Introduction
Chagas disease is an endemic Neglected Tropical Disease (NTD) in Latin America that affects an estimated 8 million people (https://www.who.int/chagas/epidemiology/en/) (Jansen et al., Reference Jansen, Xavier and Roque2018). The aetiological agent is Trypanosoma cruzi, a protozoan parasite that has a complex life cycle through which it alternates between an invertebrate insect vector and a mammalian host (Santi-Rocca et al., Reference Santi-Rocca, Fernandez-Cortes, Chillón-Marinas, González-Rubio, Martin, Gironès and Fresno2017). The establishment of a successful infection relies on the interaction between the parasite and the mammalian host cell, which can be dissected into three steps: adhesion, signalling and invasion (de Souza et al., Reference de Souza, de Carvalho and Barrias2010). After the initial binding and recognition of the parasite to the host cell, several signalling pathways are triggered in both parties to orchestrate the successful invasion, such as intracellular [Ca2+] transients and PI3k/Akt signalling, among others (Burleigh and Woolsey, Reference Burleigh and Woolsey2002; Woolsey et al., Reference Woolsey, Sunwoo, Petersen, Brachmann, Cantley and Burleigh2003; Romano et al., Reference Romano, Cueto, Casassa, Vanrell, Gottlieb and Colombo2012). Wnt/β-catenin has been recently demonstrated to be one of these pathways, participating in the regulation of the inflammatory response and parasite intracellular replication (Volpini et al., Reference Volpini, Ambrosio, Fozzatti, Insfran, Stempin, Cervi and Motran2018).
Poly(ADP-ribose) (PAR) is synthetized by poly(ADP-ribose) polymerases (PARPs) as a post-translational protein modification mainly related to nuclear processes, although it has also been localized to extra-nuclear compartments (Lafon-Hughes et al., Reference Lafon-Hughes, Vilchez Larrea, Kun and Fernández Villamil2014; Lafon Hughes et al., Reference Lafon Hughes, Romeo Cardeillac, Cal Castillo, Vilchez Larrea, Sotelo Sosa, Folle Ungo, Fernández Villamil and Kun González2017; Xie et al., Reference Xie, Zhang, Zhao, Bai, Fan, Zhu, Yu, Li, Liang, Sun, Li and Qiao2018). PAR homoeostasis has been implicated in the host cell response to T. cruzi infection (Vilchez Larrea et al., Reference Vilchez Larrea, Haikarainen, Narwal, Schlesinger, Venkannagari, Flawiá, Villamil and Lehtiö2012) as well as to other intracellular non-parasitic pathogens (Brady et al., Reference Brady, Goel and Johnson2018; Miettinen et al., Reference Miettinen, Vedantham and Pulliainen2019). During T. cruzi infection, host intracellular PAR levels raise both in vivo and in vitro (Wen et al., Reference Wen, Yin and Garg2018) and available evidence indicates that PARP-1 is crucial for the successful establishment of this parasitic infection: inhibition or silencing of PARP-1 in Vero and A549 cells leads to a marked decrease of infection levels in vitro (Vilchez Larrea et al., Reference Vilchez Larrea, Haikarainen, Narwal, Schlesinger, Venkannagari, Flawiá, Villamil and Lehtiö2012) and the absence of PARP-1 activity was proven beneficial for the maintenance of mitochondrial function in chagasic murine myocardium (Wen et al., Reference Wen, Yin and Garg2018).
Tankyrase-1 (TNKS-1) and Tankyrase-2 (TNKS-2) [telomeric repeat binding factor 1 (TRF1)-interacting ankyrin-related ADP-ribose polymerases], belong to the large PARP family and, like PARP-1 and PARP-2, are capable of synthesizing PAR in mammalian cells (Palazzo et al., Reference Palazzo, Mikoč and Ahel2017; Lüscher et al., Reference Lüscher, Bütepage, Eckei, Krieg, Verheugd and Shilton2018). Among other functions (Sbodio et al., Reference Sbodio, Lodish and Chi2002; Hsiao and Smith, Reference Hsiao and Smith2008; De Boeck et al., Reference De Boeck, Forsyth, Praet and Hogendoorn2009), TNKS positively regulate Wnt/β-catenin signalling events: TNKS-mediated poly(ADP-ribosyl)ation (PARylation) of Axin (a scaffold protein in the β-catenin destruction complex) avoids β-catenin degradation, allowing its nuclear translocation (Yang et al., Reference Yang, Tacchelly-Benites, Wang, Randall, Tian, Benchabane, Freemantle, Pikielny, Tolwinski, Lee and Ahmed2016; Mariotti et al., Reference Mariotti, Pollock and Guettler2017). Wnt/β-catenin participation in the regulation of pro- and anti-inflammatory responses, mainly in cancer models, has drawn considerable attention in the past few years (Ma and Hottiger, Reference Ma and Hottiger2016) and it has been reported that TNKS inhibitors (TNKSi) can modulate certain cytokine expression profiles (Levaot et al., Reference Levaot, Voytyuk, Dimitriou, Sircoulomb, Chandrakumar, Deckert, Krzyzanowski, Scotter, Gu, Janmohamed, Cong, Simoncic, Ueki, La Rose and Rottapel2011).
Although the role of TNKS during viral cell infections has been explored in the past (Li et al., Reference Li, Yamauchi, Kamakura, Murayama, Goshima, Kimura and Nishiyama2012; Roy et al., Reference Roy, Liu and Arav-Boger2015), to our knowledge, the possible participation of host cell Tankyrases during T. cruzi or other parasitic infections and its modulation by TNKSi has never been explored. It was hypothesized that host cell Tankyrases could potentially affect T. cruzi infection by regulating the Wnt/β-catenin signalling pathway. We demonstrate that TNKSi were detrimental to T. cruzi infection in Vero cells but most did not affect parasite viability in axenic cultures. Host cell TNKS shifted towards the plasma membrane at early stages of infection (15 min). In response to T. cruzi infection in our model, β-catenin translocated to the nucleus (6 h post-infection) and the expression of the pro-inflammatory cytokine TNF-α was upregulated. TNKSi hindered these two responses, indicating that TNKS could be modulating T. cruzi cell infection through the regulation of these signalling molecules.
Materials and methods
Parasite and mammalian cells
Vero cells (ATCC® CCL-81™) were cultivated in MEM supplemented with 10% FCS, 100 U mL−1 penicillin, 0.1 mg mL−1 streptomycin and 2 mm glutamine. Trypanosoma cruzi parasites from the Tulahuen strain stably expressing the β-galactosidase gene (clone C4) (Buckner et al., Reference Buckner, Verlinde, La Flamme and Van Voorhis1996) were maintained in culture by infection of Vero cells in MEM with 3% fetal bovine serum (FBS), 2 mm L-glutamine, 100 U mL−1 penicillin and 0.1 mg mL−1 streptomycin. Trypanosoma cruzi (Tulahuen strain) epimastigotes were cultured in Liver Infusion Tryptose (LIT) medium [5 g L−1 liver infusion, 5 g L−1 bacto-tryptose, 68 mm NaCl, 5.3 mm KCl, 22 mm Na2HPO4, 0.2% (W/V) glucose and 0.002% (W/V) hemin] supplemented with 10% FBS, 100 U mL−1 penicillin and 100 mg L−1 streptomycin for 7 days at 28°C.
Tankyrase inhibitors
XAV939 was purchased from Abcam (120897). All other inhibitors were kindly provided by Dr Lari Lehtiö (Biocenter Oulu, Faculty of Biochemistry and Molecular Medicine, University of Oulu, Finland). The reported potencies and IC50 are summarized in Table 1. The structure of the inhibitors is shown in Supplementary information Fig. S1.
Table 1. Potency of TNKS inhibitors towards PARP-1/2, TNKS-1/2 and TcPARP

In vitro IC50 values determined for activity assays using full length (FL) or truncated recombinant enzymes. Dashes indicate information is not available.
β-Galactosidase infection assay
For the relative quantification of T. cruzi infection levels, the β-galactosidase expressing Tulahuen trypomastigotes were used. Vero cells were seeded in 96-well culture plates (104 cells/well in 100 μL MEM-10% FBS) and incubated ON. Then, trypomastigotes were added (MOI: 10:1) and washed after a 24 h incubation period. New culture media was added to infected monolayers, and 96 h after the addition of parasites to the culture (post-infection, p.i.), cell culture media was removed and cells and Tulahuen β-gal intracellular amastigotes were lysed in 100 μL lysis buffer (25 mm Tris pH 8, 2 mm EDTA, 2 mm DTT, 1% Triton X-100, 10% glycerol in ultrapure MQ water) for 10 min at 37°C. Then, 100 μL 2× reaction buffer [200 mm sodium phosphate pH 8, 2 mm MgCl2, 100 mm 2-mercaptoethanol and 1.33 mg mL−1 ortho-Nitrophenyl-β-galactoside (ONPG)] was added and the reaction was allowed to proceed until yellow colour developed (1–2 h at 37°C). Abs 420 nm was measured in a Synergy HTX multi-mode microplate reader (Biotek Instruments, Winooski, USA). Each infection condition or inhibitor was tested in triplicates and in four independent experiments. The absorbance at 420 nm obtained for each condition was normalized to the value obtained for the infection in the absence of inhibitors.
Viability of T. cruzi epimastigotes and extracellular amastigotes by Alamar blue
To evaluate the viability of T. cruzi epimastigotes or extracellular amastigotes, the Alamar blue method was used. Epimastigotes (5 × 106 parasites/well in LIT culture medium) were seeded in 96-well culture plates. TNKSi were added at the indicated concentrations and incubated for 96 h at 28°C. In the case of extracellular amastigotes, parasites were incubated in LIT medium in the presence of the drugs for 48 h, following the procedure reported by Takagi et al. (Reference Takagi, Akutsu, Doi and Furukawa2019). After incubations, resazurin solution (final concentration 10 μg mL−1) was added to each well and fluorescence was measured after 2 h in a Synergy HTX multi-mode microplate reader (Biotek Instruments, Winooski, USA) using the 530–560 nm emission and 590 nm excitation filters. Each condition was tested in triplicates and in at least three independent experiments.
Vero cell viability assessment by Alamar blue assay
Vero cells were seeded in 96-well culture plates (104 cells/well in 100 μL MEM-10% FBS). After 24 h, Tankyrase inhibitors were added to cell monolayers, incubated for 96 h and processed as described above. Each condition was tested in triplicates and in at least three independent experiments.
Trypomastigotes motility assessment
Trypomastigotes from the Tulahuen β-galactosidase strain obtained from the supernatant of infected Vero cell cultures were resuspended in fresh MEM-3% FBS to a concentration of 1 × 106 parasites mL−1 and incubated for 5 h at 37°C in the presence of TNKSi. After the incubation, motile parasites were counted under light microscope using a Neubauer chamber. The percentage of motile trypomastigotes was determined by considering the number of motile trypomastigotes at the start of the incubation as 100%. Each condition was tested in triplicates and in three independent experiments.
Immunocytofluorescence
Indirect immunocytofluorescence was carried as in Lafon-Hughes et al. (Reference Lafon-Hughes, Vilchez Larrea, Kun and Fernández Villamil2014) (Lafon-Hughes et al., Reference Lafon-Hughes, Vilchez Larrea, Kun and Fernández Villamil2014). Briefly, cells were seeded on glass coverslips in 24 well plates and subjected to the corresponding treatment or infection scheme. After fixation (PFA 4% β-catenin for or PFA 3%-glutaraldehyde 0.25% for TNKS), permeabilization and blocking, cells were incubated with 1:1000 anti-β-catenin (abcam 32572, Abcam, Cambridge, UK), 1:400 anti-TNKS (GTX117417, GeneTex, Irvine, CA, USA) or 1:500 anti-T. cruzi mouse serum, kindly provided by Dr Karina Gómez. After washing, samples were incubated with the correspondent secondary antibody (1 h, RT) and/or cytopainter (ab176756, Abcam, Cambridge, UK). Nuclei were counterstained with DAPI and coverslips were mounted in Prolong Gold (Molecular Probes P36930, Eugene, OR, USA).
To evaluate PAR formation in amastigotes and trypomastigotes, parasites were collected from the supernatant of infected cultures, treated with the indicated drug for 2 h and fixed with PFA 4%. After fixation, parasites were adhered to poly-lysine coated slides, permeabilized with PBS1x-Triton X-100 0.2% and blocked with PBS1x-Tween 0.05%-BSA 3%. Parasites were incubated with 1:100 PAR detecting reagent (MABE1031, Merck Millipore, Burlington, MA, USA) and 1:500 anti-T. cruzi mouse serum. After washing, samples were incubated with the correspondent secondary antibody (1 h, RT) and nuclei were counterstained with DAPI. Images were recorded with an Olympus BX61/FV300 (Tokyo, Japan) with a Plan Apo 60×/1.42 NA oil immersion objective microscope. Original images were taken in the same conditions as reference images of controls without primary antibodies at the same microscopy session. All images in each experimental series were taken with the same setting at the same confocal session. If modified, all were subjected to the same degree of brightness/contrast adjustment and Gaussian blur filtering, including the control without a primary antibody. The ImageJ free software (https://imagej.nih.gov/ij/download.html) was used for image processing.
Subcellular fractionation
Vero cell subcellular fractions at different times p.i. were obtained using two different commercial subcellular fractionation kits. For the obtention of nuclear, cytoplasmic, internal membrane and cytoplasmic membrane fractions in TNKS localization experiments, the Plasma Membrane Protein Extraction kit (101Bio, Mountain View, CA, USA, cat# P503) was used, following the manufacturer's instructions. For the obtention of nuclear, cytoplasmic and cell membrane fractions in β-catenin relocalization experiments, the ProteoJET kit (Fermentas, Vilnius, Lithuania, cat# K0311) was used, following the manufacturer's instructions. Three independent experiments were performed.

Fig. 1. TNKS inhibitors diminished T. cruzi Tul β-gal infection in Vero cells without affecting host cells viability. (A) Experimental schedule. Vero cells were seeded and infected the following day (MOI: 10:1) in the presence of the indicated concentrations of TNKSi. After 24 h, parasites were removed and fresh medium with TNKSi was added. (B) Amastigote load in Vero cells was quantified 144 h after cell seeding (96 h p.i.) by β-gal activity assay and expressed as % control infection. Mean ± standard error is shown. Data are from four independent experiments in triplicate. (C) The effect of TNKSi on uninfected Vero cell viability after 96 h was quantified by Alamar blue assay. Mean ± standard error is shown. Data are from three independent experiments in triplicate. *P < 0.05, **P < 0.01 according to one-way ANOVA and Fisher's LSD test.

Fig. 2. Effect of TNKSi on TcPARP activity and on the viability of axenic T. cruzi cultures. (A) PAR synthesis in amastigote and trypomastigote forms was not diminished by the presence of 0.8 μ m FLALL9, 0.6 μ m MN64 nor by 1.1 μ m XAV939, as evidenced by ICF using an anti-PAR reagent (green). DAPI (blue) was used to stain nuclei and anti-T. cruzi serum (red) was used to delineate parasites. Olaparib (0.025 μ m), a known PARP-1 and TcPARP inhibitor, reduced PAR formation in both T. cruzi forms. Bar: 10 μm. (B) Quantification of PAR intensity in amastigotes treated with a PARP-1 or with TNKS inhibitors, relative to parasites in the absence of inhibitors, using ImageJ software. Mean ± standard error is shown. Data correspond to at least 30 individual parasites per condition. *P < 0.05 according to one-way ANOVA and Dunnett statistical tests. (C) TcPARP activity in epimastigotes, evaluated by Western blots with anti-PAR reagent in response to 300 μ m H2O2 (10 min) was not affected by the presence of 0.6 μ m MN64. (D) Extracellular amastigotes (1 × 106 parasites/well) were incubated in a 96-well plate in the presence of TNKSi or benznidazole (BZ) during 48 h and their viability was assessed by Alamar blue. (E) Trypomastigotes (1 × 106 parasites mL−1) obtained from infected cell culture supernatants were incubated in a 96-well plate in the presence of TNKSi or BZ for 5 h, after which motile trypomastigotes were counted in a Neubauer chamber. (F) Exponential growth phase epimastigote cultures (5 × 106 parasites/well) were incubated in a 96-well plate in the presence of TNKSi for 96 h and their viability was assessed by Alamar blue. Results shown in D, E and F are mean ± standard error from three independent experiments, each performed in triplicates and analysed by one-way ANOVA and Dunnett statistical tests. *P < 0.05.
Epimastigote protein extraction
Epimastigotes were cultured in LIT media to a 1–2 × 107 parasites mL−1 density. Parasites were collected by centrifugation and resuspended in lysis buffer (10 mm HEPES, pH 7.9, 10 mm KCl, 0.1 mm EDTA, 0.1 mm EGTA, 1 mm dithiothreitol, 0.5 mm phenylmethylsulfonyl fluoride) after which they were sonicated (1 pulse 5 s) to complete lysis. Protein concentration was determined by Bradford.
Western blot
Total protein extracts (30 μg per lane) were subjected to 10% SDS-PAGE. Proteins were blotted onto nitrocellulose membranes by semi-dry transfer and membranes were blocked and incubated with corresponding primary antibody overnight at 4°C, followed by incubation for 1 h (RT) with secondary antibody diluted in the same buffer. Western blots were developed using the Western Lightning Plus ECL Substrate (Perkin Elmer, Waltham, MA, USA) and images were taken using the GeneGnome XRQ Chemiluminescence imaging system (Syngene, Cambridge, UK). The antibodies used were: 1:2000 anti-TNKS (GTX117417, GeneTex, Irvine, CA, USA), 1:2000 anti-Lamin A/C (STJ93885, St John's Laboratory, London, UK), 1:2000 CALR3 (STJ190646, St John's Laboratory, London, UK), 1:5000 anti-PMCA (kindly gifted by Dr Gerardo Corradi, IQUIFIB-CONICET, Buenos Aires, Argentina), 1:6000 anti-β-catenin (ab3572, Abcam, Cambridge, UK), 1:1000 anti-CyclinD1 (sc-718, Santa Cruz Biotechnologies, Dallas, TX, USA), 1:1000 anti-c-Myc (sc-764, Santa Cruz Biotechnologies, Dallas, TX, USA). For PAR detection, the pan-PAR-detecting reagent MABE 1031 (Merck Millipore, Burlington, MA, USA) was used (1:4000).
TNF-α expression quantification by RT-qPCR
Total RNA was extracted from Vero cells at 0 or 24 h p.i., in the presence or absence of 0.6 μ m MN64 using Quick-zol reagent (Kalium Technologies, Bernal, Buenos Aires, Argentina), following the manufacturer's instructions. The integrity of the extracted RNA was evaluated by agarose gel electrophoresis and the amount of RNA was quantified by measuring absorbance at Abs 280 nm, using DS-11 Spectrophotometer (DeNovix, Wilmington, USA). cDNA was generated using the EasyScript Reverse Transcriptase kit (Transgen Biotech, Beijing, China). Gene expression was evaluated by real-time PCR using Mezcla Real-qPCR kit (Biodynamics, Buenos Aires, Argentina) in a RotorGene 6000 (Corbett, Germantown, USA). The primers used were Tnfa 5′-TCCCTCTTCAAGGGCCAAGG-3′(Forward) and 5′-TGGGCTCATACCAGGGCTTG-3′(Reverse); gapdh 5′-CCTCCTGCACCACCAACTGC-3′(Forward) and 5′-TTCTGGGTGGCAGTGATGGC-3′(Reverse). Cycling conditions were: 95°C for 10 min, followed by 40 cycles of 95°C 15 s, 54°C 30 s for Tnfa and 58°C 30 sec for gapdh, and 72°C 15 s. Expression was normalized to the GAPDH (gapdh) and expressed in relation to control conditions in the absence of infection. Specificity was verified by performing melting curves after each amplification.
TNF- α capture ELISA
Vero cells were seeded in six-well plates and infected with T. cruzi Tulahuen trypomastigotes, in the presence or absence of XAV939 1.1 μ m. Supernatants were collected 24 h p.i., centrifuged briefly to remove trypomastigotes, and analysed for TNF-α by capture ELISA using the OptEIA™ Human TNF ELISA Set (BD, San Diego, CA, USA, cat 555212), following the manufacturer's instructions. Quantification of TNF-α was performed by comparison against a recombinant hTNF-α standard curve.
Statistical analyses
The values obtained for the β-galactosidase infection experiments, viability determinations by Alamar blue, PAR staining, β-catenin nuclear staining, cMyc and Cyclin D1 Western blot band intensity quantification, qRT-PCR and colorimetric capture ELISA were compared by using Student's t-test or a one-way ANOVA when more than two means were considered. Calculations were carried out using GraphPad Prism.
Results
Tankyrase inhibitors diminished T. cruzi infection in Vero cells in vitro without affecting host cell viability
As a first approach to test the possible participation of host cell Tankyrases in T. cruzi infection, the effect of a panel of six TNKSi (see Fig. S1 for structures) was evaluated on T. cruzi infection in Vero cells, according to the experimental schedule displayed in Fig. 1A. The inhibitors were tested at concentrations corresponding to 100× the in vitro IC50 reported values (Table 1) (Fig. 1B).
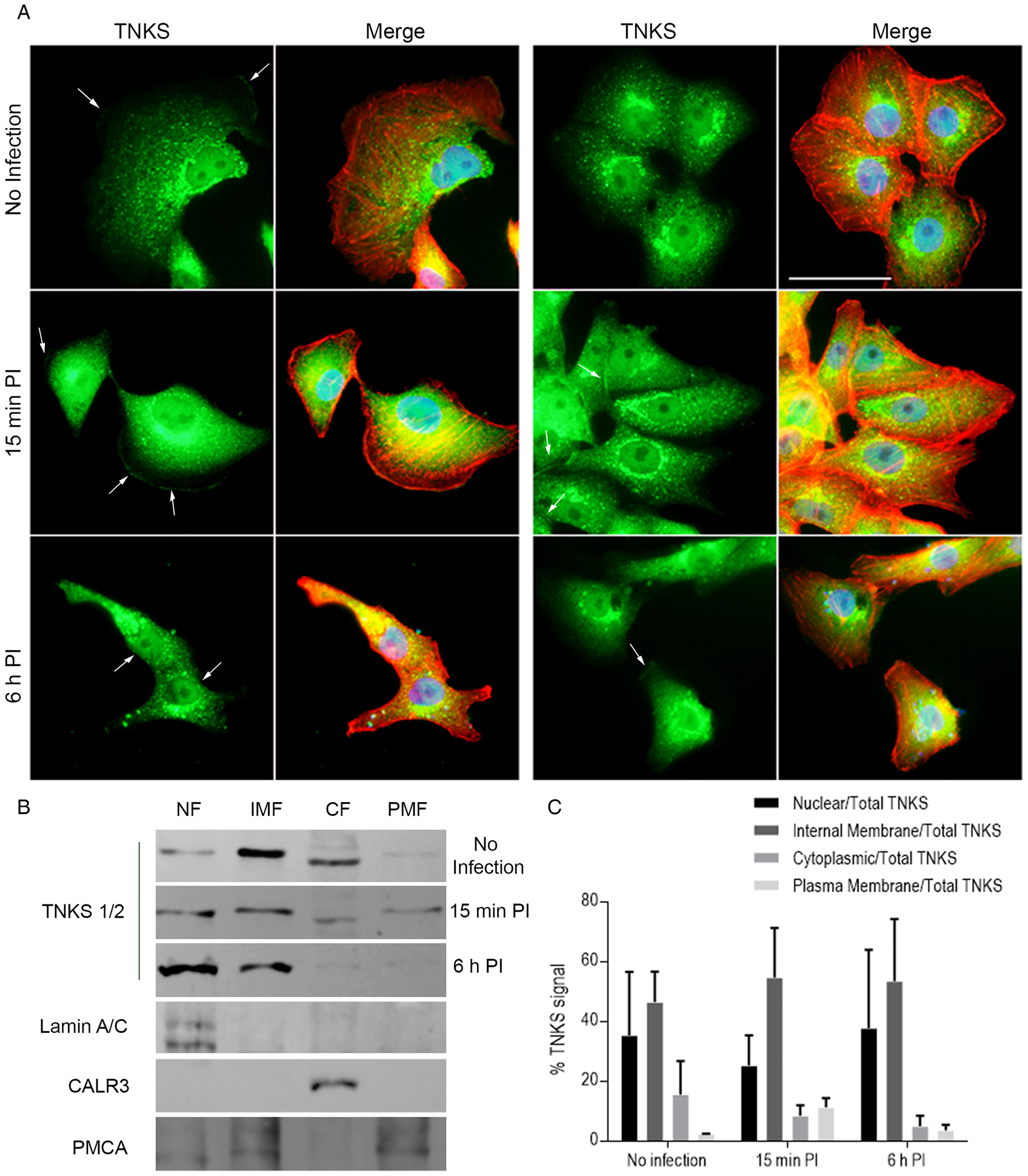
Fig. 3. Vero cell TNKS shifted towards the plasma membrane 15 min post-infection. (A) TNKS localization during initial steps of T. cruzi infection (0, 15 min and 6 h p.i.) in Vero cell monolayers was assessed by ICF using an anti-TNKS antibody (green). Nuclei were stained with DAPI (blue) and actin cytoskeleton was detected using Phalloidin (red). White arrows indicate TNKS detected at plasma membrane stretches. Bar: 50 μm. (B) TNKS subcellular localization was evaluated in nuclear (NF), cytoplasmic (CF), internal membrane (IMF) and plasma membrane fractions (PMF) by Western blot using anti-TNKS antibody at 0, 15 min and 6 h post-infection. The fractionation procedure was validated using the correspondent fraction markers, namely lamin A/C, calreticulin A (CALR3) and PMCA, for NF, CF and PMF, respectively. Representative result from two independent experiments. (C) Relative quantification of TNKS signal in the subcellular fractions over total TNKS (calculated as percentage) as evaluated by Western blot. Data from two independent experiments expressed as mean ± s.d.
Five out of the six inhibitors tested showed statistically significant decreases in the infection levels as measured by β-galactosidase activity at ~100×IC50 concentration (Fig. 1B). The largest effect was exerted by 0.8 μ m FLALL 9, which reduced infection by 39.49% compared to the infection in the absence of any compound. The rest of the drugs diminished the infection by around 30% (MN64: 32.01%, XAV 939: 35.01%, G007LK: 29.24% and OULL 9: 31.98%).
The number of cell-associated parasites was also quantified at 1 h p.i. on Vero cell monolayers that were pre-treated with 0.8 μ m FLALL 9 or 0.6 μ m MN64, as indicated in the experimental schedule in Fig. S2A. In these experiments, TNKSi were removed prior to the addition of T. cruzi trypomastigotes. Infected or uninfected Vero cell monolayers were thoroughly washed, fixed and stained using an anti-T. cruzi serum to detect trypomastigotes, which were counted under the fluorescence microscope. The number of cell-associated parasites was significantly diminished (43.96% reduction) in the presence of MN64 (Fig. S2B). The addition of FLALL 9 also caused a slight decrease (21.66%) in the amount of T. cruzi parasites adhered to and/or internalized in Vero cells, but the difference was not significant.
The observed reduction in the infection levels could be due to a decrease in the trypomastigote invasion and/or amastigote replication success but could also be explained by the loss of viable cells in the culture. To test the latter scenario, the possible toxicity of these compounds on uninfected Vero cells was evaluated using the Alamar blue method. None of the compounds at the concentrations here tested affected the growth or viability of Vero cells in our experimental conditions. Concentrations above 100 × IC50, however, reduced the viability of host cells in our model and were therefore not used for further experiments. These results indicated that the observed decrease in the infection levels at the concentrations previously tested could not be attributed to a reduction in host cell viability (Fig. 1C).
Tankyrase inhibitors did not hamper PAR synthesis in T. cruzi nor affect parasites viability
Next, it was evaluated if TNKSi were mitigating the infection of Vero cells by directly targeting the parasites or if the observed reduction of the infection was mainly due to the inhibition of host cell Tankyrases. In contrast with higher eukaryotes, T. cruzi bears only one known PARP (TcPARP) (Fernández Villamil et al., Reference Fernández Villamil, Baltanás, Alonso, Vilchez Larrea, Torres and Flawiá2008). Although a search was conducted on the available T. cruzi genomic databases using sequences of full-length human TNKS-1/2 or its catalytic and ankyrin-repeat domains as baits, no positive hits were found. We have previously reported that TcPARP is susceptible to known PARP-1/2 inhibitors but not to the Tankyrase inhibitor IWR-1, and only partially to 1 μ m XAV939 (Vilchez Larrea et al., Reference Vilchez Larrea, Haikarainen, Narwal, Schlesinger, Venkannagari, Flawiá, Villamil and Lehtiö2012). To corroborate that the compounds here used were not targeting TcPARP, the effect on PAR levels in trypomastigotes and extracellular amastigotes of FLALL 9, MN64 and XAV939, which showed the largest effects against infection, was tested. PAR levels were evaluated by immunofluorescence using a pan-PAR detecting reagent (Gibson et al., Reference Gibson, Conrad, Huang and Kraus2017) and quantified using ImageJ software. TNKSi FLALL 9 and MN64 did not cause any observable reduction in the amount of PAR present in amastigotes or trypomastigotes, as shown in Fig. 2A and B. In fact, XAV939 appeared to cause an increase in the detected PAR intensity (34% higher than untreated amastigotes). Olaparib, a well-studied PARP-1 inhibitor which we have previously demonstrated to inhibit recombinant TcPARP (Vilchez Larrea et al., Reference Vilchez Larrea, Haikarainen, Narwal, Schlesinger, Venkannagari, Flawiá, Villamil and Lehtiö2012), diminished PAR signal associated to amastigotes under the same incubation scheme by 29% when compared to parasites in the absence of any drug or treatment. The synthesis of PAR in epimastigotes in response to a genotoxic stimulus was evaluated by Western blot, in the presence or absence of MN64, one of the TNKSi that displayed the most pronounced infection diminutions. No differences in the intensity of PAR signal were observed between the hydrogen peroxide-stimulated epimastigotes in the presence of 0.6 μ m MN64 when compared to epimastigotes in the absence of TNKSi, indicating that overall TcPARP activity was not affected by this TNKSi (Fig. 2C).

Fig. 4. TNKSi diminished β-catenin nuclear translocation that occurred 6 h post-infection in Vero cells in response to T. cruzi Tul β-gal. (A) β-catenin subcellular localization was assessed by ICF, using an anti-β-catenin antibody (green), DAPI nuclear counterstain (blue) and phalloidin-546 F-actin probe (red) in (left to right) control Vero cells, T. cruzi infected, and infected in the presence of 0.6 μ m MN64. (B) Nuclear and total β-catenin signal on randomly chosen immunocytofluorescence images was quantified as RawIntDen using ImageJ software. The nuclear/total ratios obtained were normalized to values obtained for samples in the absence of infection or TNKSi. Mean ± standard error is shown. Data are from >100 cells. *P < 0.05 according to Student's t-test. (C) Western blot against β-catenin done on subcellular fraction extracts in control Vero cells, T. cruzi-infected (6 h post-infection) and T. cruzi-infected cells in the presence of 1.1 μ m XAV939. The fractionation procedure was validated using fraction markers: lamin A/C (nuclear fraction; NF), PMCA (membrane fraction; MF) and calreticulin A (cytoplasmic fraction; CF). (D) Relative quantification of nuclear β-catenin and membrane-associated β-catenin over total β-catenin, normalized to the corresponding fraction marker. Data are from three independent experiments. (E) Relative quantification of Western blot band intensity of c-Myc and (F) CyclinD1, normalized to GAPDH, in the presence or absence of infection and/or 1.1 μ m XAV939 (total extracts obtained at 24 h p.i). Mean ± standard error is shown. Data shown in E and F are from five independent experiments. *P < 0.05, according to one-way ANOVA followed by Fisher's LSD multiple comparison test.
Although TNKSi were not inhibiting PAR formation according to our results, the possibility that the TNKSi could still be affecting parasites' viability, therefore affecting Vero cell infection, needed to be considered. Hence, the effect of the compounds above tested was evaluated on different life cycle stages of T. cruzi parasites in axenic cultures.
Amastigote viability was not significantly diminished by any of the TNKSi, except for FLALL 9 (Fig. 2D). However, the viability decreased only by 8.2%, below the reduction of the infection levels of Vero cells. Benznidazole (BZ), the most employed drug to treat Chagas disease patients, was used as a control and decreased amastigotes viability by 65%. None of the TNKSi reduced trypomastigote motility either, while BZ diminished the percentage of motile parasites significantly (only 51.6% trypomastigotes retained motility when compared to controls) (Fig. 2E). None of the TNKSi showed toxicity on T. cruzi epimastigotes at the concentrations here tested either (Fig. 2F). Altogether, our results suggested that TNKSi were not decreasing infection levels by actioning on the parasites directly but rather by interfering with the functioning of the host cell.
Tankyrase relocated to the vicinity of the host cell membrane during early infection
We have recently reported that TNKS associates to the plasma membrane and is responsible for the formation of a PAR belt associated to the F-actin belt (Vilchez Larrea et al., Reference Vilchez Larrea, Valsecchi, Fernández Villamil and Lafon Hughes2021). To test if this localization changed during T. cruzi infection, the subcellular localization of TNKS during the initial steps of this parasite's host cell invasion was assessed by immunocytofluorescence. In the absence of infection, TNKS was detected in several localizations: the strongest signal was observed in a polarized perinuclear location, most likely corresponding to Golgi/ER, but it was also detected as dispersed puncta in the cytoplasm and in the nucleus (Fig. 3A, upper panels). TNKS was also detected associated to short stretches of plasma membrane (white arrows). Shortly after placing the trypomastigotes in contact with the cells, during the cell invasion step (15 min p.i.), a larger number of sections of plasma membrane associated to TNKS could be detected, which also appeared to be longer (Fig. 3A, middle panels). At 6 h post-infection, less plasma membrane-associated TNKS signals were detected, similarly to what was observed in the absence of infection (Fig. 3A, lower panels), suggesting the increase in plasma membrane localization was temporary. Western blot experiments on nuclear, cytoplasmic, internal membrane and plasma membrane fractions of Vero cells, obtained at 0, 15 min and 6 h p.i. supported the results obtained by ICF (Fig. 3B and C). Under control conditions (no infection), TNKS was mainly associated to the internal membrane and nuclear fractions. At 15 min p.i., TNKS signal associated to the plasma membrane fraction increased (from 2.4% in the absence of infection to 11.4% at 15 min p.i.), while the association to nuclear fraction diminished. After trypomastigote internalization (6 h p.i.), association to this fraction decreased (3.8%) while the association to the nuclear fraction increased (Fig. 3C). These results indicated that TNKS could be responding to T. cruzi cell invasion by changing its localization to the cell membrane, where it could exert particular functions.
β-catenin nuclear translocation in response to T. cruzi infection was reduced in the presence of TNKSi
Wnt/β-catenin signalling acts as a modulator of the inflammatory response triggered by T. cruzi infection in bone marrow-derived macrophages and supports intracellular parasite replication (Volpini et al., Reference Volpini, Ambrosio, Fozzatti, Insfran, Stempin, Cervi and Motran2018). Since TNKS is associated to the positive regulation of canonical Wnt signalling in other cellular models (Yang et al., Reference Yang, Tacchelly-Benites, Wang, Randall, Tian, Benchabane, Freemantle, Pikielny, Tolwinski, Lee and Ahmed2016; Mariotti et al., Reference Mariotti, Pollock and Guettler2017), β-catenin subcellular localization at 0 and 6 h p.i. was evaluated in the absence or presence of TNKSi by indirect immunocytofluorescence (Fig. 4A and B) and Western blot after subcellular fractionation (Fig. 4C and D).
Quantification of the nuclear/total β-catenin signal on ICF evidenced β-catenin nuclear accumulation in Vero cells 6 h post-infection (Fig. 4B). The TNKSi MN64 hampered the observed β-catenin translocation, diminishing β-catenin nuclear accumulation significantly.
β-catenin nuclear re-localization in Vero cells in response to T. cruzi infection was confirmed by Western blot on nuclear, cytoplasmic and cell membrane fractions (Fig. 4C). Relative quantification against the different subcellular fraction markers (Fig. 4D) showed that, while β-catenin association to the plasma membrane fraction appeared to decrease, its association to the nuclear fraction increased at 6 h p.i., supporting what was observed by ICF. This re-localization was consistently hindered by the presence of 1.1 μ m XAV939 across experiments, as nuclear β-catenin decreased, and membrane-associated β-catenin augmented. Although the results obtained by Western blot were not statistically significant, mainly due to variations in untreated, uninfected cultures, TNKSi diminished relative nuclear β-catenin signal during T. cruzi infection in all experiments performed.
Activation of β-catenin signalling and its translocation to the nucleus leads to the upregulation of target genes, such as c-Myc and CyclinD1 (MacDonald et al., Reference MacDonald, Tamai and He2009). Therefore, the nuclear accumulation of this protein observed during T. cruzi infection was expected to be accompanied by the increased expression of these proteins (Fig. 4E and F). Relative quantification of c-Myc on total Vero cell extracts obtained at 24 h p.i. showed that its expression was higher in infected cells than in uninfected cell cultures (2.38-fold increase). The presence of XAV939 reduced the expression of this protein significantly in infected cultures by 48% (Fig. 4E). Expression of CyclinD1 was also upregulated in our infection model (2.18-fold) and slightly decreased when infection occurred in the presence of XAV939 (12.26% reduction). These results show that TNKSi are effectively reducing β-catenin signalling during T. cruzi infection in Vero cells.
These results indicated that, similar to what occurs in macrophages (Volpini et al., Reference Volpini, Ambrosio, Fozzatti, Insfran, Stempin, Cervi and Motran2018), β-catenin nuclear concentration and its transcriptional activity in non-professional phagocytic cells increased in response to T. cruzi infection and that the inhibition of TNKS affected this behaviour negatively.
TNF-α increased during T. cruzi infection and was partially modulated by TNKSi
Since it was demonstrated that β-catenin signalling upregulated TNF-α expression during T. cruzi infection in BMM (Volpini et al., Reference Volpini, Ambrosio, Fozzatti, Insfran, Stempin, Cervi and Motran2018), the expression of this cytokine was tested by RT-qPCR in our model as a proof of concept. Cells were collected at 24 h post-infection, following the schedule in Volpini et al. (Reference Volpini, Ambrosio, Fozzatti, Insfran, Stempin, Cervi and Motran2018), in the presence or absence of MN64. TNF-α mRNA levels in Vero cells increased significantly during T. cruzi infection and 0.6 μ m MN64 led to a significant reduction in TNF-α mRNA levels, even below the ones detected in the absence of T. cruzi infection (Fig. 5A).

Fig. 5. Trypanosoma cruzi Tul β-gal infection induced expression of TNF-α, which decreased in the presence of TNKSi. (A) TNF-α mRNA level was evaluated by qPCR at 24 h p.i. in the presence or absence of 0.6 μ m MN64. Cytokine expression was normalized against GAPDH expression. Mean ± standard error is shown. Data are from three independent experiments in duplicate (B) TNF-α presence in the cell culture supernatant were evaluated by a colourimetric capture ELISA at 24 h p.i. in the presence or absence of 1.1 μ m XAV939. Sample absorbances (450 nm) were compared to a recombinant TNF-α curve. Mean ± standard error is shown. Data are from three independent experiments performed in triplicates. *P < 0.05; **P < 0.01, according to one-way ANOVA followed by Fisher's LSD multiple comparison test. Comparisons were done against the control in the absence of infection.
To corroborate the results obtained by RT-qPCR, the amount of TNF-α present in the cell culture supernatant was also quantified by capture ELISA on samples obtained at 24 h p.i., in the presence or absence of XAV939, another TNKSi that demonstrated efficacy against in vitro infection in Vero cells. Trypanosoma cruzi infection caused a mild but significant increase in the amount of TNF-α detected in the cell culture supernatant when compared to cultures in the absence of infection (3.46 vs 3.29 pg mL−1, respectively, p = 0.0033). The presence of the TNKSi XAV939 during T. cruzi infection reduced the amount of TNF-α in the culture to levels similar to the ones observed in the absence of infection (3.30 pg mL−1) (Fig. 5B). These results confirmed that TNKSi can hamper TNF-α expression during T. cruzi infection.
Because TNF-α role in the modulation of T. cruzi infection depends strongly – among other factors – on the host cell involved, we needed to confirm if this cytokine could modulate the infection levels in Vero cells. Exogenous addition of 20 ng mL−1 TNF-α markedly enhanced T. cruzi infection (Fig. S3). These results indicated that TNF-α facilitated T. cruzi infection in Vero cells at least in vitro, in agreement with what has been published for HEK293 T and LLC-MK2 cells (Pinto et al., Reference Pinto, Sales, Camargos and Silva2011). Therefore, as TNKSi reduced the expression and secretion of the pro-infection cytokine TNF-α in our model, this could be, in turn, reducing T. cruzi infection of Vero cells. These results could be hinting at one of the mechanisms that explains the ability of TNKSi to hinder T. cruzi infection in our model.
Discussion
In the present work, it was shown for the first time that inhibition of host cell Tankyrase hampered T. cruzi infection in vitro as well as nuclear accumulation of β-catenin and TNF-α expression, two signals associated to successful infections. Five out of six TNKSi here tested led to a significant decrease in the infection, with MN64 and FLALL 9 being the TNKSi that exerted the largest effect without affecting Vero cell viability at 100 × IC50 concentrations. A significant decrease in the number of cell-associated parasites was also observed when cells were incubated with MN64 only before initiating T. cruzi infection. However, parasite viability was not affected in a direct manner by most of the TNKSi here tested. Altogether, these results support that TNKSi-induced downregulation of the infection occurred through the modulation of host cell PAR metabolism and related signalling pathways.
Activation of Wnt/β-catenin pathway downregulates the activity of the β-catenin destruction complex. Upon Wnt activation, Axin is recruited to a plasma membrane signalosome complex and β-catenin is no longer targeted for degradation, enabling its accumulation in the nucleus (MacDonald et al., Reference MacDonald, Tamai and He2009). Nuclear β-catenin associates to TCF/Lef1 (T-cell factor/lymphoid enhanced factor1) and upregulates the expression of numerous genes (MacDonald et al., Reference MacDonald, Tamai and He2009). TNKS acts as a positive regulator of canonical Wnt signalling by destabilizing the β-catenin destruction complex mainly through the PARylation of Axin (Yang et al., Reference Yang, Tacchelly-Benites, Wang, Randall, Tian, Benchabane, Freemantle, Pikielny, Tolwinski, Lee and Ahmed2016; Mariotti et al., Reference Mariotti, Pollock and Guettler2017) or adenomatous polyposis coli (Croy et al., Reference Croy, Fuller, Giannotti, Robinson, Foley, Yamulla, Cosgriff, Greaves, Von Kleeck, An, Powers, Tran, Tocker, Jacob, Davis and Roberts2016). Recent findings in a T. cruzi bone marrow-derived macrophage (BMM) infection model show that β-catenin accumulates in the nucleus at 2–12 h post-infection and enhances the expression of classical targets of this pathway (Wisp1, Axin1, Ccnd1). Disturbing Wnt secretion through porcupine (PORCN) inhibition or β-catenin transcriptional activity inhibition in these macrophages diminishes the parasitic infection (Volpini et al., Reference Volpini, Ambrosio, Fozzatti, Insfran, Stempin, Cervi and Motran2018). In agreement with this report, β-catenin translocated to the nucleus at 6 h post-infection in our epithelial cell infection model. TNKSi counteracted this nuclear accumulation as well as the upregulation of genes known to be under transcriptional control of β-catenin (c-Myc and CyclinD1). Although the mechanisms through which TNKS regulates β-catenin nuclear accumulation during infection still need to be addressed, results reported for BMM (Volpini et al., Reference Volpini, Ambrosio, Fozzatti, Insfran, Stempin, Cervi and Motran2018) together with our findings suggest that TNKS could be targeted to disrupt Wnt/β-catenin signalling during T. cruzi infection. However, a crosstalk with other pathways modulating β-catenin translocation such as those activated by growth factors (Zhang et al., Reference Zhang, Zhang, Wang, Zhao, Zhang, Deng, Wu, He, Chen, Zhang, Wen, Liao, Zhang, Zhang, Liu, Yan, Luu, Haydon, Zhou and He2013) cannot be discarded and should be further explored.
Volpini et al. associated the reduction in T. cruzi infection of BMM caused by Wnt/β-catenin signalling inhibitors with a decrease in the replication rate of intracellular amastigotes (Volpini et al., Reference Volpini, Ambrosio, Fozzatti, Insfran, Stempin, Cervi and Motran2018). Although TNKSi exerted the largest detrimental effect on Vero cell infection when incubated during the whole infection cycle, a significant decrease in the number of cell-associated parasites was also registered at 1 h p.i. in the presence of MN64. This early effect might be indicating that Tankyrases are participating in early infection-triggered mechanisms during adhesion, signalling or invasion. In heart endothelial cells, YAP (Yes-associated protein) nuclear levels are altered during the early phase of T. cruzi Infection (Arun et al., Reference Arun, Rayford, Cooley, Rachakonda, Villalta, Pratap, Lima, Sheibani and Nde2020). In immortalized human Schwann cells, T. cruzi infection induces a robust activation of Akt (Chuenkova and PereiraPerrin, Reference Chuenkova and PereiraPerrin2009). Both YAP and Akt can be modulated by TNKSi (Kim, Reference Kim2018). It is therefore possible that TNKSi are also regulating YAP and Akt pathways in T. cruzi-infected Vero cells.
The observed reduction of T. cruzi infection caused by TNKSi could not be attributed to effects exerted on the parasite itself, as the drugs here tested were not cytotoxic to any of the parasite developmental forms. Trypanosoma cruzi bears a single classical PARP gene (TcPARP), structurally similar to hPARP-2, which was shown to be involved in the response to DNA damage and the regulation of the cell cycle (Fernández Villamil et al., Reference Fernández Villamil, Baltanás, Alonso, Vilchez Larrea, Torres and Flawiá2008; Vilchez Larrea et al., Reference Vilchez Larrea, Alonso, Schlesinger, Torres, Flawiá and Fernández Villamil2011). Hybrid or ‘TNKS-like’ PARP genes have been described in certain species (Citarelli et al., Reference Citarelli, Teotia and Lamb2010; Perina et al., Reference Perina, Mikoč, Ahel, Ćetković, Žaja and Ahel2014). An exhaustive search conducted on curated trypanosomes genomic databases (Berná et al., Reference Berná, Rodriguez, Chiribao, Parodi-Talice, Pita, Rijo, Alvarez-Valin and Robello2018) excluded the existence of TNKS or TNKS-like genes in trypanosomes. Although classical PARP-1 inhibitors affect TcPARP activity both in vitro and in vivo, TNKSi could not inhibit PAR formation in the different developmental stages of T. cruzi, indicating that the parasite's PARP was not affected by the TNKSi here used. This is in agreement with previous observations in which the TNKSi IWR-2 only marginally reduces TcPARP activity at 10 μ m while XAV393 only partially inhibits TcPARP at 1 μ m in vitro (Vilchez Larrea et al., Reference Vilchez Larrea, Haikarainen, Narwal, Schlesinger, Venkannagari, Flawiá, Villamil and Lehtiö2012).
The role of pro-inflammatory cytokines in T. cruzi infection depends on the host cell: while in macrophages and cardiomyocytes, cytokines such as TNF-α and INF-γ enhance anti-trypanocidal activity and reduce overall infection (Fichera et al., Reference Fichera, Albareda, Laucella and Postan2004; Volpini et al., Reference Volpini, Ambrosio, Fozzatti, Insfran, Stempin, Cervi and Motran2018), in astrocytes and epithelial cells (HEK293 T and LLC-MK2), these cytokines enhance T. cruzi infection, effect that could be counteracted by an anti-TNF antibody (Pinto et al., Reference Pinto, Sales, Camargos and Silva2011; Silva et al., Reference Silva, Mariante, Silva, dos Santos, Roffê, Santiago, Gazzinelli and Lannes-Vieira2015). In our Vero cell model, TNF-α exogenous addition increased T. cruzi infection (Fig. S3). Our results showed that T. cruzi induced TNF-α upregulation in Vero cells in an infection-promoting event. TNKS inhibition hampered the production of this cytokine and, in turn, negatively affected infection. The observed downregulation of TNF-α could partially account for the modulation of T. cruzi infection exerted by TNKSi.
TNKS was associated to various localizations in the host cell in basal conditions but appeared to shift towards the plasma membrane fraction during early T. cruzi infection (15 min). Recently, we described the existence of a PAR belt associated to the epithelial adherens junctions belt and subcortical actin in Vero cells (Lafon-Hughes et al., Reference Lafon-Hughes, Vilchez Larrea, Kun and Fernández Villamil2014), observation that was followed by the description of peripheral PAR localization in Schwann cells and oocytes (Lafon Hughes et al., Reference Lafon Hughes, Romeo Cardeillac, Cal Castillo, Vilchez Larrea, Sotelo Sosa, Folle Ungo, Fernández Villamil and Kun González2017; Xie et al., Reference Xie, Zhang, Zhao, Bai, Fan, Zhu, Yu, Li, Liang, Sun, Li and Qiao2018). Initial results pointed at TNKS as the possible PAR belt-synthesizing enzyme. Our recent report on the association of TNKS to the plasma membrane as well as the TNKS-mediated PARylation of vinculin supports those results (Vilchez Larrea et al., Reference Vilchez Larrea, Valsecchi, Fernández Villamil and Lafon Hughes2021). Both in Vero and in Schwann cells, PAR alterations are associated to actin cytoskeleton remodelling (Lafon-Hughes et al., Reference Lafon-Hughes, Vilchez Larrea, Kun and Fernández Villamil2014; Lafon Hughes et al., Reference Lafon Hughes, Romeo Cardeillac, Cal Castillo, Vilchez Larrea, Sotelo Sosa, Folle Ungo, Fernández Villamil and Kun González2017). Although the role of actin depolymerization during T. cruzi infection still remains controversial (Tardieux et al., Reference Tardieux, Nathanson and Andrews1994; Fonseca Rosestolato et al., Reference Fonseca Rosestolato, Da Matta Furniel Dutra, De Souza and Ulisses De Carvalho2002), TNKS recruitment to the plasma membrane during cell invasion by the parasite could be related to subcortical actin cytoskeleton remodelling during T. cruzi infection.
Although inhibitors with different potencies against TNKS-1 and TNKS-2 were employed, our results cannot discern the importance of each one during T. cruzi infection. A genetic approach, e.g. knockout by CRISPR/Cas9 or iRNA knockdown, could help to tackle this question. Both enzymes are remarkably similar, with 85% overall identity (Kaminker et al., Reference Kaminker, Kim, Taylor, Zebarjadian, Funk, Morin, Yaswen and Campisi2001), and it is yet unclear which of them is participating in the regulation of the Wnt/β-catenin signalling, although reported data suggest that both, TNKS-1 and TNKS-2, are playing redundant functions in the destabilization of the β-catenin destruction complex (Martino-Echarri et al., Reference Martino-Echarri, Brocardo, Mills and Henderson2016).
Evidence that Wnt/β-catenin signalling participates in the modulation of inflammation and infection-related processes is growing. For example, in epithelial cells, β-catenin can induce the secretion of pro-inflammatory cytokines (Moparthi and Koch, Reference Moparthi and Koch2019). Although T. cruzi infection induced TNF-α expression in our model and that this was downregulated by TNKSi, our results do not allow us to ascertain the connection between canonical Wnt signalling and the expression of this cytokine in this model. Evaluating the response to T. cruzi infection and TNKS inhibition of other inflammatory mediators and other β-catenin transcriptional targets could help clarify the relationship between the observed phenomena. Other mechanisms through which TNKS regulates T. cruzi infection also deserve further study.
The search for new drugs against Chagas' disease has been mainly focused on the exploration of potential therapeutic targets in the parasite itself (Beaulieu et al., Reference Beaulieu, Isabel, Fortier, Massé, Mellon, Méthot, Ndao, Nicoll-Griffith, Lee, Park and Black2010; Le Loup et al., Reference Le Loup, Pialoux and Lescure2011). However, given that the establishment of a successful infection relies on the triggering of a concerted network of signalling pathways inside the host cell, modulating these pathways can also become a valuable alternative in the quest for new effective treatments. Our results fit with recent evidence indicating that the interruption of Wnt/β-catenin signalling at different levels in the pathway can protect cell types from T. cruzi infection, at least in vitro (Volpini et al., Reference Volpini, Ambrosio, Fozzatti, Insfran, Stempin, Cervi and Motran2018).
Aiming at the development of new treatments against various types of tumours, several inhibitors targeting different steps in the Wnt/β-catenin pathway have entered clinical phase trials recently, with mixed results (Jung and Park, Reference Jung and Park2020).
A Tankyrase inhibitor, E7449, has entered into clinical trials with promising initial results regarding safety and tolerability (Plummer et al., Reference Plummer, Dua, Cresti, Drew, Stephens, Foegh, Knudsen, Sachdev, Mistry, Dixit, McGonigle, Hall, Matijevic, McGrath and Sarker2020). Therefore, efforts to understand the importance of TNKS and Wnt/β-catenin during T. cruzi infection can contribute to a drug repurposing strategy, shortening the time and diminishing the economic burden associated to de novo development of anti-parasitic drugs.
This work disclosed for the first time the anti-T. cruzi infection effects of TNKSi. Although several mechanisms may be involved, our results evidenced that TNKSi counteracted infection-induced signals: increase of nuclear-translocated β-catenin and expression of the cytokine TNF-α in Vero epithelial cells. We have previously reported that host PARP-1 and PARG inhibition or silencing diminished T. cruzi infection in epithelial cells (Vilchez Larrea et al., Reference Vilchez Larrea, Schlesinger, Kevorkian, Flawiá, Alonso and Fernández Villamil2013, Reference Vilchez Larrea, Haikarainen, Narwal, Schlesinger, Venkannagari, Flawiá, Villamil and Lehtiö2012). The results presented here indicate that host Tankyrases, also members of the PARP family, are engaged during T. cruzi infection too, adding evidence to support PAR metabolism as a piece of the signalling puzzle elicited in the host during this parasitic infection. Moreover, the promising entrance of TNKSi in clinical assays against tumours encourages further research that could eventually lead to TNKSi repurposing as anti-chagasic drugs.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182021001402.
Data
The authors confirm that all relevant data are included in the article and/or its supplementary information files.
Acknowledgements
The authors are grateful to Dr Lari Lehtiö for providing Tankyrase inhibitors and to Dr Carlos Robello for performing the detailed search for Tankyrases in the Trypanosoma cruzi genome database. We would also like to thank Dr Guillermo Alonso for carefully revising the manuscript.
Author contribution
L.L.H. and S.C.V.L. conceived, designed the study and conducted data gathering. L.L.H., S.H.F.V. and S.C.V.L. performed statistical analyses and wrote the article.
Financial support
This work was supported by Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina (Grant PCB-I. 2017-2019-SVL) and Agencia Nacional de Investigación e Innovación (ANII) Grant (|MOV_CO_2015_1_110430. 2017-2019 – LLH).
Conflict of interest
None.
Ethical standards
Not applicable.