Introduction
One of the most important factors, in assisted reproductive technology (ART), and in particular in intracytoplasmic sperm injection (ICSI), is the quality of sperm DNA – especially fragmentation (primary and secondary structure) and decondensation (tertiary structure). Oxidative stress is one of the major causes of damage to DNA and more generally to sperm quality (Kao et al., Reference Kao, Chao, Chen, Hwang, Liao and Wei2008). Even though ICSI has proven to be an effective technique there have been only a few studies that have looked at potential negative effects. In a system in which natural selection is bypassed, there may be a risk of transmission of genetic diseases (Zenzes, Reference Zenzes2000; Aitken et al., Reference Aitken, Baker and Sawyer2003; Fernández-Gonzalez et al., Reference Fernández-Gonzalez, Moreira, Pérez-Crespo, Sánchez-Martín, Ramirez, Pericuesta, Bilbao, Bermejo-Alvarez, de Dios Hourcade, de Fonseca and Gutiérrez-Adán2008). Reactive oxygen species (ROS) affect the quality of DNA, not only by provoking fragmentation but also by serving as the foundation for the formation of adducts that cause structural deformation of base pairs, in particular guanine and adenine (Badouard et al., Reference Badouard, Ménézo and Panteix2008).
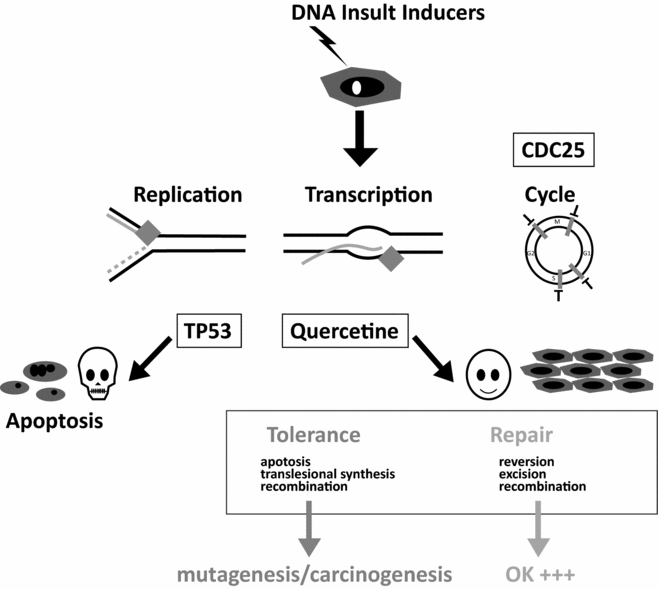
In theory, these chemical insults are repaired by the zygote, and the very early embryo, using reserves accumulated in the growing oocyte. It is known that at the time of the first cell cycle, 1.5 to 2 million repair processes are carried out (Ménézo et al., Reference Ménézo, Dale and Cohen2010a). It is important to recognize that the oocyte has a significant, yet limited, capacity to repair DNA and that this ability decreases with age (Ménézo et al., Reference Ménézo, Russo, Tosti, El Mouatassim and Benkhalifa2007a; Jaroudi et al., Reference Jaroudi, Kakourou, Cawood, Doshi, Ranieri, Serhal, Harper and Sengupta2009). Moreover, relatively little information is known about damage to oocyte DNA, excluding the case of female smokers in who it increases (presence of DNA adducts: Zenzes et al., Reference Zenzes, Puy and Bielecki1998; Zenzes, Reference Zenzes2000), thus contributing to an overall increase in the amount of DNA in need for repair. Once the capacity to repair is overwhelmed, one of two events can occur – apoptosis, which will provoke the cessation of embryonic development, or tolerance, which will let unrepaired DNA ‘pass’ and lead to mutation, a source of genetic problems and cancer. (Marchetti et al., Reference Marchetti, Essers, Kanaar and Wyrobek2007; Derijck et al., Reference Derijck, van der Heijden, Giele, Philippens and de Boer2008; Fig. 1).

Figure 1 Apoptosis/Cell cycle Arrest-Repair/Tolerance.
Attempting to reduce or even to avoid the generation of DNA damage related to ROS through consumption of anti-oxidants is often tempting (especially using vitamins A, E and C, coupled with selenium). However, if these mixtures are sometimes effective marginally in reducing DNA fragmentation in sperm, in a large number of cases, they may induce a decondensation of the nucleus (Ménézo et al., Reference Ménézo, Hazout, Panteix, Robert, Rollet, Cohen-Bacrie, Chapuis, Clément and Benkhalifa2007b), which has a negative effect on regulation of the paternal genome in the early stages of embryonic development (Rousseaux et al., Reference Rousseaux, Reynoird, Escoffier, Thevenon, Caron and Khochbin2008; Jenkins & Carrell, Reference Jenkins and Carrell2012). Early embryos are then blocked at different stages, leading to what is unduly characterized as ‘no fertilization’ syndromes.
In this paper we argue that current antioxidant treatments, given irrespectively of clinically quantified deficiencies, are potentially detrimental and that over-exposure to them is risky. Few studies have been made to correlate anti-oxidant treatments with the quality of DNA and a study of actual damage prior to any treatment is absolutely necessary in order to define effective strategies. Here we discuss potential negative effects of excessively strong anti-oxidants and suggest new treatments in relation to present day concepts on oxidative stress.
I Parameters of sperm quality
It is essential to standardize the type, reproducibility and specificity of the sperm assay (Gharagozloo & Aitken, Reference Gharagozloo and Aitken2011). Motility and number are obviously of major importance in natural conception, but in assisted reproductive technology (ART), these parameters are less important. In both cases, DNA quality parameters, i.e. fragmentation, oxidation and tertiary structure (compaction/condensation), are the key parameters for successful embryogenesis. Since the pioneering work of Evenson et al. (Reference Evenson, Darzynkiewicz and Melamed1980) on sperm chromatin structure assay (SCSA), several techniques have been designed to study DNA in sperm. Terminal deoxynucleotidyl transferase-mediated dUTP nick end-labelling (TUNEL) is the most popular for determining DNA fragmentation; it is also very reliable. To study condensation, aniline/toluidine blue staining (Hammadeh et al., Reference Hammadeh, Zeginiadov, Rosenbaum, Georg, Schmidt and Strehler2001) seems more reliable than chromomycin, which evaluates both fragmentation and decondensation (Belloc et al., Reference Belloc, Benkhalifa, Junca, Dumont, Bacrie and Ménézo2009). DNA oxidation products are numerous and can alter all the bases (Ménézo et al., Reference Ménézo, Dale and Cohen2010a). The most common insult is 8-oxodeoxyguanosine (8OH-dG): quantification is difficult especially as spontaneous oxidation may occur (Badouard et al., Reference Badouard, Ménézo and Panteix2008). Formation of malone dialdehyde (MDA) and corresponding adducts are linked to sperm immaturity/decondensation in relation to a too high content of polyunsaturated fatty acid (PUFA; Aitken et al., Reference Aitken, Wingate, De Iuliis, Koppers and McLaughlin2006) even though the final result is the product of fatty acid oxidation.
II Current treatments and their anomalies
Current treatments are primarily made using selenium, and a cocktail of vitamins (A, E, and C), occasionally including co-enzyme Q10 and superoxide dismutase (SOD).
Selenium
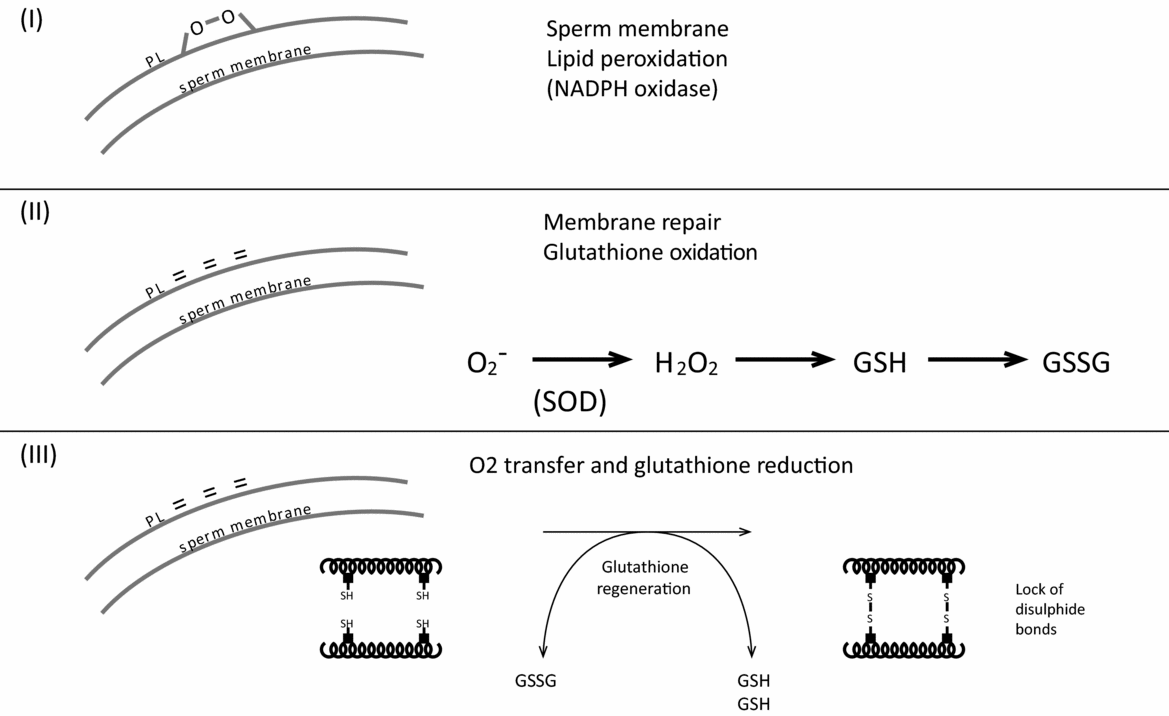
Selenium is generally prescribed for ageing. There are 25 selenoproteins in the human (male), among which the most well known are the glutathione peroxidases (GPX). For male fertility, the only reason for which selenium is prescribed, without restriction as a supplement, is its role as a co-factor for phospholipid hydroperoxide glutathione peroxidase (GPX 4, PHGPx, Ursini et al.,Reference Ursini, Maiorino and Roveri1997), which is responsible for the compaction of the spermatozoon nucleus (Fig. 2), and in fact, of the tertiary structure of the nucleus. This enzyme allows protamines at the cystine bridges to padlock the DNA. Several observations argue against the uncontrolled use of selenium supplementation in male infertility.

Figure 2 Antioxidants at too high levels block this process (importance of glutathione).
First, there is little or no selenium deficiency described in the scientific literature for the ‘Caucasian’ population. The usefulness of supplementation can therefore be questioned. Optimum concentrations range between 50 and 70 μg/ml. Within and beyond these values, the quality of the spermatozoon is altered (Bleau et al., Reference Bleau, Lemarbre, Faucher, Roberts and Chapdelaine1984). Second, even if selenium supplementation increases serum values it does not modify testicular values – as shown in animal studies (Hawkes et al., Reference Hawkes, Alkan and Wong2009; Combs et al., Reference Combs, Watts, Jackson, Johnson, Zeng, Scheett, Uthus, Schomburg, Hoeg, Hoefig, Davis and Milner2011). Third, together with aluminum, bacterial contamination, radioactivity, pesticides and nitrates, a high concentration of selenium is one of the six parameters used to regulate safety in human drinking water, according to European Economic Community (EEC) regulations.
In addition, other basic elements should be considered: selenites prompt apoptosis by generating superoxides (oxygenated free radicals) via the mitochondria (Xiang et al., Reference Xiang, Zhao and Zhong2009), which is the primary source for DNA damage in the spermatozoon due to its metabolism in relation to its energy-supplying (ox-phos) role (Koppers et al., Reference Koppers, De Iuliis, Finnie, McLaughlin and Aitken2008). In DNA repair processes, interaction may occur between the ‘zinc finger’ structure as well as damage from selenium and other elements, whose presence is essential although in trace form. These interactions have anti-carcinogenetic effects when the elements are present in weak concentrations and compromise genetic stability at strong concentration levels by affecting the methylation of DNA (Davis et al., Reference Davis, Uthus and Finley2000; Davis & Uthus, Reference Davis and Uthus2003; Hartwig et al., Reference Hartwig, Blessing, Schwerdtle and Walter2003). In a study that involved 100 patients, Li et al. (Reference Li, Zhong, Jiang, Wang, Zuo and Sha2012) found that the Cu, Mn, and Se concentrations were significantly higher in seminal plasma of an ‘abnormal sperm group’ than ‘the normal sperm group’ (P (Cu) = 0.024, P (Mn) = 0.002, P (Se) = 0.002). According to Hawkes & Turek (Reference Hawkes and Turek2001), selenium at high doses reduces sperm motility significantly through a modification of thyroid hormone metabolism. Finally, the concentration of selenium in serum is not a marker of oxidative stress, except in the case of rheumatoid type diseases (Brack et al., Reference Brack, Brack, Bonnefont-Rousselot, Dreyfus, Chapman and Kontush2012), in which it actually increases.
Co-enzyme Q10 (coQ10)
First, it is important to note that coQ10 deficiency is rare and occurs exceptionally and primarily in the case of recessive autosomal mutations, of diagnosed cancer or treatments with statins (known to induce DNA fragmentation in sperm). CoQ10 deficiencies are also present in case of neurovegetative disorders such as diabetes, muscular and cardiovascular pathologies (Villalba et al., Reference Villalba, Parrado, Santos-Gonzalez and Alcain2010). Generally speaking, coQ10 deficiency would not be expected to be a significant problem in the relatively young population exposed to ART. The second important fact is that commercially available products of coQ10 (ubiquinone) are absorbed poorly by the intestine, primarily due to its very low solubility (Liu & Artmann Reference Liu and Artmann2009; Villalba et al., Reference Villalba, Parrado, Santos-Gonzalez and Alcain2010). Therefore, the bioavailability appears to be low or close to zero. This observation is confirmed by Nadjarzadeh et al. (Reference Nadjarzadeh, Sadeghi, Amirjannati, Vafa, Motevalian, Gohari, Akhondi, Yavari and Shidfar2011) – in a double-blinded randomized study using coQ10 against a placebo there is no improvement in the quality of the spermatozoon. Marginal increases in coQ10 seminal plasma concentration may be observed with long-term (6-month) treatments, but with no improvement in fertility (Mancini & Balercia, Reference Mancini and Balercia2011) In fact, only recently with ubiquinol, a reduced form of coQ10, protection against peroxidation of the membrane lipids has been observed, which may explain a partial improvement of sperm mobility among certain patients. In other patients, this type of prolonged treatment inhibits the production of spermatozoa (reduced to 10% of the original count; T. Amar, personal communication).
Vitamin C
It is well known that vitamin C can have a pro-oxidant role in the presence of divalent cations such as iron and copper (and Se?). The most serious problem is the negative role of excessive amounts in terms of compaction, i.e. the tertiary structure of the spermatozoon nucleus. It is important to note that the quality of the tertiary structure is absolutely essential for the success of the initial cell cycles and early embryonic development, which will be blocked (Rousseaux et al., Reference Rousseaux, Reynoird, Escoffier, Thevenon, Caron and Khochbin2008; Jenkins & Carrell, Reference Jenkins and Carrell2012). In fact, in order for the disulphide bridges to close (Fig. 2), it is necessary to have an initial stage of oxidation, thus the lipids in the membrane of the spermatozoon (I) will be peroxidized. Next, glutathione will be oxidized, converting to oxidized glutathione, which will regenerate the integrity of the spermatozoon membrane (II). Subsequently, the ox-glutathione will be reduced and regenerate reduced glutathione. The process is carried out by oxidation of protamine cysteines, forming disulphide bridges, appropriately ‘bundling up’ DNA (III). In other words, it is a cascade of three oxidation/reduction processes. Vitamin C is harmful at two levels, provided of course that its concentration increases in the serum and then in the seminal plasma. First, it prevents the primary oxidation level of the spermatozoon membrane (I), then later in phase III in which it is capable of opening disulphide bridges in the protamines, owing to its strong redox potential. Similar observations have been demonstrated by Donnelly et al. (Reference Donnelly, McClure and Lewis1999) and Giustarini et al. (Reference Giustarini, Dalle-Donne, Colombo, Milzani and Rossi2008): the capacity of vitamin C to reduce the disulfide bonds of proteins is a general concern. The concentration of vitamin C in blood is not a marker of oxidative stress (de la Villehuchet et al., Reference de la Villehuchet, Brack, Dreyfus, Oussar, Bonnefont-Rousselot, Chapman and Kontush2009; Brack et al., Reference Brack, Brack, Bonnefont-Rousselot, Dreyfus, Chapman and Kontush2012), either in endocrinological or neurovegetative diseases or even in cases of cancer (in 504 patients tested). For smokers, the first treatment with ascorbic acid was proposed by Fraga et al. (Reference Fraga, Motchnik, Shigenaga, Helbock, Jacob and Ames1991), using MDA as a control, however no difference in ascorbic acid concentration was found in the seminal plasma of smokers and non-smokers (Kand’ár et al., Reference Kand’ár, Drábková and Hampl2011).
Superoxide dismutase
SOD treatments lead to the same negative results as the association of selenium with the vitamins C, A and E (Ménézo et al., Reference Ménézo, Hazout, Panteix, Robert, Rollet, Cohen-Bacrie, Chapuis, Clément and Benkhalifa2007b). This type of treatment ‘swells’ the nucleus, undoubtedly in much the same way, in other words, via cystine bonds and opening of protamines and/or the prevention of the formation of cystine bridges that ‘lock up’ DNA.
Vitamins A and E
The association of these two vitamins seems to be effective in terms of the quality of spermatogenesis in vivo (Almbro et al., Reference Almbro, Dowling and Simmons2011). Vitamin A is generally given in the form of α-tocopherol. When it is used alone, it appears active in vivo; taking on an anti-inflammatory role, but it may have a negative effect on fertilization. It is effective in vitro when it is added during manipulation and freezing of spermatozoa. Vitamin E protects membranes. Once again, it is virtually impossible to find a correlation between beta-carotene content in plasma and endocrinological and other pathologies related to oxidative stress (Brack et al., Reference Brack, Brack, Bonnefont-Rousselot, Dreyfus, Chapman and Kontush2012). However, this event is not the case for vitamin E: endocrinological psychiatric pathologies and infectious syndromes may be linked to decreased serum concentration. Generally speaking, only vitamin E, when prescribed ‘haphazardly’, solely based on a spermiogram, without taking into account the quality of the DNA or the structure of the spermatozoon, seems to improve the number and/or the motility of spermatozoa.
Finally, when looking carefully at the scientific literature, treatment with beta-carotene, vitamin A, and vitamin E may increase mortality (Bjelakovic et al., Reference Bjelakovic, Nikolova, Gluud, Simonetti and Gluud2008) and dietary supplementation with vitamin E significantly increases the risk of prostate cancer among healthy men (Klein et al., Reference Klein, Thompson, Tangen, Crowley, Lucia, Goodman, Minasian, Ford, Parnes, Gaziano, Karp, Lieber, Walther, Klotz, Parsons, Chin, Darke, Lippman, Goodman, Meyskens and Baker2011).
III New approaches
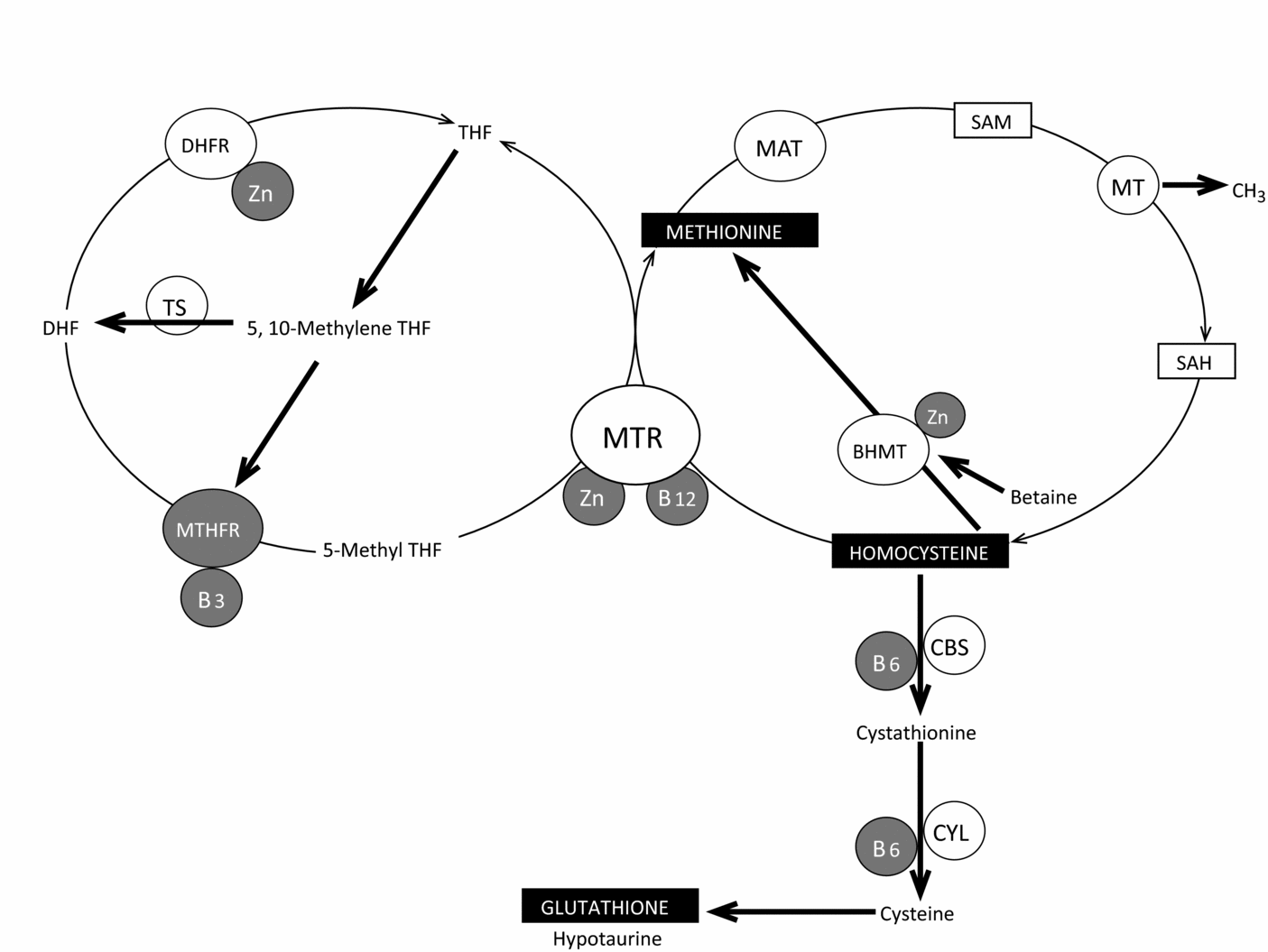
Clearly, it is important to stress that a spermiogram does not give an accurate view of the quality of the nucleus. Abnormal forms, in the most extreme cases, give an indication but correlations between fragmentation of DNA and/or decondensation are not substantiated. DNA fragmentation is related to oxidative stress, whereas non-compaction of nuclear DNA is not directly linked. DNA fragmentation of the sperm related to ROS and overall methylation of DNA are correlated significantly and inversely in infertile men (Tunc & Tremellen, Reference Tunc and Tremellen2009). A low level of folates and damage to DNA are both also strongly correlated (Boxmeer et al., Reference Boxmeer, Smit, Utomo, Romijn, Eijkemans, Lindemans, Laven, Macklon, Steegers and Steegers-Theunissen2009). Vitamin B12 concentration in seminal plasma is correlated strongly with the concentration in spermatozoa (Boxmeer et al., Reference Boxmeer, Smit, Weber, Lindemans, Romijn, Eijkemans, Macklon and Steegers-Theunissen2007). Whereas selenium deficiency has never been described for the Caucasian population, this situation is not the case for Zn – it is thought that 15% of the North American population has a deficiency in zinc (Center for Disease Control and Prevention, 2007). Zinc deficiency increases cancer risks (Ho, Reference Ho2004). Zn acts as a co-factor for at least 200 enzymes and its content in seminal plasma is also linked to sperm quality. Zinc counteracts the effects of iron and copper in the Fenton reaction, which is involved in free radical formation. Through its association with zinc finger proteins, it is involved in the regulation of gene expression and control of apoptosis. All of the Zn-dependant metallothioneins (MTs) are highly expressed in the oocyte (Ménézo et al., Reference Ménézo, Ménézo, Pluntz, Chouteau, Gurgan, Demirol, Dalleac and Benkhalifa2011a) – they are intracellular ligands of zinc and they can also counteract the negative impact of heavy metals such as cadmium. The importance of the input of MT in the support of early embryo development in the human is emphasized by the transcriptomic profile of sperm leading to pregnancy after ICSI (Garcıa-Herrero et al., Reference Garcıa-Herrero, Garrido, Martınez-Conejero, Remohı, Pellicer and Meseguer2011): most of the MT (MT1A, B, F, G, H and X) transcripts are over-expressed in the pregnant group. Finally, Zn is an important co-factor in the folate cycle, which is involved in the recycling of homocysteine to methionine (Fig. 3), both of which have a major influence on methylation reactions through S-adenosyl methionine (SAM). Intake of folate associated with zinc decreases rates of sperm aneuploidies in men (Young et al., Reference Young, Eskenazi, Marchetti, Block and Wyrobek2008).

Figure 3 Zinc, Vitamins of group B and recycling of komocysteine.
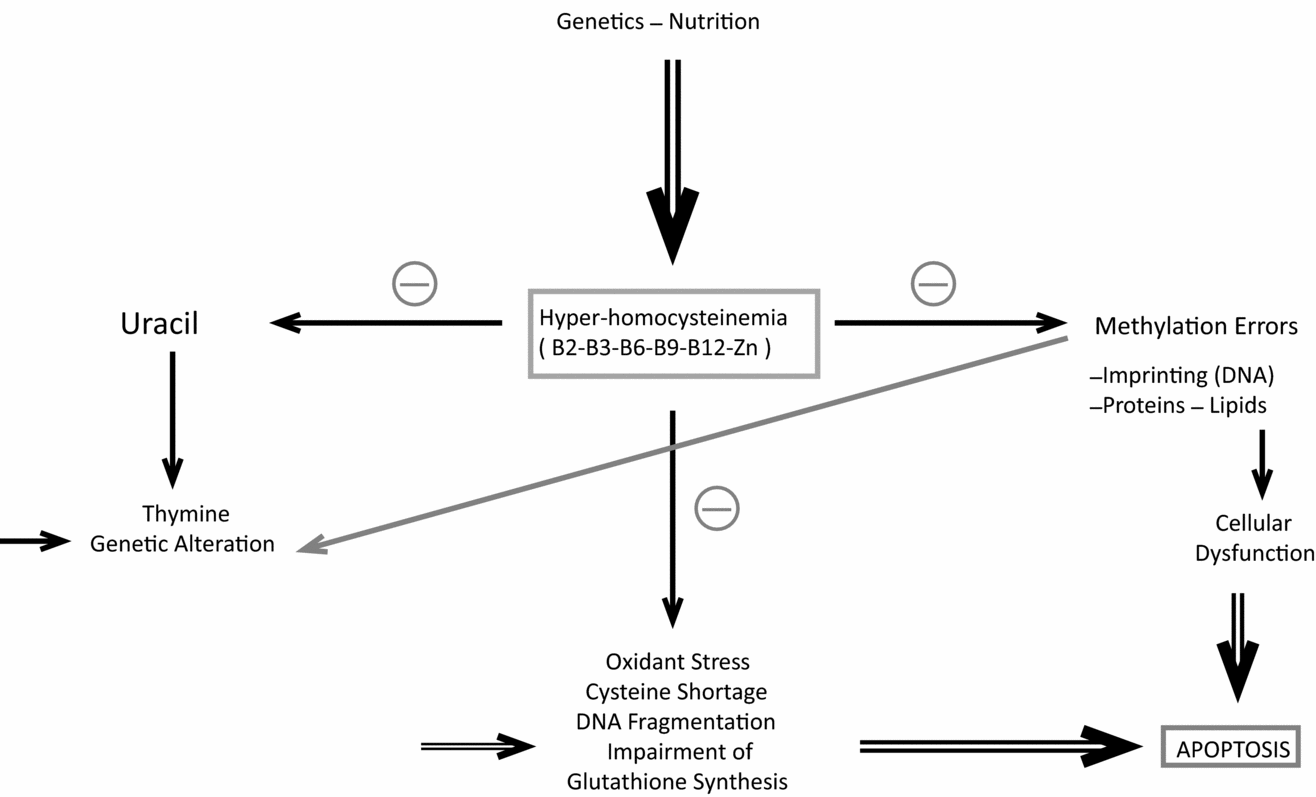
All these observations point to anomalies in homocysteine recycling (see Fig. 4) and promote zinc supplementation. Homocysteine has an extremely negative factor affect on spermatogenesis and also oogenesis (Ebisch et al., Reference Ebisch, Peters, Thomas, Wetzels, Peer and Steegers-Theunissen2006, Reference Ebisch, Thomas, Peters, Braat and Steegers-Theunissen2007; Boxmeer et al., Reference Boxmeer, Smit, Utomo, Romijn, Eijkemans, Lindemans, Laven, Macklon, Steegers and Steegers-Theunissen2009). This compound affects epigenetic regulation (methylation): behaving like dice to regulate general development (Fig. 5). It is now more and more obvious that homocysteine is both a marker and a cause of oxidant stress (Hoffman, Reference Hoffman2011; Ménézo et al., Reference Ménézo, Mares, Cohen, Brack, Viville and Elder2011b)

Figure 4 Homocysteine: epicentre of major cell biochemical problems.
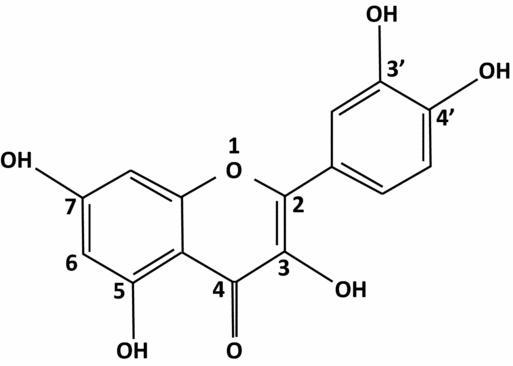
Figure 5 Structure of quercetin.
It is absolutely imperative that all group B vitamins are present, not only folic acid, in any type of fertility treatment (Figs. 3 and 4). In addition, it is important to point out some fundamental elements that are concerned in oxidative stress, spermatogenesis and their relationship with glutathione and homocysteine. Glutathione is acknowledged to protect against damage linked to free radicals. Consequently, an increase in the concentration of reduced glutathione in the environment of the spermatozoon is essential. This observation is not restricted to spermatogenesis as poor content of glutathione and/or an abnormal ratio of reduced glutathione/oxidized glutathione in serum are ubiquitous markers for various pathologies (endocrinological, psychiatric, neurovegetative, rheumatoid; Brack et al., Reference Brack, Brack, Bonnefont-Rousselot, Dreyfus, Chapman and Kontush2012).
These elements are ideal for the treatment of oxidative stress based on stimulating the synthesis of endogenous glutathione and homocysteine recycling rather than using exogenous vitamins with strong reducing capabilities, of which the pharmacokinetics are unknown.
Stimulation of synthesized endogenous glutathione: assisting the germinal cell line
Quercetin (Fig. 5), also known as quercetol, is a flavonoid of the flavonol type. Quercetin passes into the blood circulation and its metabolites have an extensive lifespan. It inhibits tumour necrosis factor (TNF) alpha and interleukin 8 involved in the inflammatory process. Glycosides that are found in certain fruits in their natural state are hydrolyzed by enzymes in the small intestine and are then absorbed. Metabolite glucuronide, sulphate and methyl, formed in the blood are slightly less active than the natural product, but have a prolonged lifespan (>10 h). It is known that quercetin has a positive impact on the germinal line, and more particularly on spermatozoa, observed in the late 1980s (Hammerstedt et al., Reference Hammerstedt, Volonté and Racker1988). It protects cells in spermatogenesis from pollutants (hydrocarbons, estrogenic hormones; Izawa et al., Reference Izawa, Kohara, Aizawa, Suganuma, Inakuma, Watanabe, Taya and Sagai2008; Li et al., Reference Li, Zhong, Jiang, Wang, Zuo and Sha2012). Moreover, when quercetin passes into the blood, it regulates and optimizes the response to oxidative stress in heavy smokers (Bøhn et al., Reference Bøhn, Myhrstad, Thoresen, Holden, Karlsen, Tunheim, Erlund, Svendsen, Seljeflot, Moskaug, Duttaroy, Laake, Arnesen, Tonstad, Collins, Drevon and Blomhoff2010). More mechanisms of action are being recognized. Foods that are rich in quercetin increase the synthesis of 15 ARN messengers that are involved in DNA repair mechanisms and four ARN messengers that are concerned with apoptosis (Bøhn et al., Reference Bøhn, Myhrstad, Thoresen, Holden, Karlsen, Tunheim, Erlund, Svendsen, Seljeflot, Moskaug, Duttaroy, Laake, Arnesen, Tonstad, Collins, Drevon and Blomhoff2010). It also regulates gamma-glutamylcysteine synthetase and glutathione synthetase expression, interacting directly on the promoters – the stage that limits synthesis of endogenous glutathione. It especially increases in the muscle (Moskaug et al., Reference Moskaug, Carlsen, Myhrstad and Blomhoff2005). Quercetin increases the endogenous natural defenses.
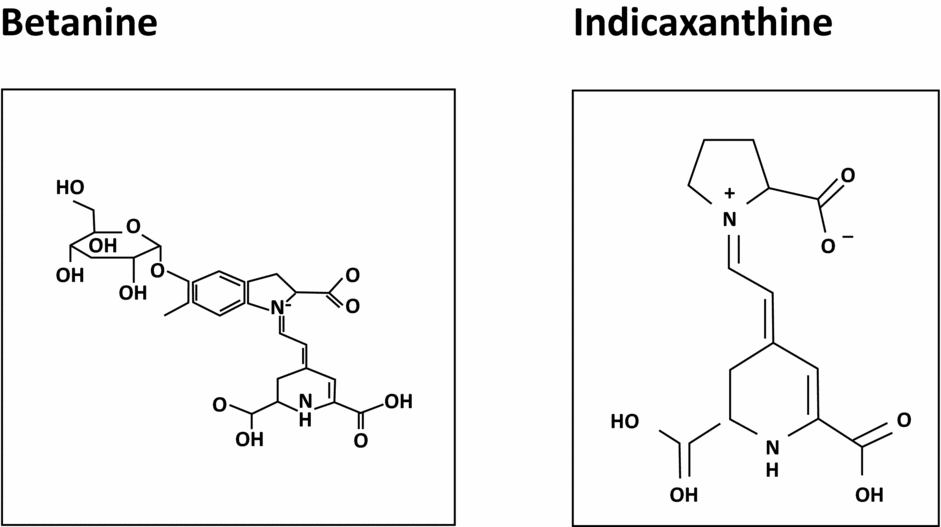
Betalains (Fig. 6)
We have seen that strong anti-oxidants have a rather negative and often random impact on spermatogenesis. However, it is also true that peroxidation of spermatozoa membrane lipids has a negative impact on mobility, especially when they are developing (Montjean et al., Reference Montjean, Ménézo, Benkhalifa, Cohen, Belloc, Cohen-Bacrie and de Mouzon2010). Betalains may be an appropriate answer to this problem through their protective effect on membrane lipids. Owing to their structure, they connect with membrane lipids and oxidize for them, then disengage. Because of their ability to carry out this task, they slow anarchistic cellular division and promote programmed cellular death of certain strains of cancerous cells in vitro (Sreekanth et al., Reference Sreekanth, Arunasree, Roy, Chandramohan Reddy, Reddy and Reddanna2007). In a sperm analysis, they significantly improve the mobility of the spermatozoa (Benkhalifa et al., Reference Benkhalifa, Cohen, Tosti, Cohen-Bacrie, Ballashova and Ménézo2008), which would support their protective effect on spermatozoon membrane lipids.

Figure 6 Chemical structure of betalains.
Betalains and quercetine are compounds of vegetable origin: one of the best sources being the prickly pear (Opuntia ficus-indica).
IV Treatments
As indicated, studies on DNA structure in the nucleus of the spermatozoon highlight two types of damage, which require different types of treatment.
DNA fragmentation
Firstly, it is the current consensus that 30% is an upper tolerable level, whatever the technique applied. Beyond this limit, problems of fertility appear, insemination becomes more uncertain than in in vitro fertilization and ICSI (Evenson et al., Reference Evenson, Larson and Jost2002; Frydman et al., Reference Frydman, Prisant, Hesters, Frydman, Tachdjian, Cohen-Bacrie and Fanchin2008; Bungum et al., Reference Bungum, Bungum and Giwercman2011). Beyond 50%, the chances of conceiving are virtually inexistent. Our experience with Procrelia Men® (Nurilia France), a combination of Opuntia extract (quercetin and betalains, group B vitamins and zinc) gave the best results, with a treatment duration of a minimum of 4 months, thus covering a full spermatogenetic cycle. Initial results show a drop in fragmentation (Benkhalifa et al., Reference Benkhalifa, Cohen, Tosti, Cohen-Bacrie, Ballashova and Ménézo2008). When fragmentation is >50%, it may be useful to treat initially with a strong antioxidant mixture (Fertibiol®), which should not be administered for more than 1 month). This event is then followed by Condensyl®, treatment over the next 2 months, re-sustaining the capacity for a correct packaging of the nucleus. Our own experience with prolonged treatment with strong anti-oxidants (Astaxanthin, coQ10 in ubiquinol form), in the case of very high levels of fragmentation, showed a strong reduction in the production of spermatozoa (down to 5% of the initial value).
Absence of nuclear condensation
This parameter may be measured by SCSA or, more simply, by permeation with aniline blue. Here the current consensus is that above 25–30%, arrests embryonic development. The observations in ICSI of no fertilization even when it is clear that a spermatozoon has been correctly injected almost always correlate with this nuclear defect. For this type of problem, Condensyl®, which primarily contains group B vitamins and zinc is especially effective for this ‘non-fertile’ group of patients (Junca et al., Reference Junca, Gonzalez Marti, Tosti, Cohen, De la Fontaine, Benkhalifa and Ménézo2012), and notably in couples, in who repeated ICSI and intracytoplasmic morphologically selected sperm injection (IMSI) were unsuccessful. In a short experiment with 27 patients, a 32% increase in condensation was observed (p < 0.0002) in association with eight pregnancies, of which five were spontaneous and three were following an ART attempt (Ménézo et al., in press). Malone dialdehyde formation, a ‘marker of oxidative stress’, is correlated strongly with sperm nucleus decondensation/immaturity (Montjean et al., Reference Montjean, Ménézo, Benkhalifa, Cohen, Belloc, Cohen-Bacrie and de Mouzon2010) and could be treated with this approach.
V Solutions for women: a further increase in oocyte competence?
Access to oocyte DNA is obviously invasive. Nevertheless a certain number of indirect observations may be considered.
Recycling homocysteine i.e. the one-carbon cycle
Many studies have shown that ovarian stimulation increases homocysteine concentration in follicular liquid (Ebisch et al., Reference Ebisch, Peters, Thomas, Wetzels, Peer and Steegers-Theunissen2006, Reference Ebisch, Thomas, Peters, Braat and Steegers-Theunissen2007; Berker et al., Reference Berker, Kaya, Aytac and Satiroglu2009). Hcy passes into the oocyte and impedes methylation while competing with methionine (Ménézo et al., Reference Ménézo, Khatchadourian, Gharib, Hamidi, Greenland and Sarda1989). As a result, there is a major risk of creating a breeding ground for imprint type disorders, among which, epigenetic anomalies (Ménézo et al., Reference Ménézo, Mares, Cohen, Brack, Viville and Elder2011b). Therefore compounds such as Zn and the entire group of B vitamins that promote homocysteine recycling, are most welcome (either in vitro or in vivo during early embryo culture). In the animal kingdom, it is important to remember that optimization of processes of methylation supports genetic stability (Cooney et al., Reference Cooney, Dave and Wolff2002) by promoting prolificacy and the quality of pups obtained. Up to J3 post conception, the human oocyte and the very young embryo are not fully equipped to recycle homocysteine, the cystathionine beta synthase (CBS) pathway is absent (Benkhalifa et al., Reference Benkhalifa, Montjean, Cohen-Bacrie and Ménézo2010) and the betaine-homocysteine methyl transferase (BHMT) pathway is expressed weekly. This situation also limits the endogenous synthesis of glutathione, which implies that, in addition to folic acid, vitamins B2, B6 and B12 can be limiting. Medically assisted procreation clearly demonstrates the problem of methylation (Katari et al., Reference Katari, Turan, Bibikova, Erinle, Chalian, Foster, Gaughan, Coutifaris and Sapienza2009; Ménézo et al., Reference Ménézo, Elder, Benkhalifa and Dale2010b). Finally, with regard to the quality of DNA, we quote Lopes’ and Zenzes’ findings (Lopes et al., Reference Lopes, Jurisicova and Casper1998; Zenzes et al., Reference Zenzes, Puy and Bielecki1998; Zenzes, Reference Zenzes2000) that damage to DNA may affect the oocyte which is not really protected in ovary, during quiescence and in the growing follicle. In smokers, tar creates the formation of adducts with DNA, detected in ovarian cells and the preimplantation embryo. Therefore, we recommend the same type of preventive treatment (e.g. Procrelia® women) that contains polyphenols, quercetin, betalains, group B vitamins and zinc, at the outset of ART.
Conclusions
In terms of oxidative stress, it is no longer possible to group every aspect, e.g. beauty, anti-ageing and hypofertility of the couple in the same category. Moreover, whatever the problem addressed, it seems that the answers are extremely unscientific. Thus, for example for selenium deficiency there is no literature available. Taken in large doses Se is proapoptotic (initiating programmed cell death) or can even be carcinogenic. The same concern exists with the Q10 Co-enzyme: deficiencies only exist in cases of serious pathologies. Vitamin C, in large doses and for prolonged treatment, has unclear effects especially when it is used to improve spermatogenesis. On the contrary, all the elements that contribute in improving the effectiveness of methylation systems, therefore improving the quality of the one-carbon cycle, are essential to improve gametes and procreation and indeed other pathologies (Hoffman, Reference Hoffman2011). Furthermore, while selenium deficiencies are negligible or inexistent, approximately 15% of the North American population have a zinc deficiency (King et al., Reference King, Shames and Woodhouse2000), with increased risks of cancer. Zinc is also an effector in the stability of the genome, which is fundamental during embryonic development, in addition to playing an essential role in homocysteine recycling. Zinc transport systems are present in the oocyte; the metallothioneins and Zn superoxide dismutase give effective protection against damage to DNA. Zinc has a pivoting role in all the processes of reproduction (as in development and lactation). In much the same way, folic acid is recommended as a supplement for the early stages of pregnancy: but why vitamin B9 only? Figure 3 clearly indicates that B2 and B3 are important players in the folic acid cycle. Vitamin B12 is an absolute necessity for the formation of methionine. B6 also takes part in the folic acid cycle and the formation of cysteine and, ‘downstream’, the formation of glutathione. Finally, it is important to take into account that there are two major types of damage to sperm DNA that require distinct treatments of differing duration. In order to recover from defects in DNA compaction, several cycles of spermatogenesis may be needed. Treating problems in spermatogenesis requires a rigid scientific approach not general assumptions and haphazard therapies, as has often been applied todate.