Introduction
Postural Orthostatic Tachycardia syndrome is a condition that affects 1–3 million patients per year in the United States with a predominance in adolescent Caucasian females. Reference Grigoriou, Boris and Dormans1,Reference Boris and Bernadzikowski2 Postural Orthostatic Tachycardia syndrome is characterised by autonomic dysfunction, with an increase in heart rate (≥30 beats/minute) when moving from supine to standing. Reference Boris, Huang and Bernadzikowski3 Many patients also experience non-specific symptoms that may or may not be related to autonomic dysfunction, such as dizziness, nausea, vomiting, fatigue, headaches, abdominal discomfort and sleep disturbances. Reference Johnson, Mack, Kuntz, Brands, Porter and Fischer4,Reference Kizilbash, Ahrens and Bruce5 Studies have suggested that the initial presentation of Postural Orthostatic Tachycardia syndrome often occurs following prolonged recovery from a viral illness or physical injury. Reference Johnson, Mack, Kuntz, Brands, Porter and Fischer4,Reference Kizilbash, Ahrens and Bruce5 However, the significant functional disability and psychological distress associated with Postural Orthostatic Tachycardia syndrome often persist after the inciting illness resolves. Unfortunately, the aetiology of Postural Orthostatic Tachycardia syndrome is poorly understood, and there is limited information on the optimal management of Postural Orthostatic Tachycardia syndrome.
The diagnosis of Postural Orthostatic Tachycardia syndrome can present a clinical challenge. Historically, many physicians have used a tilt-table test and traditional vital signs measurement to assess the presence of Postural Orthostatic Tachycardia syndrome. However, the tilt-table test has notable limitations. Tilt-table tests have been shown to lead to results inconsistent with orthostatic vital sign measurement (i.e., vitals assessed during supine followed by standing position). In a study by Plash et al., when changes in heart rate were compared in patients with autonomic dysfunction and healthy controls, orthostatic vital sign measurement was found to be superior to a 10-minute tilt-table test in accurately identifying patients with autonomic dysfunction. Reference Plash, Diedrich and Biaggioni6
The criteria traditionally used for the diagnosis of Postural Orthostatic Tachycardia syndrome is an increase in heart rate of >40 beats per minute (bpm) in patients <18 years old and ≥30 beats per minute in patients ≥18 years old. Reference Barnhill7 However, these diagnostic criteria have been criticised, and adjusting the criteria to a change ≥30 beats per minute with standing has recently been proposed. Reference Boris, Huang and Bernadzikowski3 In the current study, these updated diagnostic criteria were used in addition to the Compensatory Reserve Index to determine true physiologic autonomic dysfunction.
The Compensatory Reserve Index is a non-invasive, FDA-cleared algorithm that analyses photoplethysmograms or pulse oximetry waveforms in real time to trend subtle waveform features that correspond with varying degrees of central volume loss, from normovolemia (Compensatory Reserve Index = 1) to decompensation (Compensatory Reserve Index = 0; systolic blood pressure <80 mmHg). Many pathological states have been characterised by analysis of simple, continuous physiologic waveforms, and previous studies have demonstrated that photoplethysmogram waveforms obtained with a pulse oximeter sensor significantly change with volume loss. Reference Convertino, Ryan and Rickards8-Reference Convertino, Moulton and Grudic10 Unlike the Compensatory Reserve Index, vital signs can be unreliable in assessing decreasing volume status. Reference Brasel, Guse, Gentilello and Nirula11 Compensatory Reserve Index has a unique ability to distinguish subtle features in the waveform associated with varying tolerances to central blood volume loss. This analytical advantage is based on the relationship described by the arterial waveform (ejection wave) and peripheral vascular resistance (reflected wave). As such, all mechanisms associated with compensation for central volume loss are represented in each waveform. Thus, subtle changes in waveform features, which are detected by the Compensatory Reserve Index algorithm, allow it to differentiate individual patients (e.g., those with high or low tolerance to central volume loss) within the first 30 beats of monitoring and every beat thereafter. Reference Ryan, Batchinsky, McManus, Rickards and Convertino12-Reference Moulton, Mulligan, Grudic and Convertino14 In prior studies, Compensatory Reserve Index has been shown to accurately trend central volume loss with hemorrhage and central volume gain with blood transfusion. Reference Convertino, Howard and Hinojosa-Laborde15-Reference Convertino, Wirt, Glenn and Lein17
There is preliminary evidence that Compensatory Reserve Index can also detect physiologic changes associated with Postural Orthostatic Tachycardia syndrome. Stewart et al. described a patient with Postural Orthostatic Tachycardia syndrome whose traditional vital signs and Compensatory Reserve Index values were measured during positional changes, and this patient demonstrated a tachycardic response with orthostatic vitals and an abrupt decrease in Compensatory Reserve Index from 0.8 to 0.2 with standing. Reference Stewart, Mulligan, Grudic, Convertino and Moulton18 As Compensatory Reserve Index continuously evaluates a compendium of subtle waveform features that predictably change in response to changes in central volume status, Compensatory Reserve Index may be less influenced by anxiety than traditional vital signs such as heart rate and may be more useful for trending values over time. Reference Moulton, Mulligan, Grudic and Convertino14
More systematic research is needed to explore the utility of Compensatory Reserve Index in the diagnosis and monitoring of Postural Orthostatic Tachycardia syndrome. The inclusion of a healthy comparison group is essential to explore whether the observed changes in Compensatory Reserve Index are unique to Postural Orthostatic Tachycardia syndrome patients and can help differentiate those with Postural Orthostatic Tachycardia syndrome from those without. We hypothesise that the Compensatory Reserve Index may provide added utility in the diagnosis and management of Postural Orthostatic Tachycardia syndrome patients by identifying individuals with blunted compensatory responses to positional changes.
In addition to autonomic dysfunction, patients with Postural Orthostatic Tachycardia syndrome commonly report significant impairment in physical and social functioning, including reduced activity, socialisation and academic engagement. Reference Benrud-Larson, Dewar, Sandroni, Rummans, Haythornthwaite and Low19,Reference Anderson, Lambert and Sari20 Impairments in emotional functioning, including symptoms of anxiety and depression, are also commonly seen in patients with Postural Orthostatic Tachycardia syndrome. Reference Anderson, Lambert and Sari20,Reference Raj, Haman and Raj21 Understanding anxious and depressive symptoms in patients with Postural Orthostatic Tachycardia syndrome may be especially important given the overlap between physiological symptoms of anxiety and depression (e.g., rapid heart rate) and symptoms of Postural Orthostatic Tachycardia syndrome. Family functioning is another psychosocial factor that may contribute to our understanding of functional impairment in patients with Postural Orthostatic Tachycardia syndrome. Although not well explored in this population, family factors, including poor parent–child communication and problem-solving, are often linked to impaired functioning in youth with other chronic illnesses, such as chronic pain. Reference Palermo, Putnam, Armstrong and Daily22 Healthy family functioning may promote resilience by impacting the way patients interpret and respond to their symptoms of Postural Orthostatic Tachycardia syndrome. Thus, when evaluating a patient for the presence of Postural Orthostatic Tachycardia syndrome, it may be important to assess anxiety, depression and issues with family functioning as these could impact a patient’s perception of Postural Orthostatic Tachycardia syndrome symptoms and the resulting functional impairment. This highlights the need for a reliable and valid method to assess the extent of underlying autonomic dysfunction (or true physiologic Postural Orthostatic Tachycardia syndrome process) to best guide treatment decisions.
By providing a continuous, non-invasive, physiologic parameter that is specific, sensitive and instantaneous, the use of Compensatory Reserve Index could greatly improve our ability to guide treatment amongst youth with Postural Orthostatic Tachycardia syndrome. Other than the one case cited above, there are no prior studies that use Compensatory Reserve Index to evaluate Postural Orthostatic Tachycardia syndrome patients. Specifically, this study aims to: (1) explore associations between heart rate and Compensatory Reserve Index in a representative sample of youth with Postural Orthostatic Tachycardia syndrome, (2) describe a suggested Compensatory Reserve Index cutoff value for the diagnosis of Postural Orthostatic Tachycardia syndrome and (3) explore the demographic and psychosocial features associated with physiologic Postural Orthostatic Tachycardia syndrome in our sample.
Patients and methods
All procedures were approved by the Institutional Review Board for the University of Colorado Denver/Anschutz Medical Campus. Consent was obtained for all patients, including both parental consent and patient assent for those under 18 years. Individuals with Postural Orthostatic Tachycardia syndrome and healthy controls aged 12–21 years were prospectively enrolled from June, 2019 to March, 2020. As most study measures were only validated for use in English, only English-speaking patients were included in this study. Postural Orthostatic Tachycardia syndrome patients had been previously diagnosed by a healthcare provider and were further subdivided after their visit into those who met diagnostic heart rate criteria (Δheart rate ≥ 30) with orthostatic vitals and those who did not (Δheart rate < 30).
Patients were excluded if they were taking beta-blocker medications, had been hospitalised in the past month for a serious medical condition(s), or had orthostatic hypotension (decrease in orthostatic systolic blood pressure >20 mm Hg).
Patients, accompanied by a parent, completed one study visit comprising physiological data collection and survey responses. Patients were encouraged to drink fluids prior to the appointment, and hydration status was controlled for by measuring urine specific gravity using a urine sample and hand-held refractometer (Kibeland) prior to measurement of orthostatic vitals, with target values ≤1.015. Reference Oppliger, Magnes, Popowski and Gisolfi23 Patients with values >1.015 were asked to continue hydrating before rechecking urine specific gravity, with a maximum of three attempts at rehydration.
Traditional orthostatic vital signs were measured concurrently with Compensatory Reserve Index values. In Postural Orthostatic Tachycardia syndrome patients, heart rate is expected to increase ≥30 beats per minute within 10 minutes of moving from supine to upright position. Reference Singer, Sletten, Opfer-Gehrking, Brands, Fischer and Low24,Reference Skinner, Driscoll and Porter25 Heart rate and blood pressure (via cuff on the right upper arm) were assessed with an initial supine position for 5 minutes, followed by standing for 10 minutes and supine for 5 minutes. Compensatory Reserve Index values were continuously measured using an FDA-cleared CipherOx Compensatory Reserve Index ® M1 monitor (Flashback Technologies, Inc., Louisville, CO) attached to the patient’s left index finger. Figure 1 depicts the Compensatory Reserve Index device.

Figure 1. Demonstration of the Compensatory Reserve Index (CRI) Device.

Figure 2. Demonstration of trends in CRI and HR in a patient in the Physiologic POTS group versus a patient in the No Physiologic POTS group.
Demographic information and medical history were self-reported by patients with parental input. A number of surveys were administered on an iPad using REDCap. Reference Harris, Taylor, Thielke, Payne, Gonzalez and Conde26
The investigator-developed Symptom Severity Scale asked about the frequency of 10 common physical symptoms of Postural Orthostatic Tachycardia syndrome (fatigue, syncope, headache, palpitations, abdominal pain, nausea, brain fog, muscle weakness, exercise intolerance and insomnia Reference Boris and Bernadzikowski2,Reference Boris and Bernadzikowski27 ) on a scale of 0–3 (Not at all – 0, Several days – 1, More than half the days – 2, Nearly every day – 3). Each symptom endorsed was multiplied by its frequency (0, 1, 2 or 3) to result in a total score representing the sum of weighted items.
The Revised Child Anxiety and Depression Scale Reference Piqueras, Martín-Vivar, Sandin, San Luis and Pineda28 Generalised Anxiety Disorder and Major Depressive Disorder subscales were used; (note, there is evidence this measure retains its validity when used in adult samples Reference McKenzie, Murray, Freeston, Whelan and Rodgers29 ).
The Functional Disability Inventory, a well-validated measure of perceived activity limitations in children and adults, Reference Walker and Greene30 evaluates the difficulty of 15 daily tasks with a scale of impossible (4), a lot of trouble (3), some trouble (2), a little trouble (1) and no trouble (0). Reference Walker and Greene30 This measure was adapted very slightly to reflect the ubiquity of electronic media for today’s adolescents, e.g., modified from “Watching TV” to “Watching TV and other screens.” The Family Assessment Device – General Functioning Subscale is a 12-point scale that evaluates the “overall health or pathology in family functioning” via analysis of six dimensions of family functioning: problem-solving, communication, roles, affective responsiveness, affective involvement and behaviour control. Reference Byles, Byrne, Boyle and Offord31-Reference Miller, Epstein, Bishop and Keitner34
Statistical analysis
Patients were grouped for analysis by whether they met heart rate criteria for Postural Orthostatic Tachycardia syndrome [Δheart rate ≥ 30 from supine (minutes 2–4) to standing (minutes 3–8)] (physiologic Postural Orthostatic Tachycardia syndrome group) or not (Δheart rate < 30) (no physiologic Postural Orthostatic Tachycardia syndrome group). The no physiologic Postural Orthostatic Tachycardia syndrome group included both healthy controls and patients who had a prior diagnosis of Postural Orthostatic Tachycardia syndrome but did not meet heart rate criteria.
Data are shown as counts and proportions, means and standard deviations or medians and interquartile ranges (IQR), as appropriate. Univariate analysis was used to compare the physiologic Postural Orthostatic Tachycardia syndrome and no physiologic Postural Orthostatic Tachycardia syndrome groups, using Kruskal–Wallis for continuous data and Fisher’s exact tests for categorical data. Optimal Compensatory Reserve Index cutoffs were determined using the Youden Index from receiver operating characteristic curve analysis. Significance was set at alpha 0.05 for all analyses. The analysis was performed using SAS software 9.4 (SAS Institute Inc., Cary, North Carolina) and R software version 3.6.3, (R Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org/).
Results
Of the 64 individuals who participated, 3 were excluded who met heart rate criteria but had enrolled as healthy controls. Of the remaining 61 patients, 44 (72%) had a pre-existing Postural Orthostatic Tachycardia syndrome diagnosis; most were female (74%) and white (82%). Demographics are summarised in Table 1.
Table 1. Demographics of the Physiologic POTS versus No Physiologic POTS groups

Physiologic results
Eight patients (18%) met the criteria for physiologic Postural Orthostatic Tachycardia syndrome and 53 did not (no physiologic Postural Orthostatic Tachycardia syndrome). There were two patients in the physiologic Postural Orthostatic Tachycardia syndrome group and one patient in the no physiologic Postural Orthostatic Tachycardia syndrome group on fludrocortisone. There was one patient on midodrine, and this patient was in the no physiologic Postural Orthostatic Tachycardia syndrome group.
Median change in heart rate from supine to standing was significantly greater in the physiologic Postural Orthostatic Tachycardia syndrome group (36, IQR: 31, 38) than in the no physiologic Postural Orthostatic Tachycardia syndrome group (19, IQR: 16, 24) (p < 0.001). The associated change in Compensatory Reserve Index was significantly higher in the physiologic Postural Orthostatic Tachycardia syndrome group (0.67 (IQR: 0.62, 0.68) compared to the no physiologic Postural Orthostatic Tachycardia syndrome group [(0.51, IQR: 0.38, 0.58), p < 0.001]). The minimum Compensatory Reserve Index value with standing was significantly lower in the physiologic Postural Orthostatic Tachycardia syndrome group compared to the no physiologic Postural Orthostatic Tachycardia syndrome group [0.21 (IQR: 0.17, 0.26) vs. 0.32 (IQR: 0.25, 0.37), p = 0.007]. No individual in either group had a standing heart rate >120 beats per minute. The optimal cutoff for a change in Compensatory Reserve Index to distinguish between patients with physiologic Postural Orthostatic Tachycardia syndrome versus those without was 0.60 based on the Youden index calculation. Figure 2 demonstrates the CRI and heart rate changes with orthostatic vitals in one of the patients with Physiologic POTS and one of the patients with no Physiologic POTS.
There was no statistically significant difference in the initial urine specific gravity between the two groups (p = 0.299). Patients in the physiologic Postural Orthostatic Tachycardia syndrome and no physiologic Postural Orthostatic Tachycardia syndrome groups also had similar symptom severity scores (p = 0.260). Footnote 1 The remaining findings are summarised in Table 2.
Table 2. Physiologic characteristics of the Physiologic POTS versus No Physiologic POTS groups

Psychosocial results
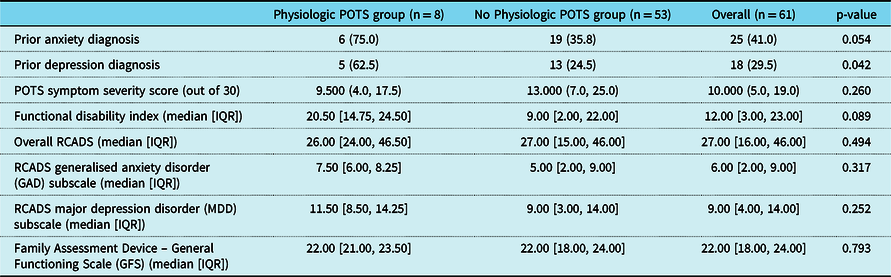
Psychosocial results are summarised in Table 3. Patients in the physiologic Postural Orthostatic Tachycardia syndrome group were more likely to report that they had been previously diagnosed with anxiety or depression (p = 0.054, 0.042, respectively), and showed a trend towards more functional impairment [20.5 (IQR: 14.8, 24.5) vs. 9.0 (IQR: 2.0, 22.0), p = 0.089]. No other significant group differences emerged.
Table 3. Survey responses of the Physiologic POTS group versus No Physiologic POTS group
Discussion
The results of the current study provide preliminary results for the utility of a novel technology, Compensatory Reserve Index, in diagnosing and monitoring Postural Orthostatic Tachycardia syndrome. In our cohort, only 18% (8/44) of previously diagnosed Postural Orthostatic Tachycardia syndrome patients met heart rate criteria for Postural Orthostatic Tachycardia syndrome, even with the updated, less stringent heart rate criteria for Postural Orthostatic Tachycardia syndrome (Δheart rate ≥ 30). We found that change in Compensatory Reserve Index and minimum Compensatory Reserve Index values significantly differed between the physiologic Postural Orthostatic Tachycardia syndrome and no physiologic Postural Orthostatic Tachycardia syndrome groups. Patients with physiologic Postural Orthostatic Tachycardia syndrome had greater declines in Compensatory Reserve Index with orthostatic vitals and lower minimum Compensatory Reserve Index values, which is likely due to their true autonomic dysfunction. In our cohort, we identified a cutoff change in Compensatory Reserve Index value associated with physiologic Postural Orthostatic Tachycardia syndrome of ≥0.60.
Our findings are exciting as we use a novel technology (Compensatory Reserve Index) in addition to traditional vital signs to detect true autonomic dysfunction. Other technologies such as the Finometer Pro have been described to evaluate the hemodynamic status (with non-invasive blood pressure monitoring) of patients with Postural Orthostatic Tachycardia syndrome, but they did not have a comparison control group. Reference Li, Liao and Wang35 Prior research in Compensatory Reserve Index has demonstrated that Compensatory Reserve Index outperforms trends in traditional vital signs in the detection of central volume loss. Reference Moulton, Mulligan, Grudic and Convertino14,Reference Stewart, Mulligan, Grudic, Convertino and Moulton18
Many factors can influence the validity of Compensatory Reserve Index, including vasoactive medications and conditions such as dehydration. Reference Gagnon, Schlader and Adams36 In the Postural Orthostatic Tachycardia syndrome patient population, dehydration is common, with a decreased blood volume in up to 70% of patients. Reference Gagnon, Schlader and Adams36 While dehydration can trigger Postural Orthostatic Tachycardia syndrome symptoms, the specific aetiology for the high rate of dehydration in Postural Orthostatic Tachycardia syndrome patients is poorly understood. It is important to differentiate dehydration and hypovolemia from autonomic dysfunction, because both may be present in Postural Orthostatic Tachycardia syndrome patients. Reference Ross, Medow, Rowe and Stewart37,Reference Sheldon, Grubb and Olshansky38 Dehydration can also exacerbate present Postural Orthostatic Tachycardia syndrome symptoms. As a result, Postural Orthostatic Tachycardia syndrome patients are counselled to drink 2–3 L of fluids daily and supplement their salt intake for a goal of 10–12 g/day. Reference Sheldon, Grubb and Olshansky38 To control for hydration status, we measured urine specific gravity and only conducted Compensatory Reserve Index measurements in hydrated patients.
The present results are useful in suggesting guidelines for a standardised way to diagnose Postural Orthostatic Tachycardia syndrome. First, patients with suspected Postural Orthostatic Tachycardia syndrome should have their hydration status evaluated with a urine specific gravity measurement. Patients who demonstrate dehydration (urine specific gravity > 1.015) should be urged to rehydrate prior to Postural Orthostatic Tachycardia syndrome evaluation. Once patients have a urine specific gravity ≤1.015, orthostatic vitals should be completed in a standardised fashion. We recommend orthostatic vitals collection during 5 minutes in a supine position followed by 10 minutes standing. This way, hydration status can be controlled for, and true autonomic dysfunction can be evaluated in this specific patient population. Second, and central to the current study, building upon recent work by Boris, Reference Boris, Huang and Bernadzikowski3 we propose that patients who have a heart rate change ≥30 and/or Compensatory Reserve Index change ≥0.60 meet the criteria for Postural Orthostatic Tachycardia syndrome.
Our results also highlight the importance of improving our understanding of psychosocial factors associated with Postural Orthostatic Tachycardia syndrome. In our study, we found that patients with physiologic Postural Orthostatic Tachycardia syndrome based on heart rate showed trends towards a higher incidence of previous diagnoses of anxiety and depression and more functional disability. These trends may suggest that individuals with physiologic Postural Orthostatic Tachycardia syndrome are more likely to have complications or comorbidities associated with their Postural Orthostatic Tachycardia syndrome (e.g., more anxiety and depressive symptoms). There are a number of explanations for this, including the impact of more severe Postural Orthostatic Tachycardia syndrome and/or the existence of predisposing vulnerability factors, and future research is needed to continue to explore these associations. Reference Benrud-Larson, Sandroni, Haythornthwaite, Rummans and Low39 At the same time, the group differences in psychosocial factors were not as pronounced as we might have expected; in effect, none of the psychosocial survey factors emerged as significantly different across groups, and family functioning scores, in particular, had identical means.
Notably, our results suggest that there exist a number of individuals who have been previously diagnosed with Postural Orthostatic Tachycardia syndrome and who experience many symptoms of Postural Orthostatic Tachycardia syndrome but who do not have true physiologic Postural Orthostatic Tachycardia syndrome. Future work should seek to better understand these patients’ experiences, including the respective contributions of underlying autonomic dysfunction, pre-existing anxiety and depression, interpersonal stressors and other health factors (e.g., physical fitness, diet and undiagnosed medical conditions) to their experience of Postural Orthostatic Tachycardia syndrome or Postural Orthostatic Tachycardia syndrome-like symptoms.
There are limitations to our findings. Our prospective enrollment was cut short due to COVID-19 resulting in a smaller number of patients than planned, and we had missing data for some patients due to a technical issue in our REDCap surveys. Thus, we may be underpowered to detect some true statistical differences. Although we attempted to recruit a representative sample, a larger sample size may help to capture more of the variability seen amongst the population of individuals with Postural Orthostatic Tachycardia syndrome. We share our findings as a pilot study with the hope that future studies can build upon these findings.
Our findings underscore the plethora of factors that can affect Postural Orthostatic Tachycardia syndrome presentation and diagnosis, including psychosocial factors and hydration status. There is a need for more physiology-based criteria to determine true autonomic dysfunction amongst Postural Orthostatic Tachycardia syndrome patients. The early results from our study highlight the potential utility of Compensatory Reserve Index in the assessment of Postural Orthostatic Tachycardia syndrome and the importance of standardising the diagnosis of Postural Orthostatic Tachycardia syndrome.
Acknowledgements
None.
Declarations
Financial Support
Institutional grant funding in the form of a microgrant from the Colorado Clinical and Translational Sciences Institute was received for nursing support for study visits (#18-2618). Additional institutional funding was also provided by the Children’s Hospital Colorado Center for Research in Outcomes in Children’s Surgery and Department of Adolescent Medicine at the Children’s Hospital Colorado.
Conflicts of Interest
Dr Moulton co-invented the Compensatory Reserve Index algorithm used in this study. The intellectual property is assigned to the Regents of the University of Colorado. Dr Moulton co-founded Flashback Technologies, Inc. in 2009 and licensed the technology from the university. He is a consultant at Flashback Technologies, has an equity interest in the company and receives royalty payments through the University of Colorado. Flashback Technologies did not sponsor this study. The remaining authors have no conflicts of interest.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. This study was approved by the Colorado Multiple Institutional Review Board (Study #18-2618 on 14 April, 2019) with informed written consent from all patients. Details that might disclose the identity of the patient reported here were removed. Data were archived and analysed anonymously. We confirm that we have read the journal’s position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.








