1. Introduction
Batfishes of the family Ogcocephalidae constitute a monophyletic assemblage of ten genera and more than 70 species, including some of the most bizarre and morphologically specialized lophiiforms (Gregory, Reference Gregory1933; Gregory & Conrad, Reference Gregory and Conrad1936; Bradbury, Reference Bradbury2003). Ogcocephalids are small to medium-sized tropical and subtropical fishes that occur from the subtidal zone down to abyssal depths (see Bradbury, Reference Bradbury1988). Members of the family differ notably from other lophiiforms in having a cavity, the illicial cavity, which opens in front of the head, into which the illicium and its terminal esca may be completely retracted through the action of a specialized musculature (see Bradbury, Reference Bradbury1967, Reference Bradbury2003). The esca is a glandular and secretory structure that apparently produces a chemical attractant that enables ogcocephalids to lure buried benthic prey (Bradbury, Reference Bradbury1988; Nagareda & Shenker, Reference Nagareda and Shenker2009). The body of batfishes is greatly depressed dorso-ventrally, with a greatly enlarged pectoral girdle that forms part of the external margin of the disk-like thoraco-cephalic sector, and the free portion of the pectoral fins protrude close to the contact area between the disk margin and the tail region. Batfishes are slow-swimming, bottom-dwelling nocturnal predators that feed on a large variety of benthic invertebrates (anthozoans, worms, gastropods, bivalves, crustaceans, bryozoans, echinoderms) and demersal fishes (Randall, Reference Randall1967; Gibran & Castro, Reference Gibran and Castro1999; Nagareda & Shenker, Reference Nagareda and Shenker2008). They ‘walk’ along the bottom searching for prey with the help of their highly specialized paired fins. Some species are characterized by having skin with bioluminescence capability (Crane, Reference Crane1968), but the ecological function of this feature remains obscure (Bradbury, Reference Bradbury1999).
The phylogenetic position of ogcocephalids within the Lophiiformes was established by Pietsch (Reference Pietsch1981, Reference Pietsch, Moser, Richards, Cohen, Fahay, Kendall and Richardson1984) who, based on morphological evidence, suggested that these fishes represent the sister group of the Ceratioidei. However, mitogenomic data seem to indicate that ogcocephalids are basal to all the lophiiform groups except for the Lophioidei, the latter representing the sister group of all the other members of the order (Miya et al. Reference Miya, Pietsch, Orr, Arnold, Satoh, Shedlock, Ho, Shimazaki, Yabe and Nishida2010). Batfishes are extremely rare in the fossil record, represented to date exclusively by otoliths (e.g. see Schwarzhans, Reference Schwarzhans2007), and very little information is therefore available about the diversification history of this extraordinary clade of lophiiform fishes. The goal of this paper is to describe a new genus and species of Ogcocephalidae from the Eocene strata of the celebrated fossiliferous locality of Monte Bolca. This new taxon, known from five specimens collected from the Pesciara cave site of the Monte Bolca locality, represents the first articulated skeletal record of the Ogcocephalidae and, more generally, the earliest known record for the family.
2. Locality and stratigraphy
The fossils described here were collected in the Pesciara cave, an extensively exploited productive site of the Monte Bolca area, in the eastern part of Monti Lessini, a few kilometres from the village of Bolca, northeast of Verona, northern Italy. The fish-bearing limestone rocks of the Pesciara cave site belong to the ‘Calcari nummulitici’, an informal unit of Eocene age that is widely distributed in northeastern Italy (see Fabiani, Reference Fabiani1914, Reference Fabiani1915; Barbieri & Medizza, Reference Barbieri and Medizza1969). The succession of the Pesciara cave consists of a cyclic alternation of finely laminated micritic limestone, with plants, fishes, soft-bodied invertebrates and crustaceans, and biocalcarenite/biocalcirudite rich in benthic fossils. Fishes are usually superbly preserved and occur in varved limestone with micritic matrix and sparse pyrite and bitumen. The taphonomic characteristics of the fossils and the sedimentary features of the laminated deposits are indicative of poorly oxygenated bottom conditions and low hydrodynamic energy. According to the classification proposed by Seilacher, Reif & Westphal (Reference Seilacher, Reif and Westphal1985), the taphonomic features and ecological spectrum of the fossil assemblage indicate that the Pesciara cave limestone represents an obrutionary stagnation deposit. Based on their benthic foraminiferan content, the fish-bearing strata of the Pesciara cave site have been referred to the Alveolina dainelli Zone (Trevisani et al. Reference Trevisani, Papazzoni, Ragazzi and Roghi2005; Papazzoni & Trevisani, Reference Papazzoni and Trevisani2006), or SBZ 11 Biozone (Serra-Kiel et al. Reference Serra-Kiel, Hottinger, Caus, Brobne, Ferràndez, Jauhri, Less, Pavlovec, Pignatti, Samsó, Schaub, SIREL, Strougo, Tambareau, Tosquella and Zakrevskaya1998), corresponding to the middle Cuisian (late Ypresian; about 50 Ma).
3. Materials and methods
The specimens documented herein were found among undescribed lophiiform material in the collection of fossil fishes from Monte Bolca of the Museo Civico di Storia Naturale di Verona (MCSNV), provisionally labelled as indeterminate members of the family Lophiidae. One of these specimens (MCSNV T492) was tentatively assigned to the Ogcocephalidae in a faunal inventory assembled by Blot (Reference Blot1980). All the specimens but one, MCSNV B28/B29, consist of well-preserved complete articulated skeletons preserved on the surface of laminated micritic limestone. The material was examined using a Wild M5A stereomicroscope equipped with a camera lucida drawing arm. Measurements were taken with a dial calliper to the nearest 0.1 mm. Standard length (SL) is used throughout. Methods for taking counts and measurements follow Bradbury (Reference Bradbury1980, Reference Bradbury1988). Osteological terminology, unless noted otherwise, follows Bradbury (Reference Bradbury1967) and Pietsch (Reference Pietsch1981). Comparative anatomical information was extracted primarily from the literature. The term ‘batfish’ is used herein as the synonym of ‘ogcocephalid’.
4. Systematic palaeontology
Subdivision TELEOSTEI sensu Patterson & Rosen, Reference Patterson and Rosen1977
Order LOPHIIFORMES Garman, Reference Garman1899
Family Ogcocephalidae Jordan, Reference Jordan1895
Genus Tarkus gen. nov.
Type species. Tarkus squirei gen. et sp. nov. from the Pesciara cave site, Monte Bolca locality, northeastern Italy; late early Eocene.
Diagnosis. Tarkus is unique among ogcocephalids in having the body moderately depressed anteriorly; large disk nearly rounded in outline; thick and stout caudal peduncle; massive and dorso-ventrally depressed neurocranium; frontals folded medially to form a shallow groove for the illicium; teeth present on jaws and palate; 18–19 vertebrae; vertebral centra with slender elongate neural and haemal spines; neural and haemal spines of the penultimate vertebra greatly enlarged forming a thick and laterally compressed plate; epural elongate and slender; illicial bone pitted with two large and rounded ventral lobes plus a small median dorsal lobe; soft dorsal fin with broad base containing eight to ten rays; anal fin containing seven to nine rays; pectoral pedicels connected to the body wall by a membrane; pectoral fin contains 13 distally branched rays; body covered with thick, slightly overlapping tubercles of two different sizes and morphologies.
Etymology. The name is taken from the suite released in 1971 by Keith Emerson, Greg Lake and Carl Palmer, which depicts Tarkus as an armoured half armadillo/half tank creature, born from an egg erupted from a volcano, and applied here in reference to the thick dermal covering of bony tubercles that characterize this genus.
Tarkus squirei sp. nov.
Figures 1–6
1972 Lophius brachysomus Agassiz, Reference Agassiz1835; Sorbini, p. 122, pl. 18, fig. 1. (misidentification)
1991 Lophius brachysomus Agassiz, Reference Agassiz1835; Frickhinger, p. 694. (misidentification)
1996 Lophius brachysomus Agassiz, Reference Agassiz1835; Long, p. 157, fig. 2. (misidentification)

Figure 1. Tarkus squirei gen. et sp. nov. Holotype. (a) MCSNV T159; (b) MCSNV T158. Scale bars 20 mm.
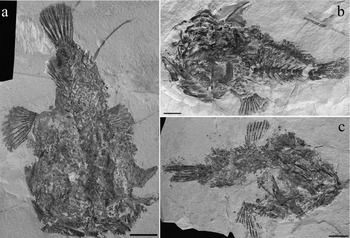
Figure 2. Tarkus squirei gen. et sp. nov. Paratypes. (a) MCSNV T371; (b) MCSNV T492; (c) MCSNV T281. Scale bars 20 mm.

Figure 3. Tarkus squirei gen. et sp. nov. MCSNV T159, dorsal view of the neurocranium. Scale bar 5 mm.
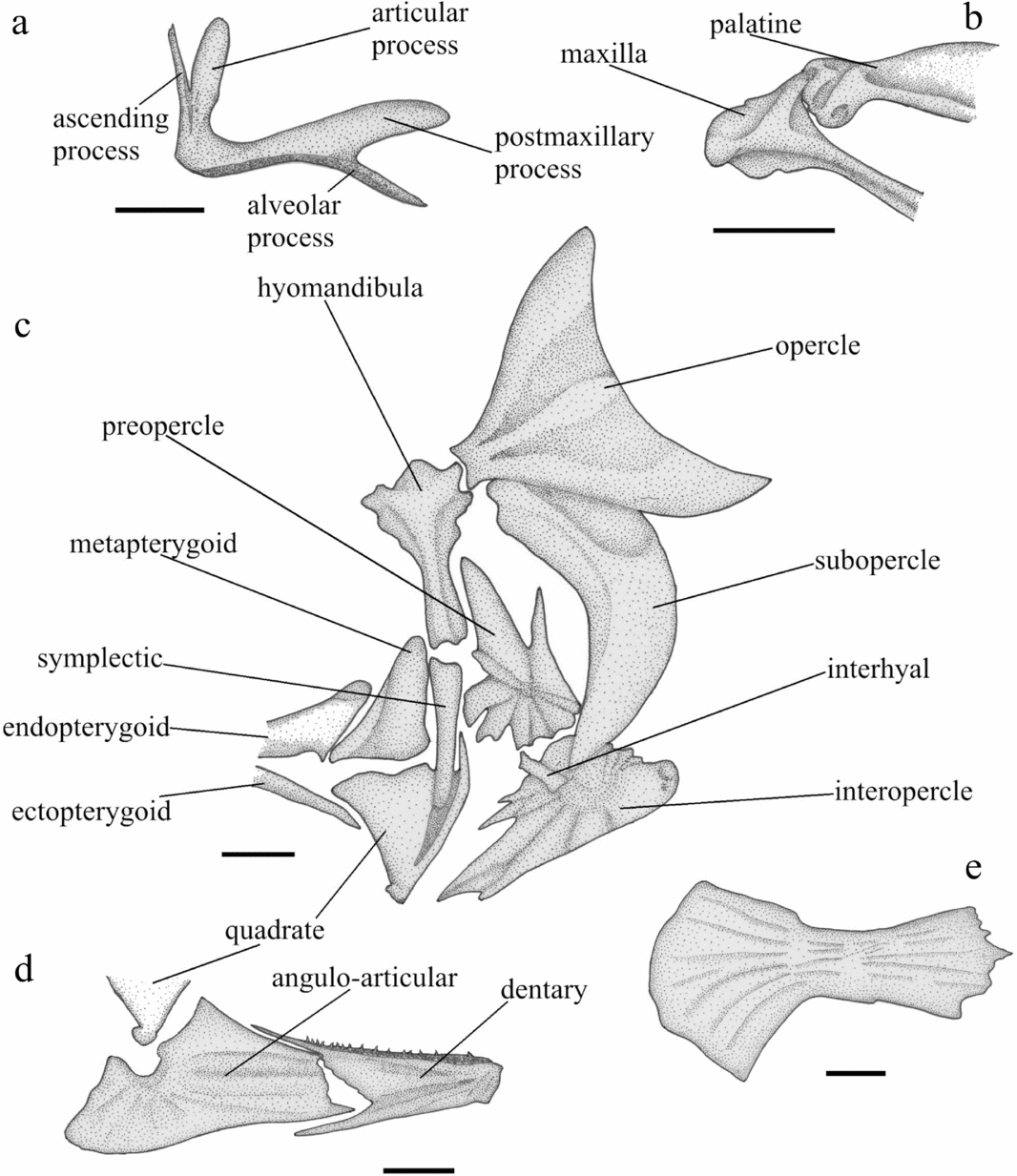
Figure 4. Tarkus squirei gen. et sp. nov. (a) MCSNV T492, right medial view of the premaxilla; (b) MCSNV T371, right medial view of the anterior portion of the maxilla and palatine; (c) MCSNV T159, right medial view of the suspensorium and opercular bones; (d) MCSNV T371, left medial view of the mandible; (e) right lateral view of the anterior ceratohyal. Scale bars 5 mm.
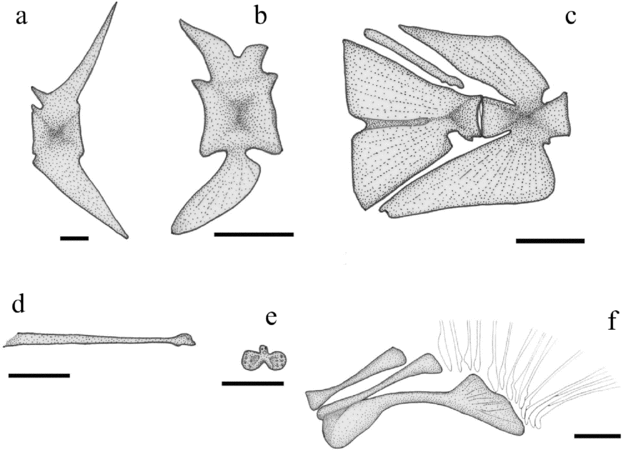
Figure 5. Tarkus squirei gen. et sp. nov. (a) MCSNV T158, left lateral view of the first caudal vertebra. Scale bar 2 mm. (b) MCSNV B28, right lateral view of the antepenultimate abdominal vertebra. Scale bar 5 mm. (c) MCSNV T371, right lateral view of the caudal skeleton. Scale bar 5 mm. (d) MCSNV T159, right lateral view of the illicial pterygiophore. Scale bar 5 mm. (e) MCSNV T159, anterior view of the illicial bone. Scale bar 5 mm. (f) MCSNV T159, right lateral view of the pectoral-fin radials. Scale bar 5 mm.
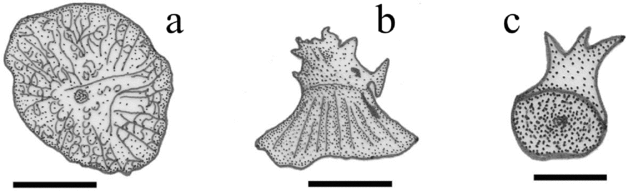
Figure 6. Tarkus squirei gen. et sp. nov. (a) MCSNV T492, dorsal view of a large dermal tubercle of the right pectoral-fin pedicel. Scale bar 2 mm. (b) MCSNV T492, lateral view of a large dermal tubercle of the dorsal portion of the urosome. Scale bar 2 mm. (c) MCSNV T371, ventrolateral view of a small dermal tubercle. Scale bar 1 mm.
Holotype. MCSNV T158/T159, complete articulated skeleton in part and counterpart, 121.2 mm SL (Fig. 1).
Type locality and horizon. Pesciara cave site, Monte Bolca locality, northeastern Italy; late early Eocene, late Ypresian, SBZ 11, Alveolina dainelli Zone.
Paratypes. MCSNV T371, complete articulated skeleton, 138.4 mm SL (Fig. 2a); MCSNV T492, complete slightly disarticulated skeleton, 162 mm SL (Fig. 2b); MCSNV T281, nearly complete partially disarticulated skeleton, 126.4 mm SL (Fig. 2c); MCSNV B28/29, moderately preserved and partially articulated skeleton in part and counterpart.
Diagnosis. As for genus, only species.
Etymology. After the British musician and composer Christopher Russell Edward ‘Chris’ Squire, commonly known by his nickname ‘Fish’.
Description Body moderately depressed anteriorly, forming a disk that appears to be nearly rounded in outline (Figs 1, 2); however, the exact original morphology of the disk, which in the fossils appears variable in size and shape depending on preservation, is difficult to define unambiguously. The rostrum is not prominent and a median horn is certainly absent. The mouth is terminal, horizontal and moderately large. The pectoral peduncles are connected by membranes to the body wall (Figs 1, 2a). The tail is stout and relatively elongate, moderately wide at its junction with the disk, gradually tapering to the caudal-fin base. The caudal peduncle is unusually strong. Predorsal distance ranges from 58.6 to 68.1 % of SL (see Table 1); preanal distance ranges from 70.6 to 77.8 % of SL. The dorsal fin has a long base, reaching to slightly less than 30 % of SL. The origin of the anal fin is placed approximately below the middle of the dorsal fin; its base ranges from 11.5 to 14.3 % of SL.
Table 1. Measurements (in per cent standard length) of Tarkus squirei gen. et sp. nov. specimens

In all the available specimens the neurocranium is exposed in dorsal view (Fig. 3). The neurocranium is compact, massive, dorso-ventrally depressed and strongly ossified. It is moderately elongate and broad, with its maximum width (measured between the two contralateral extensions of the pterotics) reaching slightly less than 85 % of its length. The bones of the skull roof are fibrous, thick and ornamented by shallow irregular pits and ridges. The frontals are large and irregular in shape. Each frontal is characterized by a compressed anterior half with nearly rounded lateral profiles, each distinctly separated and progressively divergent from its counterpart; the large posterior half of each frontal is evidently folded and depressed medially to form a shallow groove for the illicium. The posterolateral sector of each frontal largely contributes to the posterior margin of the orbit. Because of the reduced anterior extension of the frontals onto the floor of the illicial groove, the mesethmoid is largely exposed and clearly recognizable in the available material. This bone is elongate and nearly ovoid, with a rounded profile and a dome-like dorsal surface. The lateral ethmoids are columnar, with expanded ventral ends. The maximum width measured between the outer margins of the lateral ethmoids ranges between 15 to slightly less than 17 % SL. The vomer is a large irregular bone. Sockets for vomerine teeth arranged in two lateral patches are visible at least in the holotype. The parietals are large irregular bones separated from each other by an elongate supraoccipital. Each parietal articulates anteriorly with the frontal, laterally with the sphenotic, laterally and posterolaterally with the pterotic, posteriorly with the epioccipital, and (as already indicated above) medially with the supraoccipital. Each sphenotic forms a pointed anterolaterally directed process. The pterotic is thickened and flange-like laterally and massive medially. Each epioccipital articulates anteromedially with the supraoccipital, anteriorly with the parietal, laterally with the pterotic and posttemporal, and medially with its contralateral counterpart. The epioccipitals are large bones, irregular in shape, that possess a pointed and elongate process emerging from their posterodorsal margin. The posttemporal is a relatively large bone, representing a wide dorsal flange. The posttemporal is consolidated with the posterolateral corner of the neurocranium. The morphology and position of this bone seem to indicate an extremely reduced mobility in an anterodorsal–posteroventral plane that overlaps the dorsolateral surface of the epioccipital and the posterodorsal surface of the pterotic.
The premaxilla (Fig. 4a) has a narrow ascending process, a spatulate articular process that is slightly shorter than the ascending process and a large, rounded, plate-like postmaxillary process. The alveolar process is curved and tapering, with depressible conical teeth arranged in two or three rows; this process is connected by bone to the postmaxillary process for one third of its length. Overall, the ascending and alveolar processes form a right angle. The maxilla is an elongate bone with an expanded anterior head (Fig. 4b) and a large flattened expanded posterior process. The mandible apparently consists of the dentary and angulo-articular (Fig. 4d). The dentary is a thick robust bone with a massive symphyseal region and a shallow medial concavity for articulation of the adductor muscle. Two or three rows of small conical depressible teeth are present along the dorsal margin of this bone.
The hyomandibula is broad dorsally and tapers abruptly into a stout shaft with a median thickening (Fig. 4c). There are two articular heads in the dorsal portion of the hyomandibula; both articulate with the neurocranium. The posterior sector of the broadened dorsal portion of the hyomandibula is expanded to form a short condyle that articulates with a concave articular facet on the anterior margin of the opercle. The symplectic is a long and stout rod-like bone with a slightly expanded dorsal head (Fig. 4c). The quadrate is nearly triangular with a developed articular facet at its anteroventral corner; a thick pointed process emerges dorsally from the posterior margin of this bone (Fig. 4c). The metapterygoid is a laminar bone, approximately triangular in outline, with rounded corners and a slightly depressed anteroventral margin; it articulates posterodorsally with the hyomandibula, posteriorly and posteroventrally with the symplectic, ventrally with the quadrate and ectopterygoid, and anteroventrally with the endopterygoid (Fig. 4c). The endopterygoid is elongate, plate-like, with an expanded posterior end (Fig. 4c). The ectopterygoid is a thin elongate plate that contacts anterodorsally with the palatine, possibly through interdigitation (or, alternatively, through fibrous tissue). The palatine is a large curved bone; it articulates anteriorly with the concave dorsal facet of the maxilla (Fig. 4b), posteroventrally with the ectopterygoid, and posterodorsally with the endopterygoid. Palatine teeth are not visible but their original presence cannot be excluded.
The opercle is expanded and triangular in shape with a regularly concave posterior margin (Fig. 4c). The subopercle is a broad crescent-shaped bone articulating with the opercle posterodorsally, and the interopercle ventrolaterally (Fig. 4c). The interopercle is a large oblong plate, irregular in shape, broad posteriorly and progressively tapering to a point anteriorly; this bone is ornamented with moderately deep furrows that radiate from the central sector of its posterior field (Fig. 4c). The preopercle is an irregular plate characterized by having a linear posterior margin plus a number of radially disposed spinous and quadrangular processes of different sizes, each originating in the centre of the bone (Fig. 4c).
The structure of the hyoid apparatus is poorly defined owing to inadequate preservation, and for this reason it cannot be discussed in detail. The anterior ceratohyal is a robust, posteriorly enlarged plate, with a rounded posterior profile (Fig. 4e). The interhyal is a short columnar bone that appears to contact the medial surfaces of the interopercle and subopercle (Fig. 4c). Fragmented branchiostegal rays are visible in all the examined specimens.
Of the gill arches, elongate and slightly curved ceratobranchials are partially exposed in the holotype (see Fig. 1). Short, stout, conical (pharyngo)branchial teeth can be observed in the holotype and in the paratypic specimen MCSNV T371.
The vertebral column is slightly arcuate in the abdominal region (Figs 1, 2a). There are 18–19 vertebrae, including the last centrum fused to the hypural plate. There are nine abdominal vertebrae. Caudal vertebrae are interpreted herein as those that are not directly associated with anal-fin pterygiophores. The first vertebral centrum, neural arch and spine are closely articulated with the neurocranium. The robust centrum of the first vertebra is about half the length of the centra of the succeeding vertebrae. The vertebral centra are massive, rectangular in outline, longer than high (Fig. 5a, b). Neural prezygapophyses are prominent, emerging from the anteroventral margin of the neural arches. In the abdominal region, the neural spines are stout and considerably shorter than those of the caudal region. The two (or three) posterior abdominal centra bear flattened and spatulate haemal spines of gradually increasing size; these spines are posteriorly inclined and are characterized by a gently rounded profile (Fig. 5b). The haemal spines of the caudal vertebrae are large, triangular in outline, and roughly symmetrical to their counterparts (Fig. 5a). The penultimate centrum bears unusually wide and laterally compressed neural and haemal spines (Fig. 5c). The caudal skeleton consists of a wide hypural fan and a single epural (Fig. 5c). The hypural fan is a triangular, laterally compressed plate, with a shallow central furrow and a small notch along its posterior margin. Like in other lophiiforms, the hypural plate of Tarkus squirei gen. et sp. nov. represents the results of the fusion of the ural centra, preural centrum, hypurals and parhypural (see Rosen & Patterson, Reference Rosen and Patterson1969). The caudal fin contains nine elongate principal rays of which the central seven are bifurcated distally.
An incomplete disarticulated spinous dorsal fin is preserved in the holotype (Fig. 5d, e). The vestigial second dorsal-fin spine is not recognizable. The illicial bone has two large rounded ventrolateral lobes and a reduced dorsal median lobe; the lobes of the illicial bone are extensively pitted (Fig. 5e). The illicial pterygiophore is elongate, slender, cylindrical and irregularly enlarged proximally (Fig. 5d). The soft dorsal fin originates approximately at two thirds the standard body length and consists of eight to ten rays supported by seven to nine pterygiophores. The dorsal-fin pterygiophores are massive, characterized by fibrous bony tissue. The posteriormost dorsal-fin pterygiophore possesses a prominent posterodorsal crest with irregular profile. The dorsal-fin pterygiophores interdigitate with the neural spines in the upper sector of the interneural spaces (Figs 1, 2).
The anal fin consists of seven to nine closely associated rays, supported by six to eight narrow pterygiophores with expanded proximal heads.
As described above, the posttemporal is attached to the posterolateral corner of the neurocranium (Fig. 3). The supracleithrum, scapula and coracoids are difficult to recognize in the available material. The cleithrum is a very large and thickened crescent-shaped bone (Figs 1, 2); the ascending and horizontal arms of this bone are approximately of the same length. There is a single elongate and slender postcleithrum that articulates medially with the lower sector of the ascending arm of the cleithrum. The pectoral fin is supported by three moderately elongate radials (Fig 5f). The two dorsal radials are similar in shape, while the lower one is considerably larger, with broad distal and proximal ends. The two ventralmost radials are fused to one another at their proximal ends. Thirteen elongate and distally branched pectoral-fin rays interconnected by a membrane insert on the expanded distal head of the lowermost pectoral-fin radial (Figs 1, 2, 5f).
Pelvic fins and girdles are not visible in the examined specimens.
The squamation consists of slightly overlapping tubercles that cover the entire body, including the margin of the disk and the caudal, soft dorsal and anal fins. Tubercles are mainly of two sizes (Fig. 6). Relatively small conical tubercles, with simple (rarely) or multicuspidate tips, are evenly distributed over the entire body (Fig. 6c). Interspersed are very large pyramidal tubercles with irregularly rounded bases and prominent radial ridges; some of these large stiffened dermal structures possess a heavy, massive and irregularly ornamented tip with a cylindrical base emerging from the apical portion of each tubercle (Fig. 6a, b). These large tubercles appear to be scattered at least over the dorsal surface of the disk, urosome and pectoral-fin bases. Tubercles of the disk edge are no larger than the small tubercles present elsewhere on the body. Peculiar lateral-line scales have not been recognized.
5. Discussion
5.a. Comparison and relationships
The specimens documented herein show a number of features that strongly support their recognition as representatives of a new genus and species of the lophiiform family Ogcocephalidae.
Lophiiform fishes constitute one of the most heterogeneous and morphologically diverse lineages within the Acanthomorpha. All members of the 18 families belonging to this clade are characterized by a peculiar morphology of the first dorsal-fin spine, the so-called illicium, which is modified to serve as a luring apparatus and placed out on the tip of the snout (Pietsch & Grobecker, Reference Pietsch and Grobecker1987; Pietsch, Reference Pietsch2009). The monophyletic status of the Lophiiformes was demonstrated based on six synapomorphies (Pietsch, Reference Pietsch1981, Reference Pietsch, Moser, Richards, Cohen, Fahay, Kendall and Richardson1984; Pietsch & Grobecker, Reference Pietsch and Grobecker1987), of which one refers to the eggs that are spawned in an oval or scroll-shaped mucous sheath (see Rasquin, Reference Rasquin1958; Pietsch, Johnson & Arnold, Reference Pietsch, Johnson and Arnold2009), another to the external morphology (gill opening restricted to a small, elongate tube-like opening situated close to the pectoral-fin base), and the other four refer to skeletal morphology (see also Regan, Reference Regan1912; Rosen & Patterson, Reference Rosen and Patterson1969). The anatomical analysis of the fossils has revealed the presence of all four osteological characters that define the Lophiiformes (spinous dorsal fin modified to serve as a luring apparatus; epioccipitals separated from parietals and meeting on the midline posterior to the supraoccipital; caudal skeleton constituted by the fusion of the second ural centrum with the first ural and first preural centra, hypurals and parhypural; pectoral radials elongate, the ventralmost element considerably expanded distally).
Despite the general limited availability in museum collections, many of the groups of lophiiform fishes were studied in great detail in the last few decades, resulting in a thorough definition of the anatomy and systematics of the Antennarioidei (Pietsch, Reference Pietsch1981; Pietsch & Grobecker, Reference Pietsch and Grobecker1987; Pietsch, Johnson & Arnold, Reference Pietsch, Johnson and Arnold2009; Carnevale & Pietsch, in press), Ceratioidei (e.g. Pietsch, Reference Pietsch1974; Reference Pietsch2009; Pietsch & Orr, Reference Pietsch and Orr2007) and Lophioidei (e.g. Caruso, Reference Caruso1985). In this scenario of progressively increased interpretation of lophiiform biodiversity, the Ogcocephalidae certainly represents one of the least known and most obscure families of this heterogeneous and anatomically diverse clade of acanthomorphs.
In his review of lophiiform ( = Pediculati) classification, Regan (Reference Regan1912) presented a diagnosis of the family based on a limited anatomical survey of the known batfish diversity. The first comprehensive analysis of the ogcocephalid diversity was realized by Bradbury (Reference Bradbury1967), who provided a definition of the genera based on selected external and osteological features, and also recognized seven characters apparently diagnostic of the family. Subsequently, Pietsch (Reference Pietsch1981), in the context of a broad phylogenetic study of the Lophiiformes, presented a further superficial diagnosis of the family and, more recently, Endo & Shinohara (Reference Endo and Shinohara1999) published a preliminary analysis of intrafamilial relationships using a weak dataset derived from some of the morphological characters discussed by Bradbury (Reference Bradbury1967) to separate the genera. In summary, although a possible diagnosis of the Ogcocephalidae has been discussed several times, the research of the unequivocal synapomorphic features that define the family remains largely unexplored. A cursory analysis of the literature suggests that except for a few aspects of the external morphology, the only features potentially considered as exclusive to ogcocephalids concern the general structure of the illicium and the squamation pattern. In ogcocephalids the illicium represents all that remains of the spinous portion of the dorsal fin; the illicial bone is notably small and the second spine is reduced to a small splint of bone fused to or lying on the illicial pterygiophore. As far as squamation is concerned, the ogcocephalids are characterized by the presence of thick tubercles that cover partially or completely the head and body. As described in Section 4, Tarkus squirei gen. et sp. nov. possesses both the typical ogcocephalid illicium and a thick covering of tubercles on the head and body. Moreover, its attribution to the family Ogcocephalidae is also justified by the presence of several other characters, including the moderately depressed body, horizontal gape of the mouth, mesethmoid ossified as an interorbital septum and filling the interspace between the anterior processes of the frontals, lateral ethmoids stout and short, opercles very large, possession of nine caudal-fin rays, third dorsal-fin spine absent, and pectoral fins oriented in a horizontal position (see Regan, Reference Regan1912; Bradbury, Reference Bradbury1967; Pietsch, Reference Pietsch1981).
The relationships between the batfish genera are still undefined and scarcely investigated. The resolution of the cladogram proposed by Endo & Shinohara (Reference Endo and Shinohara1999) was very poor, owing to the small number of characters used, and the tree topology is only partially in agreement with the preliminary hypotheses formulated in the mitogenomic analyses of lophiiform suborders by Miya et al. (Reference Miya, Pietsch, Orr, Arnold, Satoh, Shedlock, Ho, Shimazaki, Yabe and Nishida2010). A comprehensive phylogenetic study of ogcocephalids based on morphological characters cannot be realized until a detailed anatomical study of representatives of all extant genera is available. Nevertheless, even though it is not possible to conclusively establish the precise phylogenetic position of Tarkus gen. nov., a comparative discussion of its main diagnostic features could certainly suggest convincing hypotheses about its affinities.
In her 1967 survey of ogcocephalid generic diversity, Bradbury implicitly evidenced the existence of phenetic relationships between certain genera, related to the distribution of selected features such as the general morphology of the body, disk outline, configuration of the frontals and presence of the bucklers. For example, based on the shared possession of the bucklers, triangular outline of the disk, and frontals modified with an upward outgrowth of their lateral ridges that are joined in the midline to form a tube for the illicium, a close affinity between the genera Malthopsis, Ogcocephalus and Zalieutes appears to be evident. Moreover, the genera Coelophrys and Halieutopsis seem to be aligned by sharing a number of features, including a globose body (only in one species of Halieutopsis), external surface of the frontals not deformed, absence of teeth on palate, soft loose skin, subdermal gelatinous tissue, undifferentiated tubercles over the entire body and a spine-like illicial bone, which also occur in the pelagic post-larval stage of most batfishes and have been interpreted as representing the expression of heterochronic processes (see Bradbury, Reference Bradbury1988, Reference Bradbury1999; Endo & Shinohara, Reference Endo and Shinohara1999). In this context, Tarkus gen. nov. appears to be phenetically related to the genera Halieutaea and Halieutichthys, since they share the generally depressed body, rounded disk outline, bucklers absent and frontals medially depressed to form a groove for the illicium. Moreover, Tarkus gen. nov. shares with Halieutichthys the configuration of the pectoral pedicels that are broadly attached to the body wall (Table 2).
Table 2. Summary of selected morphological features used to discriminate the genera of the Ogcocephalidae

Data from Amaoka & Toyoshima (Reference Amaoka and Toyoshima1981); Bradbury (Reference Bradbury1967, Reference Bradbury1980, Reference Bradbury1988, Reference Bradbury1998, Reference Bradbury1999, Reference Bradbury2003); Bradbury, McCosker & Long (Reference Bradbury, McCosker and Long1999); de Beaufort & Briggs (Reference de Beaufort and Briggs1962); Endo & Shinohara (Reference Endo and Shinohara1999); Ho & Shao (Reference Ho and Shao2007, Reference Ho and Shao2008); Ho, Endo & Sakamaki (Reference Ho, Endo and Sakamaki2008); Ho, Prokofiev & Shao (Reference Ho, Prokofiev and Shao2009); Ochiai & Mitani (Reference Ochiai and Mitani1956); Radcliffe (Reference Radcliffe1912); Shimazaki, Endo & Yabe (Reference Shimazaki, Endo and Yabe2004).
Despite such a superficial phenetic affinity with the Western Atlantic genus Halieutichthys, Tarkus gen. nov. is unique among ogcocephalids in having distally branched pectoral-fin rays, a high number of dorsal- and anal-fin rays, an elongate epural, and vertebrae with relatively narrow neural and haemal spines. The possession of branched pectoral-fin rays is unique within ogcocephalids and more generally is remarkably unusual in lophiiform fishes, being present only in four antennariid species of the Antennarius ocellatus Group (A. avalonis, A. ocellatus, A. sarasa, and A. senegalensis; see Pietsch & Grobecker, Reference Pietsch and Grobecker1987), but absent in all ceratioids, chaunacoids, lophioids and other antennarioids. Unbranched pectoral-fin rays represent a specialization of lophiiform fishes since most of other acanthomorphs possess branched rays. The condition observed in Tarkus gen. nov., therefore, clearly indicates that branched pectoral-fin rays independently arose at least two times in the evolutionary history of lophiiforms. The soft dorsal and anal fins of Tarkus gen. nov. contain a number of rays greater than those typical of other ogcocephalids. The dorsal fin of Tarkus gen. nov. consists of eight to ten rays, whereas in other genera the number of dorsal-fin rays ranges from zero in Halicmetus to up to seven in Dibranchus and Solocisquama (see Table 1). The structure and composition of the dorsal fin of Tarkus gen. nov., as well as of its endoskeletal support, greatly resemble those of certain lophioids, antennarioids and chaunacoids. Such a general anatomical affinity with members of the other bottom-dwelling lophiiforms, including the basal lophiid Sladenia (see Caruso & Bullis, Reference Caruso and Bullis1976), suggests that the condition observed in Tarkus gen. nov. might represent the plesiomorphic state for the ogcocephalids. This hypothesis is consistent with the structure of the caudal skeleton, which is less compact and characterized by an elongate epural and well-developed neural and haemal spines of the penultimate vertebra as in lophioids, antennarioids and chaunacoids; the caudal skeleton of extant ogcocephalids is characterized by a high degree of fusion of the elements, a very short and massive (if not fused) epural, and thick and shallow neural and haemal spines (see Fujita, Reference Fujita1990). More generally, the entire axial skeleton of Tarkus gen. nov. is different from those of extant ogcocephalids in having more elongate vertebral centra bearing slender and moderately elongate neural and haemal spines, which interdigitate only distally with dorsal- and anal-fin pterygiophores; the vertebral column of extant ogcocephalid genera is more compact, with nearly quadrate centra bearing short and stout neural and haemal spines, which are closely associated to the median-fin pterygiophores (see Garman, Reference Garman1899; Pietsch, Reference Pietsch1981).
In conclusion, the comparative analysis of the osteology of Tarkus gen. nov. clearly distinguishes it from other batfishes because of its peculiar structure of the axial skeleton (vertebral column, caudal skeleton, and dorsal and anal fins) and possession of distally branched pectoral-fin rays. The vertical development of the axial skeletal structures might be indicative of a moderately globose design of the body, definitely different from the strongly depressed physiognomy typical of most batfishes. The unusual set of characters of Tarkus gen. nov. might be related to its possible basal position within the ogcocephalids; however, a conclusive definition of the phylogenetic position of Tarkus gen. nov. cannot be properly inferred until a more detailed knowledge of the morphology of extant ogcocephalid genera becomes available.
5.b. Palaeobiological notes
Extant batfishes are distributed in tropical and subtropical seas all around the world. These fishes are known to occur primarily in outer continental shelf or upper continental slope habitats to depths of 4000 m (see Bradbury, Reference Bradbury1988). However, species of the genera Halieutaea, Halieutichthys and Zalieutes commonly inhabit tropical shallow waters, and a few species of the genus Ogcocephalus have been observed in the subtidal zone (Bradbury, Reference Bradbury1988; Bradbury, McCosker & Long, Reference Bradbury, McCosker and Long1999), where these generally reside on open-bottom habitats of rubble, sand or mud (Bradbury, Reference Bradbury1980). As documented above, Tarkus gen. nov. shows a high degree of phenetic resemblance to the extant inner shelf genera Halieutaea and (more particularly) Halieutichthys. Such a morphological affinity between Tarkus gen. nov. and the extant genera characterized by rounded disk outline is therefore corroborated by a similar bathymetric distribution.
The palaeoenvironmental and depositional setting of the fish-bearing micritic limestone of the Pesciara cave site has been discussed by many authors with different interpretative scenarios and reconstructions. According to Sorbini (Reference Sorbini1968) and Massari & Sorbini (Reference Massari and Sorbini1975), the fossiliferous limestone was deposited in a tropical coastal lagoon close to coral reefs and episodically isolated from the open ocean. Based on a broad comprehensive palaeoecological study of the ichthyofauna, Landini & Sorbini (Reference Landini, Sorbini and Cherchi1996) hypothesized that laminated sedimentation of the fish-bearing deposits occurred parallel to the coast, many dozens of metres in depth, in a silled depression that was occasionally subjected to restricted circulation and bottom anoxia. The depositional environment was located at a short distance from the coast in close proximity to coral reefs, seagrass beds and the open sea; moreover, sedimentological evidence suggests that it was subject to seasonal continental influences, possibly due to the presence of well-developed fluvial systems nearby. Papazzoni & Trevisani (Reference Papazzoni and Trevisani2006), exclusively based on facies analysis and foraminiferal palaeoecology, concluded that the fossiliferous limestone of the Pesciara cave site originated in a subtropical lagoon affected by seasonal changes of water circulation, which influenced the oxygen content on the sea bottom. In summary, although a multidisciplinary palaeoecological analysis would be desirable to properly interpret the physicochemical and ecological features of the original depositional environment, all the authors involved in the palaeoenvironmental study of the fish-bearing micritic strata of the Pesciara cave site concur to suggest that the sedimentation took place in a depressed and moderately deep basin characterized by reduced hydrodynamic energy in a tropical coastal context. Therefore, the palaeoenvironmental characters of the Pesciara di Bolca unambiguously indicate that Tarkus squirei gen. et sp. nov. was a tropical batfish species that inhabited the inner shelf area of the central-western Tethys during the early Eocene.
5.c. Concluding remarks
As pointed out by Carnevale & Pietsch (Reference Carnevale and Pietsch2006), the evolutionary history of lophiiforms is still scarcely understood, mostly because the extraordinary anatomical diversification of the various lophiiform groups makes it really problematic to decipher their origin. Moreover, the age of divergence of the lophiiform clades cannot be precisely established based on palaeontological data because representatives of these clades are extremely rare in the fossil record. However, considerable efforts in the last few years have led to the description of many new fossils and to the re-evaluation of other poorly known extinct lophiiform taxa (Bannikov, Reference Bannikov2004; Carnevale & Pietsch, Reference Carnevale and Pietsch2006, Reference Carnevale and Pietsch2009a,Reference Carnevale and Pietschb, in press; Carnevale et al. Reference Carnevale, Pietsch, Takeuchi and Huddleston2008), confirming the hypothesis by Patterson & Rosen (Reference Patterson, Rosen and Cohen1989), who, based on the phylogenetic considerations and the puzzling lophiiform fossil record known at that time, postulated that all lophiiform lineages were certainly present in the Eocene.
The Eocene existence of ogcocephalids, however, was previously testified by the otolith-based taxon Halieutaea cirrhosa from the Lutetian of southern England and Germany, and possibly also from the Bartonian of southern England (see Stinton, Reference Stinton1978; Schwarzhans, Reference Schwarzhans2007). Moreover, otoliths belonging to the Ogcocephalidae are also known from the Oligocene of Europe (e.g. Schwarzhans, Reference Schwarzhans1994) and the Miocene of Europe (e.g. Schwarzhans, Reference Schwarzhans2010) and Australia (Schwarzhans, Reference Schwarzhans1985). The specimens of Tarkus squirei gen. et sp. nov. documented herein provide the earliest evidence of articulated skeletal remains of the family Ogcocephalidae. The new genus and species described herein also fills the large gap that existed in the palaeontological record of the family, suggesting that the modern structural plan of batfishes originated before the early Eocene.
The coeval existence of lophioids, antennarioids, chaunacoids and ogcocephaloids during the Eocene makes it impossible to interpret the order of events in the phylogeny of lophiiform fishes using the fossil record (see Carnevale & Pietsch, Reference Carnevale and Pietsch2006), with negative implications in the definition of the minimum age of origin of the whole order. Recent molecular-clock analyses of divergence times proposed two different hypotheses for the origin of lophiiforms. Alfaro et al. (Reference Alfaro, Santini, Brock, Alamillo, Dornburg, Rabovsky, Carnevale and Harmon2009) and Santini et al. (Reference Santini, Harmon, Carnevale and Alfaro2009) suggested that the origin of lophiiforms is relatively consistent with the fossil record and must be searched for in the Palaeogene; on the contrary, Miya et al. (Reference Miya, Pietsch, Orr, Arnold, Satoh, Shedlock, Ho, Shimazaki, Yabe and Nishida2010) concluded that all the lophiiform clades, including the ogcocephaloids, originated in the Cretaceous during a relatively short time interval between 130 and 100 Ma ago, thereby implying the existence of a minimum ghost range of 50 Ma in the record.
Acknowledgements
We wish to thank Walter Landini (Dipartimento di Scienze della Terra, Università di Pisa) and Federica Giudice for useful discussions and support. We are particularly obliged to Drs Roberto Zorzin and Anna Vaccari (MCSNV) for access to specimens in their care and hospitality during a February 2010 visit to Verona. For reviewing the manuscript and providing many constructive suggestions for its improvement we are particularly obliged to Jean Gaudant (Muséum national d'Histoire naturelle, Paris), Graham E. Budd (Department of Earth Sciences, Uppsala University) and an anonymous reviewer.










