Respiratory syncytial virus (RSV) is a typical cause of wintertime respiratory illnessReference Falsey and Walsh 1 and is increasingly recognized in both older adults and immunocompromised patients.Reference Sundaram, Meece, Sifakis, Gasser and Belongia 2 Therefore, it may be argued that patients presenting with respiratory symptoms during the influenza season should be simultaneously tested for influenza virus and RSV. The Xpert Flu/RSV XC assay (Cepheid, Sunnyvale, CA), which detects both influenza virus A/B and RSV, demonstrates sensitivities ranging from 90.6% to 97.9% and specificities from 99.4% to 100% for RSV.Reference Griffiths, Drews and Marchant 3 – Reference Popowitch and Miller 5 The test has a short turnaround time of approximately 1.5 hours and provides semiquantitative results. During the influenza season of 2014/2015, we introduced this rapid molecular test for patient assessments presenting with an influenza-like illness to the emergency department of our university hospital. Using this strategy, we aimed to identify and isolate early patients with a transmissible viral respiratory disease requiring hospitalization. However, this strategy led to challenges from the perspectives of infection control and hospital hygiene because RSV infection might be increasingly recognized. Patients with RSV disease can trigger hospital outbreaks.Reference Huang, Chen, Shi, Chan and Huang 6 – 9 To prevent person-to-person transmission, the Centers for Disease Control and Prevention (CDC) and the Healthcare Infection Control Practices Advisory Committee recommend standard and contact precautions.Reference Tablan, Anderson and Besser 10 In our institution, we implemented a combination of droplet and contact isolation precautions for RSV (hereafter called ‘contact isolation,’ as defined in the Methods section), but only for immunocompromised patients.
The objective of this study was to critically appraise this isolation policy since the introduction of a sensitive combined molecular test with a rapid turnaround time. We aimed to review the clinical characteristics of hospitalized adults with positive RSV PCR test results and to evaluate the rate of and factors possibly associated with healthcare-associated (HAI) RSV infections.
METHODS
Patient and Microbiological Assessment
Patients eligible for this study were identified via 2 databases, 1 from the infection control unit consisting of patients isolated because of RSV infection and 1 from the microbiology laboratory with automatically collected RSV test results. We included only adult patients who were assessed with the Xpert Flu/RSV XC test on the GenXpert system (Cepheid) from nasopharyngeal swabs (UTM Viral Transport Media, Copan, Italy). The tests were performed as recommended by the manufacturer. We included patients hospitalized between January 1, 2015, and December 31, 2016, at the University Hospital Basel, an 800-bed tertiary care center in Basel, Switzerland. Demographic and clinical characteristics were collected through a retrospective medical chart review. The in-house information technology solution for patient management was used to identify the location (rooms and wards) and movements of patients (ie, from one room to another, or change of wards during hospital stay) and to track the contact patients of RSV-positive patients.
Definitions for Infection Control Measurements
Our in-house infection control policy requires only RSV-positive immunocompromised patients subject to contact isolation procedures. Patients who meet the following criteria are considered immunocompromised: lymphopenia (<0.3×109/L), stem cell transplantation (SCT) ≤1 year prior to presentation, medication with considerable immunosuppressive effect (eg, corticosteroids ≥50 mg per day, mycophenolate mofetil or tacrolimus).Reference Khanna, Widmer and Decker 11 Contact isolation precautions for RSV include the use of masks, gloves, gowns, and protective googles. Nonimmunocompromised RSV-positive patients are not contact isolated, but they are not to be placed next to an RSV-negative, immunocompromised patient.
The definition of National Healthcare Safety Network for laboratory-identified events (LabID) was used for HAIs. 12 Positive Xpert RSV test result with date of event on day 3 or later of hospitalization were HAIs. Upon identification of cases, we assessed patient charts, physician notes, and records from infection control practitioners and board-certified infectious diseases specialists regarding the likelihood of nosocomial transmission (eg, symptoms present prior to or at admission). Nosocomial transmission was considered proven if a previous negative test result was available, probable if new onset of respiratory symptoms was noted during hospitalization but the RSV PCR test was not performed at admission, and unlikely if the onset of respiratory symptoms was reported prior to hospitalization and these symptoms were attributed to RSV infection by the treating physicians. If the criteria did not fit into one of these categories, transmission was categorized as possible. Only ‘unlikely transmission’ cases according to the aforementioned definition were excluded.
Infection Control Data Collection
To evaluate the variables potentially associated with nosocomial transmission, we defined the following prior to data analyses: length of hospital stay, admittance to the intensive care unit (ICU), length of stay in ICU, number of rooms occupied during hospital stay, number of patient contacts in the same room during hospital stay, and number of ward switches during hospital stay (eg, transfers from the internal medicine ward to ICU and back to the internal medicine ward was considered as 2 switches). The emergency department was excluded from the latter variable. For comparative analyses, 3 groups were formed: (1) with HAIs, (2) community-acquired infections (CAIs), and (3) CAIs but without any isolation precautions.
Potential Nosocomial Transmission
To identify additional potential nosocomial transmission, we applied the following 4 methods. (1) All patients included in this study were screened for a hospital stay in the previous 3 months and whether, during that hospitalization, they were placed next to an RSV-positive patient. (2) We identified all contact patients staying in the same room of a RSV-positive patient. A contact patient was defined as a person who was hospitalized in the same room as an RSV-positive patient for ≥1 day. The discharge codes of the International Classification of Diseases, Ninth Revision (ICD-9) of contact patients were screened for 3 RSV-specific ICD-9 codes: 480.1, pneumonia due to RSV; 466.11, bronchiolitis due to RSV; and 079.6, RSV. (3) All contact patients were evaluated for readmission because of RSV infection after being discharged in the previous 3 months. (4) The laboratory database was matched with names of the contact patients, screened for RSV PCR test assay results, and evaluated for whether these tests were performed within the incubation time (ie, 2–12 days) after contact.Reference Falsey and Walsh 1
Statistical Analysis
GraphPad Prism 7.01 (GraphPad Software, San Diego, CA) was used for statistical analysis. Differences in group proportions were assessed by contingency tables and the χ2 test, or Fisher’s exact probability test if a frequency was<5. Differences in numbers were assessed using the Mann-Whitney U test, analysis of variance test, or Kruskal-Wallis test. A 2-tailed P-value ≤.05 was considered significant.
RESULTS
We identified 108 episodes in 106 patients with a positive Xpert RSV test result who were hospitalized during the study period (2 patients were hospitalized twice). Patient characteristics and comorbidities are presented in Table 1.
TABLE 1 Demographic Characteristics of 106 Hospitalized Patients

NOTE. SD, standard deviation; SCT, stem cell transplantation; RSV, respiratory syncytial virus; ESBL, extended-spectrum β-lactamases.
a Isolation precautions only because of RSV infection (see Figure 1).
b In 3 healthcare-associated infections, isolation precautions were applied after detection of positive RSV results.
c The number includes 3 patients with droplet isolation precautions because of influenza and 2 patients with contact isolation precautions (without mask and protective googles) because of colonization with ESBL-producing Klebsiella pneumoniae (see Figure 1).
d Obesitiy definition: ≥30 kg/m2.
e The numbers reflect the sum of active and history of malignant disease.
f Criteria defined in the Methods section.
Demographics and Comorbidities
As expected from our isolation policy, there were significantly more immunocompromised patients (68% vs 2.5%; P<.0001), particularly those with a hematological malignancy (48% vs 1%; P<.0001), in the isolation precaution group. These patients were also significantly younger than were the non-isolated patients (mean age, 62.8 years [SD, 14.1 years] vs 71.4 years [SD, 15.5 years]; P<.005).
Isolation Precautions
In 25 of 108 (23.1%) episodes, isolation precautions were applied due to a positive RSV PCR test result. In 3 of these episodes, the infections were was healthcare associated, and isolation precautions were performed after the return of a positive RSV PCR test result. The mean duration of isolation precautions because of RSV infection was 11.8 days (SD, 7.3 days).
Coinfection with influenza virus was noted in in 6 (5.6%) of 108 episodes. In 3 of these 6 episodes, contact isolation precaution was applied because of RSV. In the other 3 episodes, only droplet isolation precaution for influenza was applied because they did not fulfill the in-house policy criteria for RSV isolation precautions. In addition, 2 patients were isolated because of colonization with extended-spectrum β-lactamase-producing Klebsiella pneumoniae. Thus, in 78 (72.2%) of 108 episodes, no isolation precautions were applied (Figure 1).
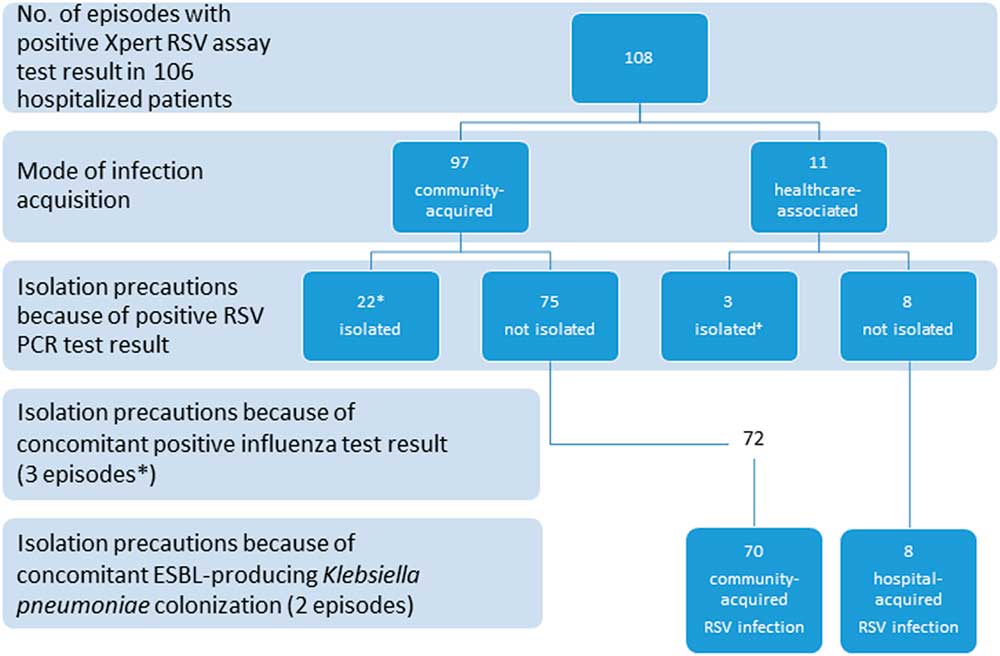
FIGURE 1 Distribution of respiratory syncytial virus (RSV) infection episodes according to mode of acquisition and isolation precautions. ESBL, extended spectrum β-lactamase. We identified 6 RSV influenza coinfected patients. For 3 episodes, contact isolation was applied because of RSV. *In the other 3 episodes, only droplet isolation for influenza was applied because the patient did not fulfill the in-house policy criteria for RSV isolation. Overall, there were 78 episodes (70 community-associated infections and 8 hospital-associated infections) without any isolation precautions during the patient’s stay.
Clinical Presentation
In 93 episodes (86.1%), an acute respiratory infection was clinically observed. The syndromes included exacerbation of chronic obstructive pulmonary disease or asthma, upper respiratory tract infection, and pneumonia. In 1 episode, conjunctivitis was diagnosed. In the remaining episodes, the diagnostic assay was performed because of shortness of breath, fever, or unconsciousness in a critically ill condition (eg, cardiac failure, pulmonary embolism, septic shock). The interpretation of the positive RSV PCR results remains difficult in these cases because the symptoms may not reflect the clinical viral disease. In 15 episodes (13.9%), antiviral treatment was administered consisting of intravenous immunoglobulin infusion and oral ribavirin. Over the 2-year study period, the crude mortality was 4 of 108 episodes (3.7%). Two of these patients may have died because of respiratory failure due to RSV disease (attributable mortality, 1.9%); both of these patients had severe underlying diseases (progressive urothelial carcinoma and Curschmann-Steinert syndrome, respectively).
Healthcare-Associated Infections
In 13 episodes, positive RSV PCR results were noted ≥3 days after admission. After a detailed review of the patient charts, the physicians’ notes, and the notes by the infection control practitioners, we concluded that transmission of RSV during the hospital stay was unlikely in 2 episodes. The remaining 11 (10.2%) episodes were categorized as HAIs (Table 2). Of these episodes, 2 were categorized as definite, 4 as probable, and 5 as possible. These 11 episodes were used for further analyses (Table 3).
TABLE 2 Episodes in Which a Positive RSV PCR Test Result Was Reported ≥3 Days After Admission

NOTE. RSV, respiratory syncytial virus; PCR, polymerase chain reaction; LOS; length of stay; Intern Med, internal medicine; IMC, intermediate care unit; ICU, intensive care unit.
a Wards 1 to 3 reflect the sequential place of hospitalization. Episodes with 2 numbers (eg, 1 d / 1 d) indicate 2 visits on the corresponding ward.
b On day 6 of hospitalization, the first RSV PCR test result was negative. On day 20, the result was positive, confirming definite healthcare-associated infection.
TABLE 3 Comparison of Predefined Variables Between Community-Acquired and Healthcare-Acquired Infection Episodes

NOTE. RSV, respiratory syncytial virus; CAI, community-acquired infection; HAI, healthcare-associated infection; IP, isolation precautions; SD, standard deviation; ICU, intensive care unit.
To evaluate the variables potentially associated with nosocomial transmission, the following comparisons were made: (1) a comparison of predefined variables between episodes with CAI (n=97) and episodes with HAI (n=11), and (2) a comparison of episodes with CAI in patients with only standard precautions (n=70) and episodes with HAI (n=11) (Figure 1 and Table 3).The mean length of hospital stay was 3.6 times longer in the HAI group (40.2 days) than in the entire CAI group (11.2 days) and 4.3 times longer in the HAI group (40.2 days) than in the CAI group with no isolation precautions (9.3 days) (P<.0001). In line with this result, patients with HAI switched both rooms and wards significantly more frequently and had significantly more patient contacts than did patients with CAI (Table 3).
Nosocomial transmission in contact patients
During 108 episodes, 106 RSV-positive patients had 447 patient contacts during their hospital stay (ie, patients who stayed in the same room). Two potential nosocomial transmissions were identified using our 4-modality approach. One patient was placed next to an RSV-positive patient in a previous hospitalization (10 days of exposure) and was readmitted with a positive RSV PCR result 13 days after discharge. Patient 4 in Table 2 was initially RSV negative and was a contact patient. She was placed next to an RSV-positive patient for 4 days, and an HAI infection was proven 9 days later. An RSV-associated ICD-9 code was not found in any of the records for other contact patients. Furthermore, 30 contact patients (6.7%) were readmitted during the study period, though none had a diagnosis of RSV infection. Within our microbiology laboratory database, 84 RSV PCR test results for 68 contact patients were identified. Sixteen tests were performed within 2 to 22 days (mean, 9.9 days; median, 7 days) after exposure to an RSV-positive patient. None of the tests revealed a positive PCR result. The other 68 tests were performed prior to contact with RSV-positive patients (ie, at admission). No outbreak was noted during the 2-year observation period.
DISCUSSION
A recent US study on RSV infection in adults reported that 80.7% of hospitalizations occurred during the winter months.Reference Pastula, Hackett and Coalson 13 It is conceivable that systematic screening of patients with an influenza-like illness who present at the emergency department, as well as the introduction of a highly sensitive multiplex molecular test, will lead to greater recognition of RSV infection.Reference Pastula, Hackett and Coalson 13 The number of episodes with positive RSV PCR test results in our single-center study is higher than that reported in previous studies.Reference Falsey and Walsh 1 , Reference Loubet, Lenzi and Valette 14 Because a different RSV test was used and was mainly performed selectively in previous years (eg, in immunocompromised patients and ordered by clinical evidence as individual PCR), we cannot draw reliable conclusions about the incidence trend of RSV infections in our center. Nonetheless, the introduction of a rapid molecular testing method raises questions regarding episodes that previously remained undetected, particularly concerning (1) handling and treatment of positive results with a viral load of unknown significance, (2) infection control procedures, and (3) in-house isolation policies. A change in isolation policy requires an analysis of costs related to isolation equipment, labor time for healthcare workers, the inability to locate isolated and nonisolated patients in the same room, and repeat testing. Hence, the introduction of highly sensitive molecular testing faces challenges from both infection control and hospital hygiene perspectives.
Similar to other observations of RSV infections in hospitalized adults,Reference Falsey and Walsh 1 , Reference Pastula, Hackett and Coalson 13 , Reference Loubet, Lenzi and Valette 14 the results of our study show that the vast majority of patients (90.6%) had at least 1 chronic underlying illness and that 68.9% were ≥65 years old. Patients for whom contact isolation was applied were significantly younger than patients without RSV-indicated isolation precautions (mean, 62.8 vs 71.4 years; P=.0048). This finding may be explained by the higher proportions of patients with hematological malignancies and a history of SCT (Table 1). The in-hospital mortality rates of RSV infection in adults in 2 recent studies were 5.1% and 8%,Reference Pastula, Hackett and Coalson 13 , Reference Loubet, Lenzi and Valette 14 respectively, while this rate was <2% in our study. In our institution, the proportion of patients treated with ribavirin and intravenous immunoglobulin infusion among immunocompromised patients was high (15 of 19 patients, 78.9%). RSV infection in immunocompromised patients, particularly in patients with a history of SCT, is associated with high mortality rates.Reference Falsey and Walsh 1 , Reference Whimbey, Couch and Englund 15 – Reference Avetisyan, Mattsson, Sparrelid and Ljungman 18 Although uncontrolled studies show conflicting results regarding the efficacy of ribavirin and intravenous immunoglobulin infusion regimen,Reference Lewinsohn, Bowden, Mattson and Crawford 19 this treatment is frequently considered beneficial and is administered to immunocompromised patients, especially SCT recipients with lower respiratory tract infections.Reference Falsey and Walsh 1 , Reference Griffiths, Drews and Marchant 3 , Reference Lehners, Schnitzler and Geis 8 , Reference Khanna, Widmer and Decker 11 , Reference Avetisyan, Mattsson, Sparrelid and Ljungman 18 , Reference Gorcea, Tholouli and Turner 20 , Reference Gueller, Duenzinger and Wolf 21 The influence of treatment on nosocomial RSV transmission has, to the best of our knowledge, not been assessed; however, the risk has been recently systematically reviewed by French et al.Reference French, McKenzie and Coope 22 In outbreaks, the transmission risk varied by hospital setting from 6% to 12% (median, 7%) in units housing immunocompromised adults and from 30% to 32% in other adult-care settings. In our population, no outbreak was noted during the 2-year observation period, and 11 (10.2%) HAIs were identified overall. In comparison to CAIs, they were significantly associated with a prolonged hospital stay (Table 3). The length of hospital stay is in line with the number of patient contacts and with the number of room and ward switches, which were all significantly associated with HAI. The length of stay has been previously identified as a risk factor for nosocomial RSV infection.Reference Hall, Douglas, Geiman and Messner 23 Moreover, it is a modifier of the effect of other risk factors for hospital infection.Reference Delgado-Rodriguez, Bueno-Cavanillas and Lopez-Gigosos 24 , Reference Beyersmann, Kneib, Schumacher and Gastmeier 25 Our observation supports the Centers for Disease Control and Prevention (CDC) recommendation to limit patient movement or transport in acute-care facilities.Reference Tablan, Anderson and Besser 10 On the other hand, HAI may contribute to a longer hospital stay. Of 11 patients with HAI infection, 7 patients required acute care for another 16 to 43 days after the positive RSV PCR test result was returned.
Immunocompromised status has been statistically associated with the risk of acquiring RSV.Reference Pastula, Hackett and Coalson 13 , Reference Loubet, Lenzi and Valette 14 Therefore, we recommend not hosting RSV-negative immunocompromised patients next to immunocompetent RSV-positive patients. Prolonged viral shedding has been shown in RSV-positive immunocompromised patients, particularly in SCT patients.Reference Lehners, Schnitzler and Geis 8 , Reference Geis, Prifert and Weissbrich 26 , Reference Lehners, Tabatabai and Prifert 27 Thus, these patients are potential sources of an outbreak8,26 and require contact isolation precautions. Our study could not properly assess how many of the HAIs could have been prevented with a more rigorous isolation policy (eg, contact isolation for all patients with a positive RSV test result, irrespective of host status). Notably, for a considerable number of patients with HAIs, respiratory symptoms were not the reason for admission and the hospital stay was not in an internal medicine ward (eg, orthopedics, urology, obstetrics, or dermatology). Hence, other transmission events likely occurred that were missed. The importance of standard precautions for all patients, healthcare workers, and visitors, cannot be overstated. The rate of 10.2% HAIs may indicate the need for improvement of our isolation policy. However, in only 6 of 11 patients was HAI was considered definite or probable, while it could not be excluded in the other 5 episodes.
Our study has several limitations, including its retrospective nature and the use of medical records for data collection, even though laboratory values for RSV test results and episodes with isolation precautions are prospectively and systematically collected within our institution. The number of nosocomial transmission cases may be underestimated because this assessment depends on the number of tests performed and patients with HAIs may have not been tested. The categorization of HAI was somewhat arbitrary, but each episode was evaluated in detail and only 2 episodes were classified as unlikely nosocomial transmission. In addition, we carefully searched for positive RSV PCR test results among 447 contact patients via 4 different search methods. Finally, the statistical results should be interpreted with caution because the absolute number of HAIs was small and there was no comparison group with RSV-negative patients.
In conclusion, the introduction of the Xpert Flu/RSV XC PCR assay for patients presenting with influenza-like illness during the winter season identified a high number of RSV infection episodes. In 70 episodes (64.8%) in 69 immunocompetent patients, no specific isolation precautions were applied. No evidence for an outbreak was noted during the study period. The mean length of hospital stay and the numbers of rooms occupied, ward changes, and patient contacts during the hospital stay were significantly longer and higher for the HAI group than for the CAI group. Our study results indicated that the introduction of a new rapid RSV PCR test, and hence, the detection of a high number of RSV infection episodes, does not necessarily require a change of existing infection control procedures, provided that immunocompromised patients are meticulously identified. Considering the increasing use of more sensitive and multiplex assays and the increasing costs related to isolation procedures, these results may have practical implications for infection control and hospital hygiene specialists.
ACKNOWLEDGMENTS
We thank Dr Claudia Horning and Clarisse Straub for data collection. We are indebted to Drs Jan Roth, Stephan Erb, and Richard A. Kuehl for critical review of and valuable comments related to the manuscript.
Financial support: No specific financial support for the generation of this study was available. A.E. received a research grant from the Swiss National Science Foundation Ambizione (grant no. PZ00P3_154709/1).
Potential conflicts of interest: All authors report no conflicts of interest relevant to this article.






