task (Aron, Reference Aron2011; Watanabe et al., Reference Watanabe, Hanajima, Shirota, Tsutsumi, Shimizu, Hayashi and Konishi2015; Wessel & Aron, Reference Wessel and Aron2013; Zandbelt, Bloemendaal, Hoogendam, Kahn & Vink, Reference Zandbelt, Bloemendaal, Hoogendam, Kahn and Vink2013; for a review, see Kenemans, Reference Kenemans2015). If alterations to preSMA are a primary driver of action control deficits in PIGD, future studies should investigate whether variations in preSMA activity predict either or both deficits in proactive control and reactive stopping control. It would also be interesting to determine if TD patients’ stopping deficits are linked to preSMA dysfunction or whether another node of the circuitry, such as the IFC or its connectivity with the STN, might account for deficits in stopping control observed in TD and PIGD patients. The importance of the STN in stopping has been indicated by van den Wildenberg (Reference van den Wildenberg, van Boxtel, van der Molen, Bosch, Speelman and Brunia2006) who showed that stimulating the STN improves SSRT in PD.
Although the connections between neural circuitries, action control, and motor subtypes needs further study, there are some potentially important clinical implications of the findings reported here and in recent studies. The findings here suggest that discussions with patients about the potential tradeoffs in action control processes could be educationally important and should be done early as predominant symptoms begin to emerge. As PIGD is associated with a more aggressive and disruptive course, conveying this insight at an early stage could have direct implications for reducing fall risk, anticipating driving difficulties, and making strategic adjustments in motor behavior when navigating complex environments that place greater demands on balancing response initiation and control (Alves et al., Reference Alves, Larsen, Emre, Wentzel-Larsen and Aarsland2006; Lyros et al., Reference Lyros, Messinis and Papathanasopoulos2008; Rajput et al., Reference Rajput, Voll, Rajput, Robinson and Rajput2009; Verbaan et al., Reference Verbaan, Marinus, Visser, van Rooden, Stiggelbout, Middelkoop and van Hilten2007; Wu et al., Reference Wu, Guo, Wei, Ou, Song, Cao and Shang2016). Notably, many of the gait complications, such as instability and freezing, are often unresponsive to dopamine therapy. As clarity around the role of abnormal cognitive control mechanisms as contributors to these gait symptoms emerges, novel treatments can be developed to improve the strategic coordination and compensation of initiation and inhibition latencies.
There were some limitations to this study. PIGD and TD groups were determined using previously published and widely accepted guidelines for defining PD motor subtypes (Jankovic et al., Reference Jankovic, McDermott, Carter, Gauthier, Goetz, Golbe and Shoulson1990; Stebbins et al., Reference Stebbins, Goetz, Burn, Jankovic, Khoo and Tilley2013). However, there is continued debate on how best to define PD motor subtypes as the expression of PD motor symptoms can change across the disease and as a function of medication state (Janvin, Aarsland, Larsen, & Hugdahl, Reference Janvin, Aarsland, Larsen and Hugdahl2003; Marras & Lang, Reference Marras and Lang2013; van Rooden, Visser, Verbaan, Marinus, & van Hilten, Reference Van Rooden, Visser, Verbaan, Marinus and van Hilten2009). While we cannot account for long-term medication effects, we used the OFF medication state to define our motor subtypes, which is often used in studies looking at PD subtypes as the most natural disease state (Jankovic et al., Reference Jankovic, McDermott, Carter, Gauthier, Goetz, Golbe and Shoulson1990; Schiess et al., Reference Schiess, Zheng, Soukup, Bonnen and Nauta2000).
Another limitation is that, while we performed subtype assessment in the OFF dopamine state, we performed the cognitive and neuropsychological testing in the ON dopamine state. When participants underwent the study procedures, they were already undergoing an extensive neuropsychological battery as part of their pre-surgery assessment for deep brain stimulation as a standard of care. Studies of dopamine effects in healthy controls have shown that a dopamine antagonist like haloperidol can lengthen SSRT (Logemann et al., Reference Logemann, Böcker, Deschamps, van Harten, Koning, Kemner and Kenemans2017). Two separate studies in PD patients, however, demonstrated that medication state does not affect SSRT (Claassen et al., Reference Claassen, van den Wildenberg, Harrison, van Wouwe, Kanoff, Neimat and Wylie2015; Obeso, Wilkinson, & Jahanshahi, Reference Obeso, Wilkinson and Jahanshahi2011), although it may have some effect on GoRTs (Claassen et al., Reference Claassen, van den Wildenberg, Harrison, van Wouwe, Kanoff, Neimat and Wylie2015) but this should be similar across paradigms. Additionally, both PD groups in the current study were on statistically similar doses of medication, arguing that the PD subgroup effect is still relevant and not affected by dopamine medication level. The effect of dopamine medications on proactive slowing awaits further investigation.
The motor subgroups were similar across a range of disease and neuropsychological variables with the exception that the PIGD group reported higher FOG symptoms. PD patients with diagnosed FOG symptoms typically display greater executive dysfunction, including exacerbated problems with motor conflict resolution and inhibition of ongoing motor actions (Bissett et al., Reference Bissett, Logan, van Wouwe, Tolleson, Phibbs, Claassen and Wylie2015; Nutt et al., Reference Nutt, Bloem, Giladi, Hallett, Horak and Nieuwboer2011; Stefanova et al., Reference Stefanova, Jecmenica Lukic, Ziropadja, Markovic, Stojkovic, Tomic and Kostic2014). FOG is inherently difficult to diagnose in the clinic or lab but we suspect both groups contained patients with FOG given the scores seen on the FOG questionnaire. In attempts to address the role of FOG, we found no correlation between proactive slowing costs or SSRT and scores on the FOG questionnaire. This suggests that the PIGD findings reported here are unlikely to be attributed to confounding FOG symptoms. However, specifying the unique and combinatorial effects of PIGD and FOG on action control processes will be an important topic for future investigation.
In conclusion, consistent with prior studies, we showed that PD patients, irrespective of subtype, are slower to abruptly inhibit already initiated actions. The need to occasionally yet unpredictably inhibit action produced significant slowing of response initiation latencies, an effect that was markedly exacerbated among PIGD patients compared to TD patients and HCs. These data support the hypothesis that PIGD patients experience exaggerated tradeoffs in going or stopping when the context dictates prioritization of one form of action control over the other. This has particular relevance clinically as it may explain the pronounced difficulty patients with PIGD experience navigating environments that require dynamic coordination of action initiation and inhibition, such as when walking through crowds or maneuvering tight spaces. Therapeutically, efforts to promote slower, more deliberate mobility strategies in such situations would potentially be helpful for patients with PIGD, especially given their propensity toward acting on impulsive motor tendencies and slower speed to inhibit action abruptly.
ACKNOWLEDGMENTS
Dr. Tolleson has done consulting work for Medtronic, Acadia, and Teva. Dr. Phibbs has also done consulting for Medtronic and Teva. No other conflicts of interest to report. There is no funding to report for this manuscript.