INTRODUCTION
Loa loa is a human filarial parasite that affects about 3 million people living in the endemic regions of the central African rainforest belt (Fain, Reference Fain1981; Boussinesq, Reference Boussinesq2006). It is usually a mild disease. Common symptoms include pruritis, calabar oedema and eye problems caused by subconjunctival migration of adult worms. Loiasis is the third cause of rural hospital visits in endemic areas (Boulestiex and Carmes, Reference Boulestiex and Carme1986).
In endemic areas, microfilariae are found in about 30% of individuals, the remainder either being amicrofilaraemic or having occult loiasis (Van Hoegaerden et al. Reference Van Hoegaerden, Chabaud, Akué and Ivanoff1987; Dupont et al. Reference Dupont, Zué-Ndong and Pinder1988; Pinder et al. Reference Pinder, Leclerc and Everaere1992). Diagnosis based on classical microfilarial detection methods may therefore underestimate the prevalence of infection. Studies on the mechanisms of amicrofilaraemia have used crude extracts of different life-cycle stages, which makes the data difficult to interpret. Better-characterized antigens are needed to study L. loa-specific immune responses, but parasite material is difficult to obtain in sufficient quantities. One way to circumvent this problem is to clone and express the genes coding for L. loa proteins by using recombinant DNA technology. We have previously cloned a gene encoding a L. loa 15 kDa ladder antigen composed of several repetitive regions (Ajuh et al. Reference Ajuh, Akue, Boutin, Everaere and Egwang1995). Some repeats have strong homology with the Brugia polyprotein (Poole et al. Reference Poole, Grandea, Maina, Jenkins, Selkirk and McReynolds1992), while others, like the R3 region, seem to be L. loa specific (Touré et al. Reference Touré, Egwang, Wahl, Millet, Bain and Georges1997). Structurally, both the L. loa and the Brugia polyprotein show strong homology with ABA-1, the Ascaris allergen (Spence et al. Reference Spence, Moore, Brass and Kennedy1993). Although immunological studies have been performed on the Brugia polyprotein (Paxton et al. Reference Paxton, Yazdanbakhsh, Kurniawan, Partono, Maizels and Selkirk1993), they focused on the repeat region most strongly conserved among species. Interest in the nematode polyprotein is growing, for several reasons. First, it has been shown to be important for lipid transport (Timanova et al. Reference Timanova, Muller, Marti, Bankov and Walter1999). Second, it can induce immune protection (Vercauteren et al. Reference Vercauteren, Geldhof, Vercruysse, Peelaers, Van Den Broeck, Gevaert and Claerebout2004). Third, it can be used as a diagnostic tool (Touré et al. Reference Touré, Egwang, Wahl, Millet, Bain and Georges1997). Fourth, it is also involved in parasite evasion of the host immune system and in immunomodulation (Tezuka et al. Reference Tezuka, Imai, Muto, Furuhashi and Fujita2002). This molecule is therefore attractive as a target for chemotherapy, vaccination and diagnosis. The multiple roles of the polyprotein point to structural or subunit diversity. A sequence of L. loa genomic DNA cloned in a lambda gt11 library (Egwang et al. Reference Egwang, Pinder and Akue1990) presents interesting structural features, as it contains a polyprotein repeat subunit with strong homology to other nematode polyproteins, and other subunits with less homology to other nematode polyproteins, including subunits within the L. loa polyprotein family. This points to corresponding functional diversity. Yet there are few data on the L. loa polyprotein, and particularly the repeat region 3 (R3), which seems to be L. loa specific. Here we report preliminary immunological characterization of the recombinant L. loa repeat region R3.
MATERIALS AND METHODS
Plasmid DNA construction; expression and purification of the recombinant 15 kDa protein (15r3)
Genomic DNA (500 ng) extracted from L. loa by the phenol/chloroform method after parasite lysis at 65°C in buffer containing 50 mm Tris-HCl, pH 8, 100 mm NaCl, 50 mm EDTA, 1% SDS, 30 mm β-mercaptoethanol and 150 μg of proteinase K (Davis et al. Reference Davis, Dibner and Battey1986) was used for PCR with primers specific for the L. loa R3 repeat (based on the sequence reported by Ajuh et al. Reference Ajuh, Akue, Boutin, Everaere and Egwang1995) containing an additional EcoR1 cutting sequence, as follows: forward primer 5′-CTT AAG AAT CAG GCA AAT AAT GGC ACA AAA-3′, reverse primer 5′-CTT AAG CGT TTT CTT CTC ACC AGC TGT CT-3′. PCR was run under the following conditions: extensive denaturation at 97°C for 7 min; followed by 25 three-step cycles with denaturation at 94°C for 1 min; annealing at 60°C for 1 min; extension at 72°C for 2 min per cycle, and a final extension step of 10 min at 72°C. The product was cloned using TA cloning methods and the PCR 2·1 vector (Invitrogen). The clone containing the 396bp insert of L. loa was grown and the plasmid DNA isolated by routine mini-plasmid preparation methods. The L. loa 396-bp insert was cut out using EcoR1 and electrophoresed on 1·6% agarose gel, then purified with Geneclean (Biobrop) and subcloned into the pTrcHis expression vector digested with EcoR1 (Invitrogen). The resulting plasmid containing L. loa R3 was designated pTrcHis-15r3. Competent TOP10 E. coli cells were transformed with pTrcHis-15r3. Expression of the recombinant protein (15r3) was then induced with 0·1 mm isopropyl β-D-thiogalactopyranoside (IPTG) for 5 h, and the bacteria were harvested by centrifugation. The soluble bacterial extract was used for purification of the 15r3 recombinant protein, expressed as fusion polypeptides with 6 tandem histidine residues, in denaturing conditions, by nickel-chelating chromatography according to the manufacturer's instructions (Probond Resin, Invitrogen, Carlsbad, USA). The purity of the recombinant protein was checked by use of SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and Coomassie blue staining. The protein concentration of the eluate was measured with the Bio-Rad assay (Bradford, Reference Bradford1976).
Immunization of Balb/c mice with Loa loa recombinant fusion protein 15r3
Ten mice were immunized by intraperitoneal injection of SDS-PAGE slices containing the 15r3 protein emulsified in Freund's incomplete adjuvant. Each mouse received 500 μl of the suspension once a week for 3 consecutive weeks, and was then rested for 1 week before the last injection. Four non-immunized mice served as negative controls. Three days after the last injection, blood samples were collected by cardiac puncture and centrifuged for 5 min at 500 g. Sera were aliquoted and stored at −20°C until use. Specific IgG antibodies were quantified with a standard ELISA procedure.
Immunolocalization of the polyprotein subunit in adult Loa loa
Worms were cryofixed on copper grids at −190°C in a Leica EM CPC apparatus. They were then dehydrated and infiltrated with methanol and uranyl acetate according to the method of Edelmann and Ruf (Reference Edelmann and Ruf1996). Cryofixed worms were transferred to containers that were filled with increasing alcohol concentrations during a freeze-drying process, starting with 5 baths for 12 h each with methanol plus 0·5% uranyl acetate at −90°C, then 5 baths in pure methanol at −60°C for 3 h each; 4 baths of 1 h each in embedding medium composed of methanol mixed with Lowicryl K4M resin at −35°C, and a final 1-h bath in pure Lowicryl K4M resin. The following day the worms were transferred to embedding moulds filled with freshly made Lowicryl K4M resin, and polymerization was allowed to proceed under UV light (360 nm) for 72 h. The resin blocks were cut into sections. The presence of worms was verified on sections obtained with an ultramicrotome using a glass cutter, stained with toluidine blue and examined under a light microscope. Ultrathin sections obtained with a diamond cutter were placed on copper grids and used for immunolocalization, after drying. Immunolocalization with colloidal gold was performed according to the method of Kiss and MacDonald (1993) as follows. The copper grid containing worm sections was placed on Parafilm deposited in a Petri dish in humidified air at room temperature, and then incubated with 100 μl of phosphate-buffered saline (PBS), 0·685 mm NaCl, 1% BSA (buffer A) to block non-specific sites, for 90 min. This was followed by incubation for 90 min with either monospecific anti-15r3 or normal mouse serum in buffer A, followed by 20 rinses for 10 min each in buffer A. The sections were then incubated with 100 μl of goat anti-mouse antiserum coupled with 20 nm colloidal gold beads, diluted 1/40 in buffer A. This was followed by 10 rinses in buffer A and 5 rinses in 50 mm Tris-HCl, 0·685 mm NaCl, 1% BSA (buffer B). The complex was then fixed with 1% glutaraldehyde for 3 min, washed 10 times in PBS, and rinsed with distilled water. Contrast enhancement was obtained with 5% uranyl acetate for 20 min then lead citrate for 12 min. The specimens were then examined in an electron microscope.
Loa loa crude antigen preparation
Microfilariae were purified on Percoll gradients as described elsewhere (Van Hoegaerden and Ivanoff, Reference Van Hoegaerden and Ivanoff1986). L3 stages were obtained by dissection of naturally infected chrysops. Adult and L3 crude antigens were obtained by homogenization in PBS, while microfilariae were disrupted by sonication in PBS. All these antigens were boiled in SDS-PAGE sample buffer containing 2% SDS and 100 mm dithiothreitol in Tris-HCl buffer, pH 6·8, for 3 min and loaded on gels for electrophoresis.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)
Ten μg of detergent-soluble extracts of L. loa adult worm, microfilariae, and L3 or 15r3 were separated by standard SDS-PAGE (Laemmli, Reference Laemmli1970) on 15% acrylamide mini-gels (6×9 cm) in reducing conditions. Then 10 μg of each antigen was loaded in each well after boiling for 3 min in SDS-PAGE sample cocktail. Gels were run for 1 h at 200 volts at room temperature, and then the gels were stained with Coomassie blue, destained with a mixture of 7% acetic acid and 20% methanol, and dried.
Western blotting
After SDS-PAGE, the gels were equilibrated at room temperature in blotting buffer containing 25 mm Tris-HCl, 192 mm glycine and 20% methanol and proteins were electrophoretically transferred to nitrocellulose paper (NCP) as described elsewhere (Towbin et al. Reference Towbin, Staehelin and Gordon1979). The paper was stained with Ponceau S (Sigma Chemical Co., St Louis, MO, USA) to visualize the bands, cut into strips, and then blocked for 1 h in TBS-T-3% BSA. After 3 washes in TBS-T, each strip was incubated overnight at 4°C with mouse serum diluted 1/1600 in TBS-T-1% BSA. After washing as above, the paper strips were incubated for 1 h with anti-human IgG or anti-mouse IgG (Fc-specific) conjugated to alkaline phosphatase, diluted 1/1000 in TBS-T-1% BSA. This was followed by another washing step and incubation in the substrate solution containing 5-bromo-4-chloro-3 indolyl (BCIP, 0·015 mg/ml) and nitroblue-tetrazolium (NBT, 0·03 mg/ml) in 1 m Tris-HCl, 500 mm MgCl2 buffer, to visualize bound antibodies.
Human sera
Sera (Table 1) were obtained from 155 individuals of both sexes in an area of Gabon endemic for L. loa and Mansonella perstans, another filarial species. The individuals mostly originated from southeast of Gabon in Haut 0goue and the nearest province of Ogoué Lolo (Lastourville) where other filarial like O. volvulus and M. streptocerca are also prevalent. Their ages ranged from 6 to 85 years (mean 46 years). Of these subjects, 103 were amicrofilaraemic for L. loa, 4 were occult infected, 7 were endemic controls, while 41 had detectable L. loa microfilariae (mf) in their peripheral blood. M. perstans was present in 123 of the subjects. To study immune responses, the population was subdivided according to L. loa status, regardless of M. perstans status, as amicrofilaraemic (no microfilariae, but adult worms revealed by ocular passage), occult infected (clinical signs such as calabar oedema, without L. loa adult or microfilariae found), highly microfilaraemic (microfilaria >100/ml), weakly microfilaraemic (microfilaria <100/ml) and endemic controls (individuals with no evidence of L. loa infection despite long-term exposure). Other samples were obtained from people attending the hospital for extraction of ocular adult L. loa by an ophthalmologist. Others were obtained from individuals participating in filarial field surveys conducted in villages in the endemic region. Samples from individuals living in other parts of the world were also included, as follows: Comoros: 13 individuals with Wuchereria bancrofti microfilariae or immunochromatographic test (ICT) positivity; Yemen: 12 individuals with documented Onchocerca volvulus infection; Gambia/Senegal: 10 individuals with Mansonnella perstans monoinfection living in an area where L. loa is not endemic but where other filarial and intestinal nematodes are present; Europe: 10 European donors who were visiting filaria-endemic areas for the first time. All samples were obtained with the subjects' written informed consent, and the protocol was approved by the CIRMF's ethics committee.
Table 1. Characteristic of the study population

a Represents individuals positive by ICT test only.
b Except some zoonosis (Dirofilaria species …).
c Zero (0) is the number of occult or endemic controls in the sample.
d Absence of data (—).
e None existent in this region or sample (no).
Enzyme-linked immunosorbent assay (ELISA)
Specific anti-15r3 IgG was measured with a modification of the standard method (Engevall and Perlmann, Reference Engevall and Perlmann1971). Briefly, microtitre plates (Immunolon II, Dynatech Laboratories, Chantilly, VA) were coated with 100 μl/well of 15r3 at 10 μg/ml diluted in carbonate-bicarbonate buffer, pH 9·6. Another plate was coated with a control protein (histidine tag fused to bacterial protein). After overnight incubation at 4°C, the plates were washed 3 times for 10 min at room temperature with 50 mm Tris-HCl (pH 7·4), 200 mm NaCl and 0·05%Tween 20 (TBS-T). The plates were blocked with 200 μl/well of 5% BSA in TBS-T at room temperature for 1 h. After 3 washes in TBS-T, the plates were incubated with 100 μl of human serum diluted 1/5 with TBS-T containing 1% BSA (as determined by the checkerboard method). The plates were incubated for 1 h at room temperature and then washed 3 times with TBS-T. In another step, plates were incubated for 1 h with mouse anti-human IgG1 (HP 6012) 1/2000, IgG2 (HP 6014)1/2000, IgG3 (HP 6050) 1/1000 or IgG4 (HP 6025) 1/7500. This step was followed by 3 washes in TBS-T. Mouse anti-human IgG Fc-specific antiserum conjugated to alkaline phosphatase (Sigma, St Louis) was added at 1/1000 dilution and incubated for 1 h at room temperature. The plates were washed 3 times with TBS-T. The reaction was revealed with p-nitrophenyl phosphate (Sigma) diluted in diethanolamine buffer, pH 9·8, and optical density (OD) read at 405 nm with a Pasteur LP 400 reader. The background OD obtained with the histidine tag control protein was subtracted to give a net OD, used as a reactivity index for the filarial-specific antibodies. Total IgG against L. loa was detected by ELISA, with a crude extract of adult worms, as previously described (Akue et al. Reference Akue, Devaney, Wahl and Moukana2002).
Statistical analysis
Median IgG subclass titres in the different groups were compared using the non-parametric Mann-Whitney U test. Spearman's correlation test was used to identify correlations between specific IgG subclass titres and microfilarial density or IgG and age. Differences with P values <0·05 were considered significant.
RESULTS
Expression and purification of 15r3 protein
Expression of the recombinant 15r3 protein was induced with IPTG, and the kinetics of production was monitored in hourly E. coli extracts. A band of 25 kDa, not present before induction of E. coli cells containing pTrcHisA-15r3 plasmid (Fig. 1, line b), was seen 1 h after induction. The intensity of the band reached its maximum after 5 h (Fig. 1, line d). In an extract of E. coli containing non-expressing plasmid pTrcHisB-r3, no such band was seen either before (Fig. 1, line a) or after 5 h of induction (Fig. 1, line c). The 25 kDa product was eluted from E. coli lysates through a nickel column, which binds fusion proteins with a histidine tail (Fig. 1, line e). No such product was eluted from cell lysates containing the non-expressing pTrchisB-r3 plasmid.

Fig. 1 Expression and purification of pTrcHisA-15r3 from E. coli. Coomassie blue staining of a 15% SDS-PAGE gel showing the control plasmid pTrcHisB-r3 product before induction (a) and 5 h (c) after induction with IPTG; recombinant pTrcHisA-15r3 product before induction (b) and 5 h (d) after induction with IPTG; purified 15r3 fusion protein (e) eluted from the nickel column and loaded onto the gel (10 μl). The arrow on the right indicates the position of the 15r3 recombinant protein. Molecular weight markers are shown on the left in kDa.
Immunization of BALB/c mice and reactivity of anti-15r3 antibodies against native antigens from Loa loa developmental stages
All 10 mice immunized against the recombinant 15r3 antigen produced abundant antibodies. Their pooled sera were used to check for the existence of a native protein in crude extracts of different developmental stages of L. loa by Western blotting. The product obtained with the non-expressing plasmid pTrcHisB-r3 was used as a negative control. Negative mouse serum did not recognize any bands (Fig. 2, line a and c), whereas immunized mouse serum did not recognize the 25 kDa antigen in extracts of E. coli containing the non-expressing plasmid (Fig. 2, line b). IgG from immunized mice reacted strongly with the 25 kDa antigen (Fig. 2, line d). Furthermore, anti-15r3 from immunized mice strongly recognized a band at 38 kDa in adult worm extracts (Fig. 2, line f) and at 38 kDa and 20 kDa in microfilaria extracts (Fig. 2, line g), but not in L3 extracts (Fig. 2, line e).

Fig. 2. Reactivity of 15r3-immunized mouse serum (Western blot). PTrcHisB-r3 control protein (lanes a, b); pTrcHisA-15r3 fusion protein (lanes c, d); antigen extract from Loa loa L3 (e), adult worm (f) and microfilariae (g) were electrophoresed in 15% SDS-PAGE and transferred to nitrocellulose paper. Western blotting was performed using mouse anti-15r3 (lanes b, d, e, f, g) at 1/1600 dilution and normal mouse serum at the same dilution (lanes a, c). The position of the recognized antigens is marked with an arrow at 38, 25 and 20 kDa on the right, from the top to the bottom of the gel. Molecular weight markers are indicated on the left in kDa.
Ultrastructural location of the native molecule
With mouse anti-15r3 antibodies (Fig. 3 panel A), gold particles were seen in the cuticle (C) and, to a lesser extent, in the hypodermis (H). Many particles were located around the cortical layer between the hypodermis and internal structures. In contrast, no gold particles were seen in cross-sections of the same adult worm probed with control mouse antibodies or with gold-conjugated goat anti-mouse antiserum (Fig. 3 panel B).

Fig. 3. Immunolocalization of the Loa loa polyprotein subunit in an adult worm. Adult L. loa worms were cryofixed and cut into thin sections and then probed with mouse anti-15r3 antibodies diluted 1/100 and goat anti-mouse gold (20 nm)-conjugated antiserum diluted 1/40 (Panel A); or normal mouse serum plus goat anti-mouse gold-conjugated antiserum (Panel B). Arrows indicate gold spot. C, cuticle; H, hypodermis. Electron micrograph (×15 000 for anti-15r3 antibodies, ×12 000 for normal mouse antiserum).
Reactivity of human sera
15r3-specific IgG, IgE and IgM
All 155 individuals were first tested for total 15r3-specific IgG, IgM and IgE. No specific IgE or IgM was detected. Specific IgG was present, with no significant difference between amicrofilaraemic and microfilaraemic individuals (0·792 vs 0·786; Interquartile range (IQR): 0·767–0·624 respectively; P=0·822). Differences were found between European donors and both L. loa endemic populations (amicrofilaraemic 0·792 vs 0·246; IQR: 0·767–0·092 respectively; P=0·005; microfilaremic 0·786 vs 0·246; IQR: 0·624–0·092 respectively, P=0·003).
IgG subclasses
We examined the influence of parasitological status, age, microfilarial density and other infections on IgG subclass titres.
Influence of Loa loa infection status on IgG subclass titres
No significant difference was found between strongly and weakly microfilaraemic subjects, amicrofilaraemic subjects and endemic controls with respect to specific IgG1 (P=0·15), specific IgG3 (P=0·12) or IgG2 (P=0·08). No specific IgG4 was detected.
Influence of age
This analysis focused only on the population sampled in the same area and thus subject to the same transmission intensity (Akue et al. Reference Akue, Devaney, Wahl and Moukana2002). The population was split into 7 age groups, as follows: 6–10 years (n=9), 11–20 (n=13); 21–30 (n=19); 31–40 (n=8); 41–50 (n=13); 51–60 (n=7) and 61–80 years (n=18). No relationship was found between age and the titre of specific IgG1 (Fig. 4A-b) or specific IgG3 (Fig. 4D-b) [r=−0·005, n=87, P=0·967; r=0·120, n=86, P=0·270 respectively]. In contrast, the titre of specific IgG2 (Fig. 4C-b) increased from age 20 years to age 60 years, then declined or plateaued from 61 years onwards (r=0·240, n=87, P=0·025).

Fig. 4. Variation of anti-15r3 reactivity and total IgG subclasses according to age. Anti-15r3 (b) and anti-crude Loa loa extract (a) were measured by ELISA in an endemic Gabonese population aged from 6 to 80 years (age groups 0–10; 11–20; 21–30; 31–40; 41–50; 51–60; 61–80). Each dot represents the geometric mean for a given specific IgG subclass, and the two short bars on some curves indicate missing data for these age groups. Panel A=IgG1; B=IgG4; C=IgG2; D=IgG3.
Using the adult L. loa crude extract, the level of IgG1 (Fig. 4A-a) and IgG2 (Fig. 4C-a) were high but not related to age (r=−0·149, n=105, P=0·235; r=−0·051, n=105, P=0·685 respectively). The IgG4 titre (Fig. 4B-a) was high and age-independent (r=0·08, n=105, P=0·486), contrasting with the lack of specific IgG4 against the recombinant antigen 15r3 (Fig. 4B-b). Similar results were obtained for IgG3 (Fig. 4D–a) (r=0·169, n=105, P=0·179) although the level of this subclass was low.
Relationship between microfilarial density and 15r3-specific IgG titres
All 41 Gabonese microfilaraemic individuals were included in this analysis. No correlation was found between microfilarial density and the level of specific IgG1 (r=0·078; P>0·05) or IgG3. In contrast, the 15r3-specific IgG2 titre (Fig. 5) correlated with microfilarial density (r=0·400; P=0·02). No correlation was found between the levels of 15r3-specific IgG1 (r=0·080; P=0·1) or IgG2 (r=0·02; P=0·1) and M. perstans microfilarial density. Furthermore, the levels of specific IgG1 (P=0·080) and IgG2 (P=0·6) did not differ between M. perstans-positive and -negative individuals.

Fig. 5. 15r3-specific IgG2 levels measured by ELISA (expressed as OD) correlated with the number of microfilariae per ml in microfilaraemic individuals (n=41).
Anti-15r3 antibody cross-reactivity with other filarial species in human sera
Fourteen samples from Gabonese subjects (Fig. 6) were selected on the basis of L. loa monoinfection. These subjects had L. loa microfilariae or ocular passage of adult worms and no signs of co-infection by other filarial species such as M. perstans, which was highly prevalent in the same population (79%). Samples from individuals in other African countries (Gambia and Senegal) exposed to M. perstans and to other helminth infections were used as controls. Positivity for each subclass was assumed at a mean control optical density (OD) plus 2 standard deviations. As shown in Fig. 6, the mean level of 15r3-specific IgG1 was significantly higher in L. loa-monoinfected subjects than in Comorian, Yemeni and European donors (P=0·01) but not in Gambian/Senegalese controls. The level of 15r3-specific IgG2 was also significantly higher in the Gabonese subjects than in all the control groups, including Gambian and Senegalese individuals (P=0·01). Only Europeans had a significantly lower level of IgG3 than the Gabonese individuals (P=0·03).

Fig. 6. Cross-reactivity of anti-15r3 antibodies with filariae in human samples. The level of specific IgG1, IgG2 and IgG3 was measured by ELISA using recombinant antigen 15r3. Each dot represents the level of antibodies expressed as optical density (OD) minus background OD obtained with non-specific protein (pTrHisB-r3). Short horizontal bars represent the median values of the group. The long horizontal bar represents the positivity cut-off. Eur (European=no infection); Com (Comorian=W. bancrofti); Yem (Yemeni=O. volvulus), Gam (Gambian /Senegal=M. perstans and other helminths), Gab (Gabon=L. loa).
We then assessed the specificity and sensitivity of each 15r3-specific IgG subclass for L. loa infection with respect to other filarial (M. perstans, O. volvulus, W. bancrofti n=35) helminths and to uninfected controls (Table 2). IgG3 was the least sensitive (33%) and least specific (90%) IgG subclass, as 2 of the 10 samples from European donors cross-reacted with 15r3. IgG2 was the most specific subclass (100% vs uninfected sera, 91% vs other filarial infections). However, its sensitivity (70%) was lower than that of IgG1 (88%), which, in turn, was less specific than IgG2 (60% vs other filarial infections). Total IgG was less specific (70%) than any of the subclasses (3 of the 10 European donors cross-reacted with 15r3).
Table 2. Comparative specificity and sensitivity of various Loa loa specific IgG subclass detection test
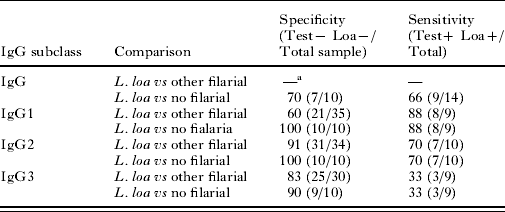
—a Not done.
DISCUSSION
We report the antigenic and immunogenic properties of a recombinant antigen corresponding to a specific region of the 15 kDa ladder of L. loa (Ajuh et al. Reference Ajuh, Akue, Boutin, Everaere and Egwang1995). The antigen is soluble and therefore suitable for use in immunoassays. Immunization of mice against the filarial recombinant protein 15r3 induced a strong humoral response, suggesting good immunogenicity. In addition, serum from humans infected naturally by L. loa reacted with the recombinant protein, suggesting its antigenicity. Immunolocalization showed that the L. loa polyprotein subunit was located in the cuticle and hypodermis, like an homologous antigen found in D. immitis (Poole et al. Reference Poole, Grandea, Maina, Jenkins, Selkirk and McReynolds1992). The antibodies produced in response to 15r3 recognized 2 major bands corresponding to 38 kDa and 20 kDa adult and microfilarial native antigens. The actual size of the native antigen recognized differed from the expected size, which increases in increments of 15 kDa per subunit (Ajuh et al. Reference Ajuh, Akue, Boutin, Everaere and Egwang1995). This difference may be explained by glycosylation of the native molecule (Ajuh et al. Reference Ajuh, Akue, Boutin, Everaere and Egwang1995), contrary to the recombinant molecule expressed in a prokaryotic cell.
The negativity of L3 extracts for anti-15r3 reactivity, despite the known distribution of the 15 kDa ladder among the different life-cycle stages of L. loa, raises the possible existence of several ladder repetitive antigen families in this species. The diversity of the polyprotein and the role played by the carbohydrate or possible post-translational modification of this protein are not known. Expression in a eukaryote vector might help to answer these questions.
In the absence of IgM and IgE responses to 15r3, we focused on the distribution of specific IgG subclasses. The parasitological status of individuals in L. loa endemic regions did not appear to influence this distribution. Age did not affect total or type 1, 2, 3 or 4 IgG levels. The observed differences might be due to variations in exposure to infective bites at certain ages or during specific activities. Paradoxically, IgG4 reacted strongly with the L. loa crude extract but not with the recombinant protein. Similar results were obtained in an endemic population in Cameroon, where a recombinant O. volvulus infective-stage antigen was recognized by IgG1, 2 and 3 but not by IgG4 (MacDonald et al. Reference MacDonald, Turaga, Harman-Brown, Tierney, Benett, Mc Cathy, McSimonek, Enyong, Mokatte and Lustigman2002). Specific IgG4 has been shown to be produced in abundance during chronic stimulation (Aalberse et al. Reference Aalberse, Van Der Gaa and Van Leeuwen1993), and its level has been shown to be high in L. loa microfilaraemic and amicrofilaraemic subjects (Akue et al. Reference Akue, Egwang and Devaney1994). However, like specific IgG4, IgG2 is considered as a blocking antibody, due to its weak capacity for complement fixation and monocyte binding. Dafa'Alla et al. (Reference Dafa'Alla, Ghalib, Abdelmageed and William1992) found a correlation between this subclass and the amicrofilaraemic state in onchocerciasis. Importantly, we found that specific IgG2 levels correlated with the density of Loa microfilariae, but not with M. perstans microfilariae, a species also prevalent in the study population. The correlation between L. loa microfilariae and anti-15r3 IgG2 is therefore specific to loaisis. The potential diagnostic utility of 15r3 was examined by analysing reactivity with IgG, IgG1, IgG2 and IgG3. Cross-reactions occurred with serum from individuals infected by other filariae or helminths and with some sera from European donors. This would lead to false-positive results in a diagnostic test. This problem occurred with IgG and IgG1, while IgG3 was the least sensitive subclass. However, IgG2 showed high specificity (91%) and promising sensitivity (70%). The latter could be improved by using new methods or antigens (Burbelo et al. Reference Burbelo, Roshan, Klion, Iadarola and Nutman2008). One alternative would be to use a cocktail of species–specific antigens in combination with other IgG subclasses (Klion et al. Reference Klion, Vijaykumar, Oei, Martin and Nutman2003).
These findings may have important implications for filariae control programmes now being implemented in Central Africa. Indeed, the risk of encephalopathy during ivermectin therapy for O. volvulus infection in L. loa co-infected subjects (Boussinesq et al. Reference Boussinesq, Gordon, Gardon-Wendel, Kamgno, Ngoumou and Chippaux1998) makes it important to develop a reliable test for L. loa before engaging in mass treatment campaigns for O. volvulus infection in this region.
ACKNOWLEDGMENTS
CIRMF is supported by the Gabonese State, Total-Gabon and Ministère Français de la Co-operation. We thank the Département d'Anatomie Pathologie, Faculté de Medicine, Université François Rabelais de Tours, for electron microscopy.










