INTRODUCTION
The Laboratoire de Mesure du Carbone 14 (LMC14) and its Pelletron tandem AMS unit ARTEMIS are dedicated to radiocarbon (14C) measurements for the research programs of five French institutions. About 4500 samples, comprising unknown samples, standards and blanks, are measured every year, including more than 200 samples dedicated to specific LMC14 research programs. Over many years, we have improved our protocols in order to extend our range of dated samples. Various types of materials are prepared and measured, such as organic matter, carbonate (Dumoulin et al. Reference Dumoulin, Comby-Zerbino, Delqué-Količ, Moreau, Caffy, Hain, Perron, Thellier, Setti, Berthier and Beck2017), dissolved inorganic carbon in water (Dumoulin et al. Reference Dumoulin, Caffy, Comby-Zerbino, Delqué-Količ, Hain, Massault and Moreau2013), and archaeological iron alloys (Leroy et al. Reference Leroy, L’Héritier, Delqué-Kolic, Dumoulin, Moreau and Dillmann2015).
Recently, we have investigated the preparation of bone samples. Existing protocols to extract the collagen include modified Longin (Longin Reference Longin1971; Brown et al. Reference Brown, Nelson, Vogel and Southon1988), ultrafiltration (Bronk Ramsey et al. Reference Bronk Ramsey, Higham, Bowles and Hedges2004; Brock et al. Reference Brock, Bronk Ramsey and Higham2007), single amino acid/hydroxyproline extraction (Marom et al. Reference Marom, McCullagh, Higham and Hedges2013) and ninhydrin (Nelson Reference Nelson1991; Tisnérat-Laborde et al. Reference Tisnérat-Laborde, Valladas, Kaltnecker and Arnold2003). In this study, we have selected two methods: the ninhydrin and a modified Longin protocols.
The aim of this paper is to compare the efficiency of these two collagen-extraction processes according to the bone age, the preservation condition, and the collagen content. For this study, bones of known ages including three mammal specimens of the Fifth International Radiocarbon Intercomparison and 14C-free background samples have been used. Bone preservation has been characterized by elemental analysis and Fourier transform infrared spectrometry in attenuated total reflection mode (ATR-FTIR). Elemental analysis directly provides concentrations of carbon and nitrogen while the second technique quantifies the collagen content (Lebon et al. Reference Lebon, Reiche, Gallet, Bellot-Gurlet and Zazzo2016).
The 14C results of the bones prepared with the two processes—modified Longin and ninhydrin techniques—are presented and the best protocol to be applied according to the different types of bone samples is discussed.
MATERIAL AND METHODS
Bone Samples
A bone is a porous network of mineralized fibers, made up of 20–30% by weight of organic matter (collagen), and 60–70% by weight of mineral matter (hydroxyapatite). Water (10% by weight) and other chemical elements in very small quantities, such as sulfur, are also present. It is possible to visually distinguish two different porosities in a bone: the cortical bone is compact whereas the trabecular bone is very porous (Figure 1). For 14C dating of archaeological samples, the compact cortical bone is chosen and the trabecular bone is removed. This porous part is more altered by diagenesis and can trap a lot of contaminants (Dauphin et al. Reference Dauphin2015). However, Ubelaker and Parra (Reference Ubelaker and Parra2011) found that for modern bone, the trabecular tissue actually gave better dates than the cortical. However, this is only due to faster turnover of collagen in the trabecular material.

Figure 1 Sample showing the compact cortical bone and the spongy trabecular bone.
In this study, different bone samples have been chosen because of their species (mammoth, horse, whale) and ages (Table 1). Three VIRI samples E, F, and I (Scott et al. Reference Scott, Cook and Naysmith2010) and different samples with known ages have been selected. They originate from different archeological sites and are sufficiently varied to offer the possibility to see which preparation method is the most suitable according to the bone characteristics. The Scladina bone specimens come from layer 4b, which is older than 120,000 years according to thermoluminescence dating (Debenham Reference Debenham1998).
Table 1 Bone samples selected for this study.

* Expected age is an average value of two previous results measured at LSCE and Groningen laboratories.
** GrA-40485.
Collagen-Preservation Study
To determine if a bone can be dated, the preservation condition of the collagen has to be estimated. Several researchers (Gillespie et al. Reference Gillespie, Hedges and Wand1984; DeNiro Reference DeNiro1985; Ambrose et al. Reference Ambrose1990; van Klinken Reference Van Klinken1999; Bocherens et al. Reference Bocherens, Drucker, Billiou and Moussa2005; Beck et al. Reference Beck, Cuif, Pichon, Vaubaillon, Dambricourt Malassé and Abel2012) have demonstrated that the percent of nitrogen (%N) for whole bone can be a useful prescreening technique to identify bones suitable for 14C dating. According to Brock et al. (Reference Brock, Higham and Bronk Ramsey2010), the acceptable limit to prepare a bone sample is %N > 0.7–0.8%. Below this value, the collagen is not well-preserved enough for dating.
The exogenous contamination rate (%Cexcess) can be determined in a bone by comparing the measured percent carbon (%C) with the theoretical %C. According to Person et al. (Reference Person, Bocherens, Mariotti and Renard1996) and Bocherens et al. (Reference Bocherens, Drucker, Billiou and Moussa2005), the following equations were defined:
The classic method for measuring the concentrations of C and N is based on a carbon and nitrogen elemental analyzer. We uses a Thermo Fisher Scientific Flash 2000 series.
The detection and quantification of the collagen content have been also measured by ATR-FTIR with a Vertex 70 spectrometer (Bruker) equipped with a ATR-GoldenGate accessory (Specac). The relative collagen content of the bone samples is calculated by measuring the area ratio of the amide I and phosphate peaks of the IR spectrum. The amide I and the phosphate band areas are measured between 1710 and 1590 cm–1 and between 1110 and 940 cm–1, respectively, according to the methodology developed by Lebon et al. (Reference Lebon, Reiche, Gallet, Bellot-Gurlet and Zazzo2016). The limit of detection of this method is 0.5 wt% for N (corresponding to ~3 wt% of collagen) and the limit of quantification is 0.7±0.2 wt% (~4±1.2 wt% of collagen).
Elemental analysis and ATR-FTIR are carried out on the same samples for both methods of preparation. In addition, the ATR-FTIR method is applied to the different steps of the collagen extraction by analyzing the bone powder, the residue collected on the glass filter, and the extracted collagen.
Collagen Extraction
A mass of 1 or 2 g is cut with a small electrical saw from the cortical part of each bone (compact porosity). The trabecular bone (spongy porosity) is removed. Mechanical cleaning of the bone surface is carried out by sand blasting (27 µm diameter aluminum oxide) to remove macro-contaminants such as sediment. An aliquot of a few milligrams of bone is first drilled out to evaluate the bone preservation by elemental analysis and ATR-FTIR spectrometry. The rest of the sample is used for the collagen extraction. Two processes of collagen extraction—the modified Longin and the nynhidrin methods—are tested and compared.
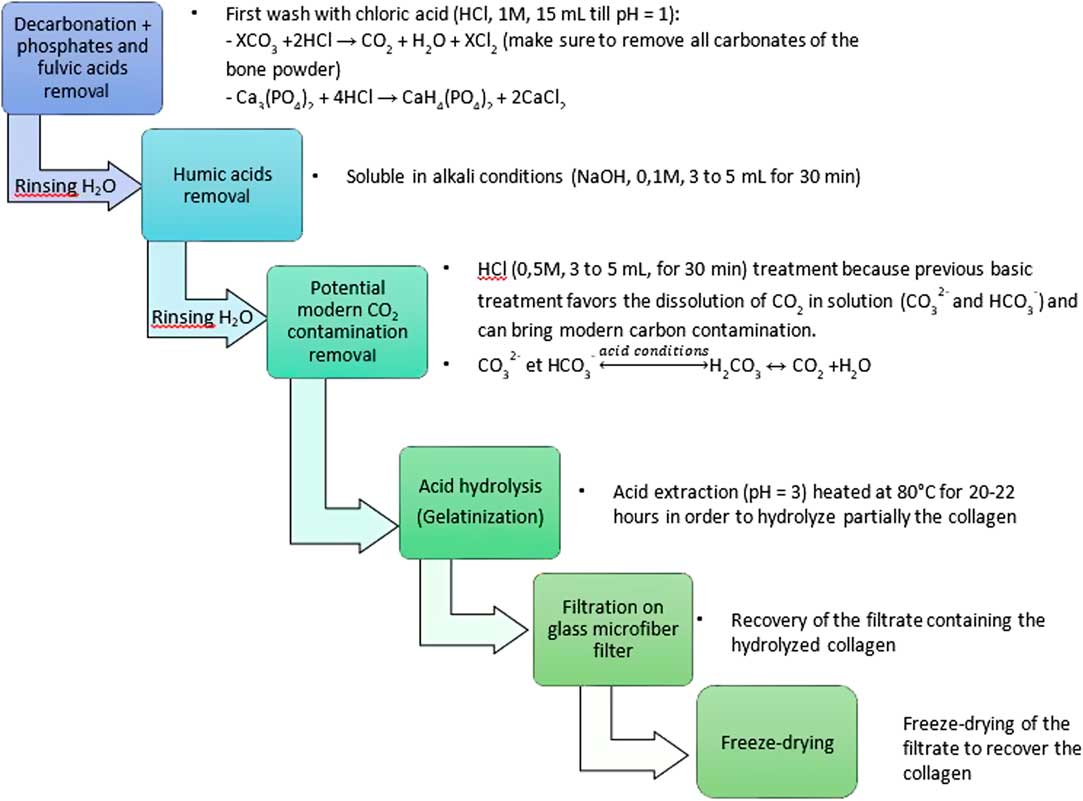
The modified Longin method, used in many laboratories, is described in Figure 2. The bone powder is treated at room temperature with 1M HCl to remove carbonates, phosphates, and fulvic acids. The duration depends on the sample weight and its carbonates content. The acid solution in changed several times until the pH of the solution remains acid (pH = 1). Then, after three washes with deionized water, the humic acids are removed in a 0.1M NaOH alkali solution for 30 min. After three more water washes, a last 0.5M HCl treatment is applied. The collagen residue is finally washed with deionized water until a neutral pH is reached. After the ABA (acid-base-acid) pretreatment, the collagen is gelatinized in a pH3 solution at 80°C for 20–22 hr and then filtrated on a clean glass microfiber filter (GE-Whatman, 0.7 µm). The collected gelatin is freeze dried. Then, 2–3 mg of pure collagen is placed in a quartz tube with an excess of CuO (300–400 mg) and a 1-cm Ag wire. The quartz tube is then sealed under vacuum (5.10–6 mbar) and heated at 850°C for 5 hr in an external oven. The CO2 gas is separated from H2O on a vacuum line using a dry ice/alcohol trap (–78°C). The sample is then cryogenically collected with liquid nitrogen (–196°C) into vials to be transferred to the graphitization lines (Vogel et al. Reference Vogel, Southon, Nelson and Brown1984; Dumoulin et al. Reference Dumoulin, Comby-Zerbino, Delqué-Količ, Moreau, Caffy, Hain, Perron, Thellier, Setti, Berthier and Beck2017).

Figure 2 Detailed steps of the modified Longin method.
The ninhydrin method is described in Figure 3. In this method, a ninhydrin solution (50 mg of ninhydrin in 2 mL of sodium citrate) is first added to the sample for 10 min at 100°C after a first acid pretreatment (1M HCl at room temperature). This first ninhydrin step reacts only with the free amino acids weakly bound to the collagen and suspected as being a contaminant (Tisnérat-Laborde et al. Reference Tisnérat-Laborde, Valladas, Kaltnecker and Arnold2003). After this step, the solution has a pink color and the collagen has to be rinsed with deionized water until the solution is clear and no color remains.

Figure 3 Detailed steps of the ninhydrin method.
The collagen residue is then hydrolyzed with 2 ml 6M HCl solution at 100°C overnight to break the collagen molecules into free amino-acid molecules. The solution is filtrated on a clean glass microfiber filter (GE-Whatman-0.7 µm), then rinsed and evaporated under nitrogen three times to remove as much acid as possible.
The filtrate containing free amino acids from the collagen is placed in a special glass setup under vacuum until 10–5 mb and before proceeding to the CO2 extraction. A second solution of 50 mg of ninhydrin dissolved in 2 mL of sodium citrate is introduced with a syringe through a septum and heated at 100°C (Figure 4a). The reaction is quite exothermic and the boiling has to be controlled with the valve above. The produced CO2 passes through two successive “water traps” (dry ice and ethanol at –78°C) to remove the water and is collected with a liquid nitrogen trap at –196°C in vials (Figure 4b) before the graphitization step (Vogel et al. Reference Vogel, Southon, Nelson and Brown1984; Dumoulin et al. Reference Dumoulin, Comby-Zerbino, Delqué-Količ, Moreau, Caffy, Hain, Perron, Thellier, Setti, Berthier and Beck2017).

Figure 4 (a) First ninhydrin introduction through the septum and (b) CO2 collection line.
After preparation of the collagen, combustion, purification, and graphitization, the samples are measured by the ARTEMIS accelerator mass spectrometer (Moreau et al. Reference Moreau, Caffy, Comby, Delqué-Količ, Dumoulin, Hain, Quiles, Setti, Souprayen and Thellier2013).
RESULTS AND DISCUSSION
Collagen-Preservation Test
The results concerning the preservation of the bones are presented in Table 2. Elemental analysis directly measures % N and % C values. % Ctheory and the % Cexcess are calculated according to the above equations. We observe that the VIRI samples and Buzha 02 are well preserved with a nitrogen content around 3% indicating that a suitable quantity of collagen remains in the sample (around 20%). The nitrogen content of BK3-08-05 and Sc91 is around 0.7% showing that the collagen is degraded, close to the acceptable limit for a 14C measurement. In all cases, the samples are not highly contaminated because the contamination rates (%Cexcess) are between 1 and 2%, lower than the maximum accepted value of 5–10 %.
Table 2 Comparison of elemental analysis and ATR-FTIR analysis for the different bone samples. Two sub-samples, labelled ① and ② were analyzed to check the homogeneity of the collagen preservation.

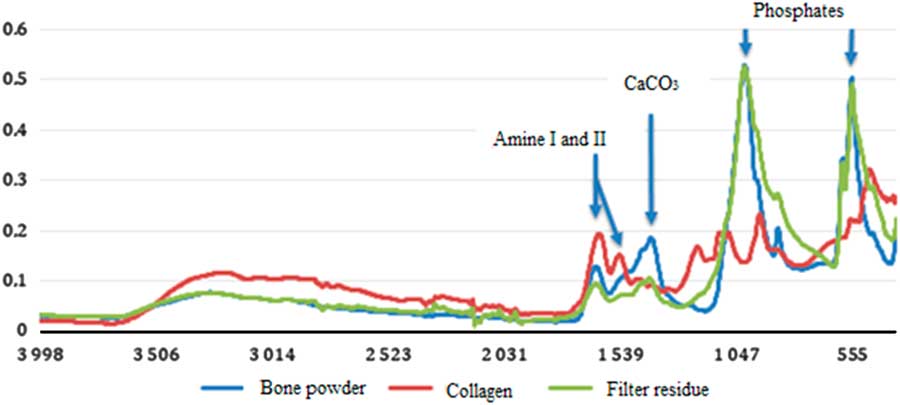
The ATR-FTIR analysis provides the % N and the collagen content calculated from the amide I: phosphate peak ratio of the IR spectrum (Figure 5). The results of the ATR-FTIR and elemental analyses are close to each other in most of the cases. The IR method estimates directly the quantity of collagen remaining in the bone and the elemental analysis gives the %Cexcess corresponding to the contaminant to be removed.

Figure 5 ATR-FTIR spectra of not pretreated bone powder (blue curve), the residue on the glass filter (green curve), and the purified collagen (red curve) after ABA pretreatment of sample Buzha 02. (Colors refer to online version.)
The ATR-FTIR analysis also provides information on the different steps of the pretreatment (Figure 5). In the non-pretreated bone powder, the presence of carbonates, phosphates as well as amide I and II (from the collagen) are observed. The residue on the filter contains traces of collagen that has not been extracted by the hydrolysis step (gelatinization) and the insoluble phosphates. In the extracted collagen, the carbonates and phosphates peaks are very weak and the amide I and II peaks are well defined. These results show that the major part of the non-collagen components are removed by the chemical pretreatment.
14C RESULTS AND DISCUSSION
The results are presented in Table 3. For both methods, samples expected as background samples (Aliquot 10, Sclayn, and Sc91) do not have any background correction. All other samples get a background correction. The modified Longin results are obtained by subtracting the Aliquot 10 value (0.336±0.014 pMC) and the ninhydrin results are obtained by subtracting the Sclayn value (0.582±0.026 pMC).
Table 3 14C dating results for the two different methods compared to consensus values or previously measured values.

* Previous measurements from LSCE.
When using the Longin method, the results are very close to the expected values. All the VIRI sample results are in accordance with the consensus values. In addition, the value obtained for Aliquot 10 is in the age range for an old sample. Only one sample, Sclayn, appears too young for a background sample. This is probably because some contaminants remain in the collagen before combustion. For this sample, the result obtained by the ninhydrin method is better.
With the ninhydrin method, the results for VIRI E and VIRI I samples are in accordance with the consensus values. Unfortunately, not enough CO2 was collected after the second ninydrin step for VIRI F and Aliquot 10. For the old samples Sc91 and Sclayn, the values are superior to 40,000 BP as expected.
Even if the two methods are in accordance each other and in agreement with the consensus values for 2σ uncertainty, the ninhydrin results are slightly older than the Longin results. At this stage, it is difficult to determine if the ninhydrin method gives better results for old bones or if the method introduces a bias. It is possible that the improvement in the old age range could be an artifact.
The modified Longin process seems to be applicable for almost all bones and requires only small quantities of bone material. This method is easy to implement and does not require special glassware or setup. Furthermore, it is possible to proceed to several measurements with only one chemical pretreatment. The limit of this method is probably for strongly contaminated bones because some contaminants (such as free amino acids) cannot be fully removed. For that reason, some laboratories add an ultrafiltration step at the end of the protocol (Brown et al. Reference Brown, Nelson, Vogel and Southon1988; Bronk Ramsey et al. Reference Bronk Ramsey, Higham, Bowles and Hedges2004; Brock et al. Reference Brock, Bronk Ramsey and Higham2007; Wood et al. Reference Wood, Bronk Ramsey and Higham2010). In this study, we use the ninhydrin method proposed by Nelson (Reference Nelson1991). This method appears to be particularly recommended for heavily contaminated bones since the first step allows the removal of the free amino acids weakly bound to the collagen. This treatment should have an equivalent effect to the ultrafiltration which removes contaminants under 30 kD. The use of the ninhydrin method can be very useful to date certain samples which cannot be accurately cleaned with modified Longin and can be an interesting alternative to the ultrafiltration protocols when consolidants and glues like PVA (polyvinyl acetate) are difficult to remove. We have shown that the collagen extraction by the ninhydrin method is well appropriate for almost all bones. However, we have not tested this method on heavily contaminated bones yet. This interesting aspect of the method will be investigated in a future study. However, the disadvantage of this method is the specific glassware setup necessary to undertake the ninhydrin reaction and the use of this harmful chemical product. Another shortcoming is that the ninhydrin method requires larger quantities of sample (around 1g) for only one possible measurement. It is not suitable for very small samples as only the carboxylic function COOH of the amino acids contributes to create CO2.
The best approach would be to choose the better collagen extraction method according to the bone characteristics. In most cases, both methods are applicable. As its implementation is easier, the modified Longin process could be preferred, particularly when the amount of bone is small and the sample is not too contaminated.
CONCLUSION
In this paper, preliminary results comparing two preparation methods for dating bone are presented: a modified version of the Longin method and the ninhydrin protocol have been tested on bones of different types and ages. The preservation condition of the bones was first tested by using elemental analysis and ATR-FTIR spectrometry. Elemental analysis has directly provided the %C and %N contents, ATR-FTIR has quantified the collagen content and has allowed an assessment of the pre-treatment efficiency.
The modified Longin process can be used for almost all bones because the implementation is simple and requires only small sample quantities. However, some small contaminants might not be fully removed and it can be a problem for older contaminated bones. To overcome this problem, ultrafiltration or single amino acid/hydroxyproline extraction can be used. In this paper, an alternative solution based on the ninhydrin method has been tested. In this protocol, free amino acids weakly bound to the collagen and suspected as being a contaminant should be removed efficiently by the first ninhydrin step. We have succeeded in extracting the collagen, but more contaminated bones would be necessary to confirm this point. Bones coming from museum collections or modern bones artificially contaminated with “aged” consolidant could be further investigated to assess the ninhydrin method efficiency. The main disadvantage of this method is the use of special glassware and the requirement of a large amount of sample (around 1 g) for only one possible measurement. The 14C results obtained with the ninhydrin method are coherent with the expected values for 2σ uncertainty even if some results seem slightly older than those obtained with the Longin protocol.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the colleagues of the LSCE for their help and particularly E. Kaltnecker for her precious advice during the laboratory experimentations. The authors thank the MNHN for providing access to the plateau de spectrométrie infrarouge. We also thank the ORAU (Oxford) for giving us the mammoth bone (Aliquot 10). Two anonymous reviewers are also thanked for their valuable remarks. The LMC14 is funded by five French organizations: CEA, CNRS, IRD, IRSN and MCC. This is LSCE contribution number 6068.