Management Implications
Foliar sprays of triclopyr amine or ester formulations are widely used for selective control, but the new triclopyr choline and acid formulations have not been evaluated. Previous work reported the effectiveness of imazapic, but the generally more selective imidazolinone, imazamox, has not been tested. In field studies conducted at three locations in Florida, effective Ardisia crenata (hen’s eyes) control was demonstrated with multiple herbicide options, which included a triclopyr acid formulation and imazamox. Repeated annual treatments were necessary to control new recruitment from the seedbank and the established multilayered canopy of A. crenata infestations. There were differences among triclopyr formulations. The acid and ester formulations provided better control compared with the amine and choline formulations. Imazamox controlled A. crenata with repeated annual application, but flumioxazin did not. These results identify triclopyr acid (4.04 kg ae ha−1) and imazamox (2.24 kg ae ha−1) as new treatment options for managing highly invasive A. crenata in the southeastern U.S. Coastal Plain.
Introduction
Hen’s eyes (Ardisia crenata Sims), also known as coral ardisia, was introduced into Florida as an ornamental shrub in the early 1900s (Dozier Reference Dozier1999; Hutchinson et al. Reference Hutchinson, Langeland and Meisenburg2011; Niu et al. Reference Niu, Long, Wang, Shen, Ye, Mu, Cao, Wang and Bradshaw2012; Sellers et al. Reference Sellers, Langeland, Ferrell, Meisenburg and Walter2007). There are more than 500 species within the genus Ardisia that are native to tropical and subtropical regions of eastern Asia, where some are ornamental or used for food and medicine (Hutchinson et al. Reference Hutchinson, Langeland and Meisenburg2011; Kobayashi and de Mejía Reference Kobayashi and de Mejía2005). In 1982, A. crenata was recognized as escaped from cultivation into native habitats in Florida (Langeland et al. Reference Langeland, Cherry, McCormick and Craddock Burks2008; Wunderlin Reference Wunderlin1982) and is now listed as a Category 1 invasive exotic plant by the Florida Exotic Pest Plant Council (2019) and a noxious weed by the Florida Department of Agriculture and Consumer Services (2016). Ardisia crenata occurs throughout the Coastal Plain region of the southeastern United States (EDDMapS 2019; Niu et al. Reference Niu, Long, Wang, Shen, Ye, Mu, Cao, Wang and Bradshaw2012; Wunderlin and Hansen Reference Wunderlin and Hansen2019).
Ardisia crenata grows primarily in moist areas, such as hardwood hammocks and mixed pine–hardwood forests. This evergreen shrub is shade tolerant, grows to 1.8 m (6 ft) in height, and can grow in multistem clumps (Langeland et al. Reference Langeland, Cherry, McCormick and Craddock Burks2008; Sellers et al. Reference Sellers, Langeland, Ferrell, Meisenburg and Walter2007). Leaves can be up to 21-cm long, are alternate, waxy, and dark green on top. Fruit of A. crenata is a bright-red, one-seeded drupe up to 8 mm in diameter. Copious quantities of fruits can be produced within 2 yr from germination. Seeds from A. crenata can germinate within 40 d in acidic or alkaline soils (pH 4 to 10), with germination rates between 84% and 98% (Langeland et al. Reference Langeland, Cherry, McCormick and Craddock Burks2008). This invasive shrub can dominate the forest understory, displacing native plant communities (Ewe et al. Reference Ewe, Overholt, Kirton, Lai, Ahmad and Ulaganathan2006; Langeland et al. Reference Langeland, Cherry, McCormick and Craddock Burks2008).
Hutchinson et al. (Reference Hutchinson, Langeland and Meisenburg2011) tested the performance of 10 herbicide treatments on A. crenata. They determined triclopyr to be effective when applied as either the amine or ester formulation. These herbicides have since become the primary recommended herbicides to control this plant (Miller et al. Reference Miller, Manning and Enloe2013). Recently, two new formulations of triclopyr have been registered for use in the United States. These include a choline salt formulation (Vastlan®, Dow AgroSciences, Indianapolis, IN 46268) and an acid formulation (Trycera®, Helena Agri-Enterprises, Collierville, TN 38017). These two formulations warrant testing, as both convey potential advantages over the amine and ester formulations. The choline formulation is a 0.48 kg ae L−1 (4 lb ae gal−1) formulation and has reduced risk for eye injury compared with the amine. Reduced product volume needed and increased applicator safety would both be substantial benefits. The triclopyr acid formulation is a lower concentration formulation (0.34 kg ae L−1 [2.87 lb ae gal−1]) and is labeled for aquatic use. This may confer an advantage over the ester formulation in seasonal wetlands where A. crenata is abundant and the use of the ester formulation is limited. Additionally, both choline and acid formulations have reduced potential for volatility compared with the ester formulation.
Despite these potential advantages, few published studies have compared these triclopyr formulations for invasive plant control. Langston et al. (Reference Langston, Peterson, Burch, Flynn, Cummings, Halvstedt, Nelson and Brinkworth2015) reported no differences between the choline and amine formulations for control of several hardwood species, including sweetgum (Liquidambar styraciflua L.), white oak (Quercus alba L.), southern red oak (Quercus falcata Michx.), black cherry (Prunus serotina Ehrh.), and water oak (Quercus nigra L.). Dias et al. (Reference Dias, Banu, Sperry, Enloe, Ferrell and Sellers2017) tested the four formulations in greenhouse dose–response studies and determined differences in their performance on four broadleaf crops. Such formulation differences in a controlled greenhouse study with highly sensitive crops suggest additional studies in natural environments are warranted, especially on difficult to control species.
Imazamox, which will control invasive waxy-leaved species such as Chinese tallowtree [Triadica sebifera (L.) Small] (Enloe et al. Reference Enloe, Loewenstein, Streett and Lauer2015) and wild taro [Colocasia esculenta (L.) Schott], has not been tested for efficacy on A. crenata. Imazapic, another imidazolinone herbicide, was effective for control of A. crenata, but damaged adjacent native vegetation (Hutchinson et al. Reference Hutchinson, Langeland and Meisenburg2011). The greater selectivity of imazamox compared with imazapic would be of considerable interest due to greater non-target vegetation safety. A similar rationale can be made for flumioxazin, which is widely used for selective weed control in aquatic and non-crop environments and provides both foliar and soil activity.
The objectives of this study were to compare the efficacy of the four triclopyr formulations, imazamox, and flumioxazin for control of A. crenata and examine repeated annual applications of each herbicide to control regrowth and subsequent seedling recruitment.
Materials and Methods
Study Sites
The study was conducted at three forested sites across north and central Florida that contained abundant populations of A. crenata in the understory (Figure 1). Study sites were at Lake Griffin State Park in Fruitland Park (28°51′01.9″N, 81°53′33.6″W), San Felasco Hammock Preserve State Park in Alachua (29°42′51.42″N, 82°27′39.16″W), and the North Florida Research and Education Center (NFREC) in Quincy (30°32′44.00″N, 84°35′40.68″W). The Lake Griffin State Park site was established on the edge of a forested basin swamp with a dense shrub cover of A. crenata. Common overstory species included pond cypress (Taxodium ascendens Brongn.), blackgum (Nyssa sylvatica Marshall), red maple (Acer rubrum L.), laurel oak (Quercus laurifolia Michx.), and L. styraciflua. The site is characterized by extended inundation much of the year, but low water conditions for several years have allowed A. crenata to dominate the shrub layer. The San Felasco Hammock site was in an upland hardwood forest with an overstory of pignut hickory [Carya glabra (Mill.) Sweet], southern magnolia (Magnolia grandiflora L.), oak (Quercus spp.), and southern sugar maple [Acer floridanum (Chapm.) Pax]. Ardisia crenata cover was patchy, with few other shrubs present. The NFREC site was established in a mixed pine–hardwood forest, predominantly loblolly pine (Pinus taeda L.) and Q. nigra in the overstory with patches of established A. crenata in the understory.
Figure 1. Ardisia crenata study site locations.
Experimental Design
Eight herbicide treatments and a nontreated check were tested in separate, uniform studies at the three locations using a completely randomized deign (Table 1). The Lake Griffin and Quincy study sites included thirty-six 4.6 by 4.6 m (15 by 15 ft) plots with treatments assigned to four replications, whereas the San Felasco site included twenty-seven 3.1 by 9.1 m (10 by 30 ft) treatment plots with three replications. The plot layout was different at the San Felasco site to account for differences in the density and size of the A. crenata infestation. Before the first herbicide application (0 d after the first treatment = 0DAT1), percent cover of established A. crenata (greater than 8-cm high) was visually estimated within a 3.1 by 3.1 m (10 by 10 ft) measurement plot in the center of each treatment plot at the Lake Griffin and Quincy sites, whereas the entire plot was used at San Felasco to sample established A. crenata infestation. Seedling A. crenata (less than 8-cm high) percent cover was sampled within two permanent 1 by 1 m (3.3 by 3.3 ft) quadrats within each measurement plot across all sites. The categorization of A. crenata plants into established or seedling and an 8-cm-height cutoff between the categories followed the protocol of Hutchinson et al. (Reference Hutchinson, Langeland and Meisenburg2011) for consistency. Two observers independently estimated percent cover for established and seedling A. crenata, and average cover was calculated for each category.
Table 1. Eight herbicide treatments applied in February 2016 and 2017 were compared with a nontreated check at three study sites for effectiveness in controlling Ardisia crenata.

a A nonionic surfactant at was added 0.5% v/v.
b A methylated seed oil was added at 1% v/v
The first herbicide treatments at all sites were applied in February 2016; the dormant season timing was chosen to reduce impact to non-target vegetation. Herbicides were applied using a CO2-pressurized backpack sprayer with a single adjustable cone nozzle at a pressure of 276 kPa (40 PSI) to attain a uniform 374 L ha−1 (40 gal ac−1) application volume. The spray solution was measured for each plot individually to ensure the target application volume. A nonionic surfactant at 0.5% v/v was included with all herbicide treatments except imazamox. Methylated seed oil at 1% v/v was included with each imazamox treatment as recommended by the manufacturer. The second applications were made at all study sites in February 2017 using the same treatments, rates, and application methods.
Percent cover was assessed at 12 mo after the first herbicide treatment (12MAT1) and 12 mo after the second treatment (12MAT2) using the same techniques as at 0DAT1. Herbicide effectiveness was quantified by determining the percent control of A. crenata between each posttreatment cover assessment and the pretreatment cover assessment (0DAT1). Percent control at 12MAT1 and 12MAT2 was calculated using Equations 1 and 2, respectively:
 $$12{\rm{MAT}}1\;{\rm{percent}}\;{\rm{control}} = {\rm{ }}{{12{\rm{MAT}}1\;{\rm{percent}}\;{\rm{cover}} - 0{\rm{DAT}}1\;{\rm{percent}}\;{\rm{cover}}} \over {0{\rm{DAT}}1\;{\rm{percent}}\;{\rm{cover}}}} \times 100$$
$$12{\rm{MAT}}1\;{\rm{percent}}\;{\rm{control}} = {\rm{ }}{{12{\rm{MAT}}1\;{\rm{percent}}\;{\rm{cover}} - 0{\rm{DAT}}1\;{\rm{percent}}\;{\rm{cover}}} \over {0{\rm{DAT}}1\;{\rm{percent}}\;{\rm{cover}}}} \times 100$$
 $$12{\rm{MAT}}2\;{\rm{percent}}\;{\rm{control}}{\mkern 1mu} = {{12{\rm{MAT}}2\;{\rm{percent}}\;{\rm{cover}} - 0{\rm{DAT}}1\;{\rm{percent}}\;{\rm{cover}}} \over {0{\rm{DAT}}1\;{\rm{percent}}\;{\rm{cover}}}} \times 100$$
$$12{\rm{MAT}}2\;{\rm{percent}}\;{\rm{control}}{\mkern 1mu} = {{12{\rm{MAT}}2\;{\rm{percent}}\;{\rm{cover}} - 0{\rm{DAT}}1\;{\rm{percent}}\;{\rm{cover}}} \over {0{\rm{DAT}}1\;{\rm{percent}}\;{\rm{cover}}}} \times 100$$
Statistical analyses were conducted using SAS-JMP (SAS Institute 2016). Data did not meet normality assumptions, so they were natural log transformed. ANOVA to examine the effects of site, herbicide, and their interaction and multiple comparisons using Fisher’s protected LSD were conducted at P ≤ 0.05.
Results and Discussion
Percent Control of Established Ardisia crenata
There was a difference in pretreatment established A. crenata cover (0DAT1) among sites (P < 0.01), but not among treatment plots at each site (P = 0.65). Average percent cover at 0DAT1 was higher at Lake Griffin (76%) than at Quincy or San Felasco (57% and 49%, respectively) (Table 2). These differences may be indicative of invasion stage or abiotic differences between sites. The same herbicide treatments were applied at all sites irrespective of A. crenata infestations.
Table 2. Mean percent cover of established (>8-cm high) Ardisia crenata at 0 d after treatment 1 (0DAT1), 12 mo after treatment 1 (12MAT1), and 12 mo after treatment 2 (12MAT2).

At 12MAT1, there were no differences among sites (P = 0.32) in terms of average control of established A. crenata. However, herbicide treatment and the interaction of site and herbicide treatment had an effect on percent control of A. crenata at this assessment (both P < 0.01). The interaction indicated that herbicide treatment effects were different among sites for the high rate of imazamox (P = 0.01), triclopyr amine plus flumoxazin (P < 0.01), and nontreated check treatments (P = 0.04) (Table 3). The high rate of imazamox was most effective at the Quincy site (83% control) when compared with the San Felasco and Lake Griffin sites (66% and 50% control, respectively). Triclopyr amine plus flumioxazin did not control A. crenata at the Quincy site (−30% control) and was not different from the San Felasco site (17% control). However, at the Lake Griffin site, the same treatment reduced A. crenata cover by 50%. Increased A. crenata cover (as indicated by negative percent control values) was observed in the nontreated check at all three sites; however, at the Quincy site the increase (−49% percent control) was greater than at the Lake Griffin and San Felasco sites (−16% and −8% control, respectively).
Table 3. Mean percent control of established (>8-cm high) Ardisia crenata at 12 mo after treatment 1 (12MAT1) and 12 mo after treatment 2 (12MAT2). a
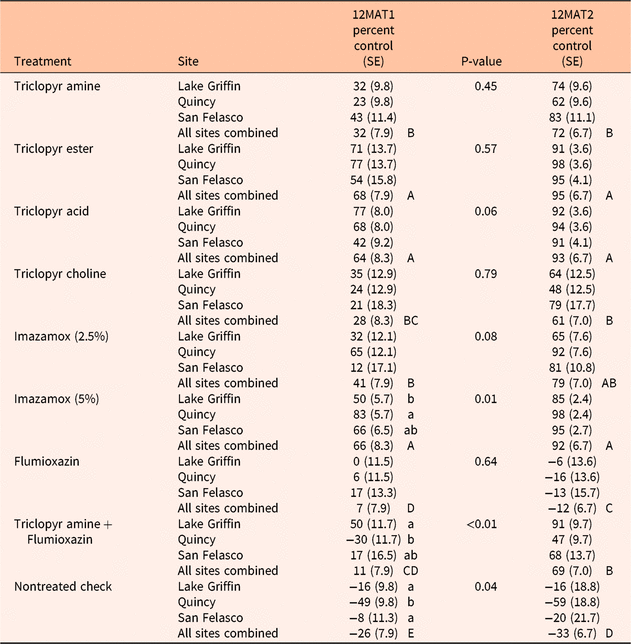
a At each assessment, treatment means across all three sites followed by the same capital letter are not different at P ≤ 0.05 using Fisher’s LSD. For the 12MAT1 evaluation, site means within a treatment followed by the same lowercase letter are not different at P ≤ 0.05 using Fisher’s LSD. There was an interaction (P < 0.01) between site and treatment only at 12MAT1.
Although there was an interaction between site and herbicide treatment for the 12MAT1 data, additional examination of the herbicide treatment main effect elucidated additional important information. Control of A. crenata occurred across all herbicide treatments at 12MAT1 when compared with the nontreated check (Table 3). Triclopyr ester, 5% imazamox, and triclopyr acid provided greater percent control than any other herbicide treatment (68%, 66%, and 64% control, respectively). Flumioxazin and triclopyr amine plus flumioxazin were the least effective in controlling A. crenata at 1 yr after application (7% and 11% control, respectively). Control levels achieved at 1 yr after a single application were not acceptable with any treatment, warranting retreatment.
At 12MAT2, only herbicide treatment had an effect on A. crenata percent control (P < 0.01). The lack of site effect (P = 0.10) and the site by herbicide treatment interaction (P = 0.11) indicate that herbicide effectiveness in controlling established A. crenata was similar across all sites at 1 yr after the second application. All herbicide treatments except flumioxazin provided control of A. crenata compared with the nontreated check (Table 3). Ardisia crenata percent cover increased in the nontreated check and flumioxazin treatments by 33% and 12%, respectively. Triclopyr ester, triclopyr acid, and 5% imazamox resulted in greater control of A. crenata (95%, 93%, and 92%, respectively) than any other treatments except the low rate of imazamox (79% control). Triclopyr choline (61%), triclopyr amine (72%), and triclopyr amine + flumioxazin tank mix (69%) all controlled A. crenata to a lesser extent and were not different in their performance.
Percent Control of Seedling Ardisia crenata
Average percent cover of seedling A. crenata before the first treatment (0DAT1) was not different among sites (P = 0.64) and ranged from 19% to 33% (Table 4). At 12MAT1, site and herbicide treatment were significant factors (P < 0.01 and P = 0.05, respectively), but there was no site and herbicide treatment interaction (P = 0.07). Control of seedling A. crenata was greater at Lake Griffin (81%) when compared with San Felasco or Quincy (33% and 47% control, respectively).
Table 4. Mean percent cover of seedling (<8-cm high) Ardisia crenata at 0 d after treatment 1 (0DAT), 12 mo after treatment 1 (12MAT1), and 12 mo after treatment 2 (12MAT2).

Across all sites, all herbicide treatments and the nontreated check reduced cover of seedling A. crenata, with triclopyr choline, resulting in the lowest percent control (32%) and triclopyr ester the greatest percent control (73%) (Table 5). We hypothesize that the reduced seedling percent cover in the nontreated check was due to seedling growth into the established category (established A. crenata increased by 44%). Seedling growth into the established category may also have impacted seedling cover in the other treatments.
Table 5. Mean percent control of seedling (<8-cm high) Ardisia crenata at 12 mo after treatment 1 (12MAT1) and 12 mo after treatment 2 (12MAT2). a
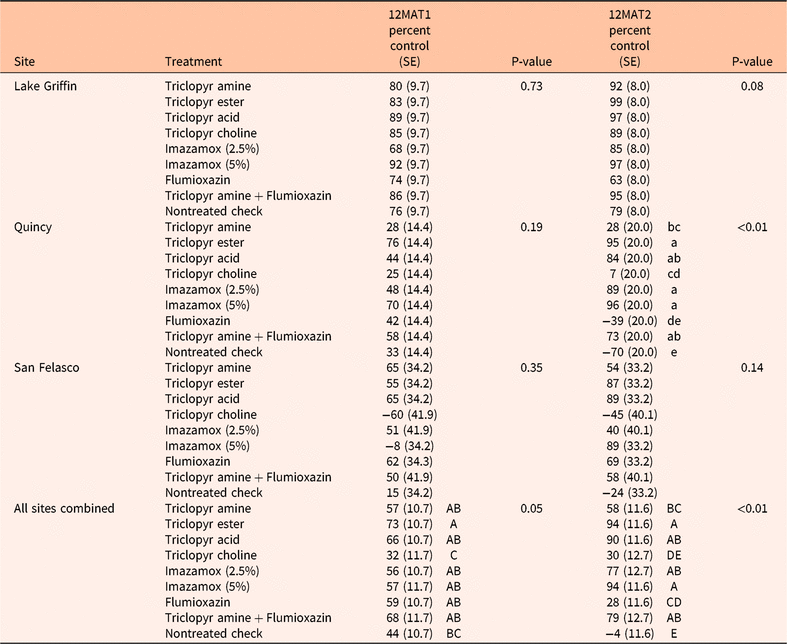
a Treatments differed only at the Quincy site at 12MAT2 (P < 0.01), but when means from all three sites were combined, treatments differed at both 12MAT1 (P = 0.05) and 12MAT2 (P < 0.01). For each assessment, treatment means for the Quincy site followed by the same lowercase letter and all sites combined (12MAT1 and 12MAT2) followed by the same capital letter are not different at P ≤ 0.05 using Fisher’s LSD.
The 12MAT2 results indicated an effect of site (P < 0.01), treatment (P < 0.01), and site and herbicide interaction (P < 0.01). The interaction indicated that herbicide treatment effects were different among sites for the high rate of imazamox (P < 0.01), flumioxazin (P = 0.02), and nontreated check treatments (P = 0.04). At the Lake Griffin and Quincy sites, the high rate of imazamox gave 97% and 96% control, respectively, whereas at the San Felasco site, only 89% control was obtained. At Quincy, negative percent control (−39%) was observed with flumioxazin; however, 63% and 69% control occurred at Lake Griffin and San Felasco, respectively. There was an increase in percent cover of seedling A. crenata in the nontreated check at the Quincy and San Felasco sites (−70% and −24% control, respectively); whereas 79% control was obtained at the Lake Griffith site. The Lake Griffin site is subject to flooding, which could have influenced the greater percent cover reduction. Annual rainfall data retrieved from a nearby National Oceanic and Atmospheric Administration weather station 9 km from Lake Griffin indicated above average rainfall (145 cm) in 2017 with 3 mo (June, August, September) of greater than 26.7 cm rainfall (NOAA 2019). Ardisia crenata is reported to be susceptible to root rot when growing in inundated conditions (Langeland et al. Reference Langeland, Cherry, McCormick and Craddock Burks2008), and the combination of herbicide treatment with flooded conditions likely enhanced control.
Similar to the 12MAT1 assessment, at 12MAT2 percent control of seedling A. crenata was greater at Lake Griffin across all treatments (88% control) compared with San Felasco or Quincy (46% and 40% control, respectively), which again could be the result of the impact from flooding. Across sites, all herbicide treatments except triclopyr choline resulted in greater percent control of seedling A. crenata compared with the check (Table 5). The triclopyr choline treatment effect at 12MAT2 was similar to the effect at 12MAT1. The most effective treatments at 12MAT2 for controlling seedling A. crenata were the high rate of imazamox, triclopyr ester, triclopyr acid, triclopyr amine plus flumioxazin, and the low rate of imazamox (94%, 94%, 90%, 79%, and 77% control, respectively). A treatment effect (P < 0.01) was observed at 12MAT2 at the Quincy site (Table 5), with negative percent control in the nontreated check and flumioxazin treatment.
At the 12MAT1 assessment, treatments had an effect on percent control of established A. crenata cover, but remaining cover of both established plants and seedlings warranted a second herbicide application. Higher herbicide application rates, especially for triclopyr, should be investigated to determine their effectiveness in a single application. Ardisia crenata seeds present in the seedbank and the few established plants and seedlings present at 12MAT2 would warrant a longer repeated-application study to evaluate control of emerging seedlings. A 3% triclopyr amine solution (product containing 0.36 kg L−l) is generally recommended over the ester formulation for A. crenata control in Florida natural areas. This is because amine has lower volatility and greater tolerances by native plants (Hutchinson et al. Reference Hutchinson, Langeland and Meisenburg2011). This study compared four triclopyr formulations applied at the same 4.04 kg ha−1 rate, which was set by the standard 3% triclopyr amine treatment when applied at 374 L ha−1 (40 gal ac−1). The triclopyr ester and choline formulations contain 25% more triclopyr than the amine and were applied at 2.25%, whereas the acid formulation was applied at 3.14%. Recommendations by other authors have included greater concentrations of foliar herbicides but have not specified application volumes or target rates of herbicides per hectare. Miller et al. (Reference Miller, Manning and Enloe2013) recommends a foliar application of glyphosate or triclopyr ester at a 5% product solution (both products having 0.48 kg L−l) with a surfactant, whereas Sellers et al. (Reference Sellers, Langeland, Ferrell, Meisenburg and Walter2007) recommends a 4% triclopyr amine or 3% triclopyr ester foliar application.
The results of this study differ from those reported by Hutchinson et al. (Reference Hutchinson, Langeland and Meisenburg2011), who determined that both triclopyr amine and ester formulations at an identical herbicide rate of 5.43 kg ae ha−1 (10.8 g ae L−1) resulted in excellent control of A. crenata at 12MAT. The primary difference between our study and Hutchinson et al. (Reference Hutchinson, Langeland and Meisenburg2011) was the application volume, which was much higher in the Hutchinson et al. (Reference Hutchinson, Langeland and Meisenburg2011) study (503 L ha−1) than in our study (374 L ha−1). This issue of herbicide application volume in relation to concentration should be examined further. Low-volume backpack foliar (LVBF) herbicide applications tend to use more concentrated solutions that are applied sparingly. LVBF applications require less water to be carried by applicators and increase the land area that can be treated by a tank. This approach is in contrast to the present, in which 374 L ha−1 of spray solution were applied, nearly twice what is recommended using LVBF. However, typical A. crenata infestations are multilayered, with well-established shrubs that have a dense understory of seedlings and juveniles. Successful coverage of all canopy layers is difficult with low application volumes, and this may explain the better control in the Hutchinson et al. (Reference Hutchinson, Langeland and Meisenburg2011) study compared with the present study.
The present study also points to the need for a better understanding of the relationships among herbicide rate, application volume, and plant coverage needs. Surfactants should also be examined further, in particular the use of methylated seed oil versus conventional polyethoxylate surfactant materials to improve herbicide uptake through the waxy leaf cuticle of A. crenata. Seed oil emulsions have become the standard practice for management of many waxy-leaved species, in part because oil emulsions slow the drying time on the leaf surface and may solubilize the cuticle wax. Further examination of these factors in controlled environment studies would be extremely useful in understanding this complex relationship for water versus oil-soluble formulations.
Acknowledgments
This project was funded by the Florida Fish and Wildlife Conservation Commission’s Invasive Plant Management Section. We would also like to acknowledge San Felasco Hammock Preserve State Park and Lake Griffin State Park for their support in conducting this study.








