Acute rheumatic fever is a non-suppurative complication of tonsillopharyngitis caused by group A β-haemolytic streptococci. Its clinical findings become evident when the immune response to streptococcal antigen affects the heart, joints, central nervous system, skin, and subcutaneous tissue. Acute rheumatic fever and rheumatic heart disease continue to cause a large global burden of morbidity and mortality, predominantly in low-income countries.Reference Watkins, Johnson and Colquhoun1
Jones criteria, which has been used in the diagnosis of the disease since 1944, was last updated in 2015 and acute rheumatic fever frequencies in various populations were classified into low, medium, or high risk followed by updates added to the diagnostic guideline that there should be some differences between them in terms of acute rheumatic fever diagnostic criteria.Reference Gewitz, Baltimore and Tani2 Other than the overt carditis, subclinical carditis detected by echocardiography was also regarded as a major finding in all populations upon new criteria.Reference Gewitz, Baltimore and Tani2 In all communities, a fever ≥ 38°C and erythrocyte sedimentation rate ≥ 30 mm/hour and a C-reactive protein value ≥ 3 mg/dl are regarded among minor criteria.Reference Gewitz, Baltimore and Tani2,Reference Eroğlu3
The frequency of acute rheumatic fever in our country is not known exactly and the available data are published as local studies. In the study conducted in Ankara from 1980 to 2009, the incidence of acute rheumatic fever was reported as 37/100,000 between 1980 and 1989, while it was 60/100,000 between 1990 and 1999 and 21/100,000 between 2000 and 2009.Reference Orün, Ceylan and Bilici4 According to revised Jones criteria, Turkey is among moderate- to high-risk communities in terms of acute rheumatic fever frequency.Reference Eroğlu3
In our study, we aimed to retrospectively evaluate the clinical and laboratory characteristics of patients with acute rheumatic fever who were hospitalised in our paediatric clinic between 2008 and 2018. Our secondary aim is to determine the frequency of subclinical carditis and the side effects of the drugs used in the treatment.
Materials and Methods
The study was designed as a retrospective and observational method. The data of all patients hospitalised with the diagnosis of acute rheumatic fever between January, 2008 and December, 2018 in the Pediatric Clinic were extracted from the patient files and recorded in the database created for the study. Ethics committee approval was obtained from the Dr Sadi Konuk Training and Research Hospital with the protocol number of 2018/104.
Database including age, gender, month of admission, year of admission, complaint on admission, history of upper respiratory tract infection, history of acute rheumatic fever in the family, examination findings, laboratory findings at the time of admission (C-reactive protein, erythrocyte sedimentation rate, leucocyte count, antistreptolysin O levels and anti-deoxyribonuclease B, in particular), telecardiography, electrocardiography, echocardiography, whether newly diagnosed or was a recurrent attack, duration of hospitalisation, treatment type, and need for intensive care were gathered from the medical records of patients and entered to the case forms.
During the 10-year period of the study, echocardiographic examinations were performed by same one experienced paediatric cardiologist with the device Vivid 7 Pro (GE Vingmed Ultrasound, Horten Norway) in all patients. The criteria for valvular regurgitation using orthogonal planes were a colour jet observed in at least two separate planes, a colour jet length of at least 1 cm, and a colour jet mosaic with a peak velocity greater than 2.5 m/s. The mitral regurgitation and aortic regurgitation detected by colour Doppler was considered grade 1 if the jet length was 1.5 cm; grade 2 if 1.5–2.9 cm; grade 3 if 3–4.4 cm; and grade 4 if 4.5 cm.Reference Otto and Otto5 Grade 1 was considered as mild, grade 2 as moderate, and grades 3 and 4 as severe regurgitation.Reference Helmcke, Nanda and Hsiung6,Reference Perry, Helmcke, Nanda, Byard and Soto7
The patients included in the study were diagnosed with acute rheumatic fever according to the Jones criteria modified by the American Heart Association in 1992 before 2015 and according to the revised Jones criteria after its revision in 2015.Reference Gewitz, Baltimore and Tani2,Reference Dajani, Ayooub and Bierman8
The distribution of the diagnosis frequency of the patients by seasons and years was examined. The relationship of gender and age with the frequency of major symptoms was evaluated. It was aimed to determine the frequency of subclinical carditis in addition to the distribution and severity of valve involvement in patients with carditis.
In patients with tachycardia and respiratory distress and left ventricular dilatation and significant valve insufficiency on echography, heart failure treatment was given upon the recommendation of a paediatric cardiologist. The distribution of the drugs used in the treatment and their side effects were evaluated. In the diagnosis of toxic hepatitis, the patients were evaluated and diagnosed by a paediatric gastroenterohepatologist. Patients whose aspartate aminotransferase and alanine aminotransferase values were 10 times normal, did not increase bilirubin, and no other viral agents were found in their examinations were considered as toxic hepatitis.
Statistical analysis
Descriptive statistics: categorical variables were given as numbers and percentages, numerical variables were given as mean, standard deviation, minimum, maximum, and median. The ratios of the categorical variable among the groups were tested with chi-square analysis with Fisher’s exact test. SPSS 15.0®IBM for Windows program was used for statistical analysis. p-Values of <0.05 were considered statistically significant.
Results
During the study, medical records of 102 patients with complete data were included to the study and data forms were filled up. While 56.9% of the patients included in the study were male (n = 58), the mean age of the children was 10.7 ± 1.9 years (range 5–15 years, shown in Fig 1). In the family history of the patients, it was found that 12.7% (n = 13) had acute rheumatic fever in the first- and second-degree relative.

Figure 1. Distribution chart of patients according to the admission age.
The frequency of upper respiratory tract infection history within 1–5 weeks before admission was 39.2% (n = 40). Most of our patients (95.1%, n = 97) were hospitalised due to the first attack of acute rheumatic fever, only five of them (4.9%) were diagnosed with recurrent attacks.
Distribution of patients by seasons was as follows: spring (38.2%), winter (32.3%), summer (15.7%), and autumn (13.7%), respectively. According to the years of hospitalisation, highest number of patients were hospitalised in 2012, 2008, and 2018 (n = 15, n = 14, and n = 13, respectively) (Fig 2). The distribution of demographic and laboratory characteristics of children with acute rheumatic fever is presented in Table 1. The mean hospitalisation period of them was 9.2 ± 4.5 days (range 6–30 days). The mean, minimum, and maximum level and distribution of C-reactive protein, erythrocyte sedimentation rate, leucocyte count and antistreptolysin O values of our patients were shown in Table 1. Anti-deoxyribonuclease B levels measured were 547 U/ml and 624 U/ml (normal value <200 U/ml) in two patients. When complaints of cases on admission are evaluated, there was more than one symptom association in 35.3% (n = 36). The distribution of complaints in order of frequency was as follows: joint pain and swelling (arthritis) in 61.8% of cases, joint pain (arthralgia) in 25.5%, fever in 16.7%, and weakness in 12.7%. Choreiform movements were detected in 2.9% (n = 3, 2 boys and 1 girl). Most common associations noted in patients with two complaints were arthritis and fever in 6.9% (n = 7), joint pain and fever in 3.9% (n = 4) and oint pain with weakness in 3.9% (n = 4), respectively. The most common examination findings detected in patients at the time of admission were murmur in 64.7% (n = 66), arthritis in 61.8% (n = 63), and arthralgia in 25.5% (n = 26), respectively (Table 1).

Figure 2. Distribution chart of the patients according to the years of hospitalization.
Table 1. Distribution of demographic and laboratory characteristics of children with acute rheumatic fever

* There are patients with more than one complaint.
** One or more complaints of shortness of breath, chest pain, palpitations.
*** There are patients with multiple examination findings.
**** Other symptoms of cardiac involvement: Tachycardia, tachycardia, dysponea, orthopaedics, venous fullness, gallop rhythm, any or more of the signs of arrhythmia.
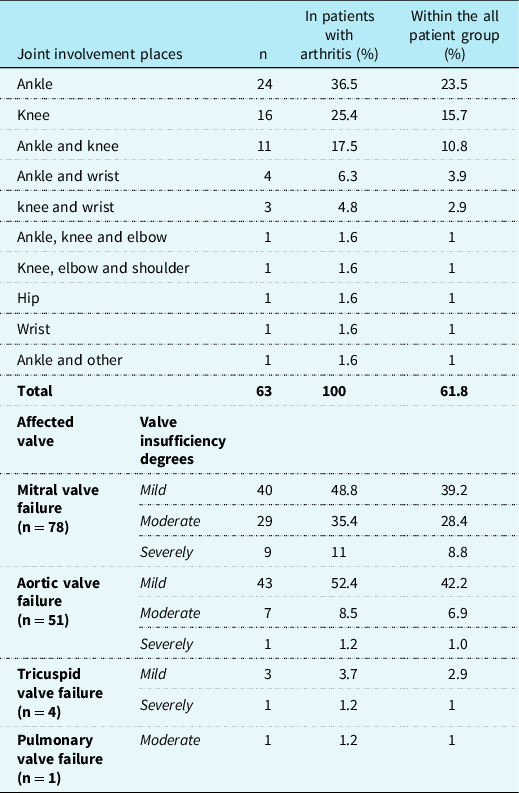
The distribution of joint involvement sites of patients with arthritis is presented in Table 2. It was observed that the most commonly involved joint was ankle in 65.1% followed by knee joint in 50.8% and wrist joint in 12.7%. Hip and shoulder involvement was rare. Telecardiograms of the patients were normal in 96.1% (n = 98) and cardiomegaly was detected in only four patients. Additionally, pathological electrocardiography findings were observed in 18 patients (17.6%). The most common electrocardiography finding was PR prolongation (n = 16, 15.7%). One patient had Mobitz type 2 block, and one patient had ST depression and T negativity. Carditis findings were detected in 80.4% (n = 82) of our patients via echocardiographic evaluation. Eleven of these patients (13.4% of carditis cases and 10.8% of all patients) were subclinical carditis cases without examination findings suggesting carditis. From the carditis patients, 41.5% (n = 34) had one valve involvement, 53.7% (n = 44) had two valves involvement, and 4.9% (n = 4) had three valves involvement, whereas two patients had signs of myocarditis in addition to valvular involvement (2.4% of carditis cases).
Table 2. Distribution of joint involvement sites of arthritis and degrees of valve involvement via echocardiography in children with acute rheumatic fever
In our study, the relationship of arthritis and carditis to gender and age was compared. It was found that the frequency of carditis was higher in female patients (88%) compared to male patients (74%) with a borderline statistical significance (p: 0.056). There was no statistically significant relationship between the gender and age distributions of patients with and without arthritis and those with and without carditis (p > 0.05). No subcutaneous nodules were detected in any of our patients, only one male patient had erythema marginatum that regressed within hours during hospitalisation.
When the echocardiographic findings of patients with carditis were evaluated in terms of valve involvement, it was noted that the most frequently involved valve was the mitral valve which was followed by aortic valve. Mostly, the involvement was at mild regurgitation level in both valves. The most rarely involved valve in our study was pulmonary valve. Table 2 shows the type of valve involved and the degrees of valve insufficiency according to the echocardiographic findings of patients.
The treatment methods applied to patients are evaluated; paracetamol (15 mg/kg/dose, maximum of 60 mg/kg/day, orally) was given to 21 patients for mild or moderate pain before confirming the diagnosis of acute rheumatic fever. Acetylsalicylic acid treatment (80–100 mg/kg/day) was applied to patients with only arthritis (n = 20) and mild carditis findings (n = 25, in 30.5% of carditis cases) (total n = 45, 44.1%). Since toxic hepatitis developed in four of these patients during the treatment, acetylsalicylic acid was ceased, and methylprednisolone treatment (0.8–1.6 mg/kg/day) was started. Along with these cases, 59 of the patients (57.8%, in 72.0% of carditis cases) diagnosed with carditis were treated with methylprednisolone. Anti-congestive therapy including furosemide (1–2 mg/kg/day) and/or angiotensin-converting enzyme inhibitor (enalapril 0.1 mg/kg/day) was required in 22% (n = 18) of cases with carditis (for patients with systemic ventricular dysfunction), whereas inotropic treatment was needed in 6.1% (n = 5) with carditis cases and paediatric intensive care required in four of them (4.9% in carditis patients and 3.9% of the entire patient group). None of the patients died. All of the patients diagnosed with chorea (n = 3) had carditis findings on echocardiography. In one of them, methylprednisolone treatment was given together with chorea treatment (haloperidol) because of high acute-phase reactants. One of the other two patients received only haloperidol (0.25–0.50 mg/day), and the other received valproic acid (15–20 mg/kg/day) and haloperidol. Aboriginal secondary prophylaxis treatment was initiated by administering Benzathine benzylpenicillin G (child ≤27 kg: 600,000 units; >27 kg: 1,200,000 units, intramuscular injection, single dose) at a dose suitable for their weight in all patients hospitalised with a diagnosis of acute rheumatic fever.
Recurrence attack was detected in five patients. The reason for recurrence in all five patients was non-compliance with secondary antibiotic prophylaxis and they were having their second episode. Of the recurrent cases, three were female and two were male. Carditis was detected in three patients during the second attack, while only one patient had cardiac involvement during the first attack.
Discussion
Although the incidence of acute rheumatic fever decreases with the improvement in living conditions and health services in developed countries, acute rheumatic fever is still the most important cause of acquired heart diseases in children and young adults in underdeveloped and developing countries. It is estimated that there are at least 15.6 million people with rheumatic heart disease in the world and 282,000 new cases are added to it each year, whereas 233,000 deaths are associated with it annually.Reference Khaled, Yosef, Petty, Laxer, Lindsley and Wedderburn9
The incidence of acute rheumatic fever differs by country as it has been reported having a frequency of 7.5 in Israel, 8.7 in India, 1.25 in Slovenia, and 4.1 in Italy per 100,000 people.Reference Vinker, Zohar, Hoffman and Elhayany10–Reference Breda, Marzetti, Gaspari, Del Torto, Chiarelli and Altobelli13 Ethnicity is also important in susceptibility to disease. In New Zealand, where acute rheumatic fever is common, the annual incidence varies greatly according to ethnic origin; It has been found to be 40.2/100,000 in Maori people, 81.2/100,000 in Pacific islands, and 2.1/100,000 in other races.Reference Milne, Lennon, Stewart, Vander Hoorn and Scuffham14 An incidence of 194/100,000 has been reported in Australian Aboriginal peoples.Reference Lawrence, Carapetis, Griffiths, Edwards and Condon15,Reference Karthikeyan and Guilherme16 A limited number of studies conducted in our country reported that the incidence of acute rheumatic fever is decreased as 21/100,000 over time.Reference Orün, Ceylan and Bilici4,Reference Karademir, Demirçeken, Atalay, Demircin, Sipahi and Teziç17 It is known that the frequency of acute rheumatic fever does not differ between genders.Reference Watkins, Johnson and Colquhoun1,Reference Karthikeyan and Guilherme16 However, in our study, the male sex ratio was slightly higher, and similar to our study, there are studies reporting a higher rate in male gender.Reference Özer, Olgu Hallıoğlu, Özkutlu, Çeliker, Alehan and Karagöz18,Reference Bostan and Çil19
The frequency of acute rheumatic fever is high between the ages of 6 and 15 years, as in group A β-haemolytic streptococci pharyngitis.Reference Watkins, Johnson and Colquhoun1,Reference Kumar, Sharma and Thakur11,Reference Karthikeyan and Guilherme16,Reference Bostan and Çil19 Approximately 5% of all cases are children younger than 5 years of age.Reference Dajani, Ayooub and Bierman8 The age distribution and average of our patients were compatible with the literature data and we did not find acute rheumatic fever in a child younger than 5 years of age in our study. The history of upper respiratory tract infection within 1–5 weeks is important in patients with acute rheumatic fever. In studies from Turkey, the presence of a history of upper respiratory tract infection in 1–5 weeks is reported at a rate of 39–41%.Reference Özer, Olgu Hallıoğlu, Özkutlu, Çeliker, Alehan and Karagöz18,Reference Bostan and Çil19 Similarly low rates were found in our study. The low rate of previous upper respiratory tract infection may be attributed to the inability of the patient and their relatives to remember the infection attack due to the asymptomatic time between infection and acute rheumatic fever symptoms.
In the incidence studies of acute rheumatic fever, it was found that some cases had recurrent attacks. In the studies of Özer et alReference Özer, Olgu Hallıoğlu, Özkutlu, Çeliker, Alehan and Karagöz18, it was reported that 11 (8.9%) of 129 patients diagnosed with acute rheumatic fever had recurrent attacks and the reason for recurrence in 10 patients was non-compliance with secondary antibiotic prophylaxis in Turkey. In the same study, it was shown that the incidence of carditis was higher (at a rate of 90%) in patients with recurrent acute rheumatic fever attacks.Reference Özer, Olgu Hallıoğlu, Özkutlu, Çeliker, Alehan and Karagöz18 In our study, five patients were diagnosed with recurrent acute rheumatic fever attacks and the reason for recurrence was non-compliance with secondary antibiotic prophylaxis in all patients.
The incidence of acute rheumatic fever in the Northern hemisphere varies seasonally. While the highest frequency is observed in winter, it can be encountered in spring, autumn, and rarely in summer in order of frequency.Reference Çağatay, Yıldız, Temel, Arslan and İnalhan20,Reference Pirinççioğlu, Alyan and Kanğın21 This is due to the higher prevalence of streptococcal pharyngitis in the Northern hemisphere in winter, spring, and autumn.Reference Khaled, Yosef, Petty, Laxer, Lindsley and Wedderburn9 Also in our study, it was observed that most of our patients were diagnosed in the winter and spring months.
It has been reported that in children diagnosed with acute rheumatic fever, an elevation of C-reactive protein is observed at a rate of 72–100% and the rate of high erythrocyte sedimentation rate was found to be between 73% and 88.8% during the acute period.Reference Özer, Olgu Hallıoğlu, Özkutlu, Çeliker, Alehan and Karagöz18–Reference Pirinççioğlu, Alyan and Kanğın21 Similarly, high rates of C-reactive protein value and high sedimentation rate were found during the acute period in our study.
Antistreptolysin O elevation can be detected in 83% of patients with acute rheumatic fever.Reference Watkins, Johnson and Colquhoun1 There are studies reporting that the antistreptolysin O cut-off value is 200 Todd units/ml and 320 Todd units/ml.Reference Özer, Olgu Hallıoğlu, Özkutlu, Çeliker, Alehan and Karagöz18,Reference Çağatay, Yıldız, Temel, Arslan and İnalhan20,Reference Pirinççioğlu, Alyan and Kanğın21 The mean antistreptolysin O value was found to be 295 ± 92 Todd units/ml in the first episode acute rheumatic fever patients and 301 ± 83 Todd units/ml in the recurrent acute rheumatic fever cases, and according to the cut-off values of 200 and 320 Todd units, the frequencies of high antistreptolysin O values were reported as 86 and 48.6%, respectively.Reference Pirinççioğlu, Alyan and Kanğın21 The average antistreptolysin O value (1009 ± 644 U/ml) and antistreptolysin O elevation rates (antistreptolysin O value was >300 U/ml in 96.1% and >400 U/ml in 85.3%) of the patients in our study population were higher.
It should be kept in mind that acute rheumatic fever may rarely present with findings that suggest gastrointestinal system disease such as abdominal pain and vomiting. This may cause some difficulties to diagnose it in patients without joint complaints. In a similar study, complaints at presentation were evaluated in children with acute rheumatic fever, and the frequency of abdominal pain was found to be 13.3%.Reference Pirinççioğlu, Alyan and Kanğın21 Although at a lower rate, the presence of gastrointestinal findings in three patients in our study supported this observation.
In our study, when the examination findings of patients on admission are evaluated; the rate of arthritis is similar to literature knowledge.Reference Özer, Olgu Hallıoğlu, Özkutlu, Çeliker, Alehan and Karagöz18,Reference Bostan and Çil19 However some studies have reported higher rates of arthritis (81.4–91.4%).Reference Çağatay, Yıldız, Temel, Arslan and İnalhan20–Reference Karaaslan, Oran, Reisli and Erkul22 It is known that acute rheumatic fever arthritis primarily affects large joints such as the knee, ankle, wrist, and elbow joints.Reference Watkins, Johnson and Colquhoun1 A study inquiring joint distribution in children with acute rheumatic fever reported that knee joint involvement was found at a rate of 64.4%, ankle joint involvement at a rate of 62.2%, and wrist joint involvement at a rate of 33.3%.Reference Çağatay, Yıldız, Temel, Arslan and İnalhan20 Our study was compatible with the literature in terms of the most commonly involved joints.
Electrocardiography findings play an important role in the diagnosis of acute rheumatic fever. It is known that the rate of PR interval prolongation as an electrocardiography finding in the acute period in children is between 7.45 and 17.7%.Reference Özer, Olgu Hallıoğlu, Özkutlu, Çeliker, Alehan and Karagöz18,Reference Çağatay, Yıldız, Temel, Arslan and İnalhan20,Reference Pirinççioğlu, Alyan and Kanğın21 Our study revealed that almost one-fifth of the cases had pathological electrocardiography findings.
Traditionally, rheumatic heart disease was diagnosed by auscultating for a heart murmur in those with a history of acute rheumatic fever. Currently, echocardiography is mandatory in all patients with suspected or definite acute rheumatic fever, as echocardiography is more sensitive and specific than auscultation for acute rheumatic carditis. The World Health Organization has recommended echocardiographic screening for rheumatic heart disease in the high-prevalence regions in 2004.23,Reference Reményi, Wilson and Steer24 These screening programmes have been demonstrated that the necessity of distinguishing physiological and pathological echocardiographic findings. In 2012, the World Heart Federation published standardised screening guidelines and minimal criteria for a diagnosis of rheumatic heart disease on echocardiography.Reference Reményi, Wilson and Steer24 Three categories are defined on the basis of assessment by 2D, continuous-wave, and colour Doppler echocardiography: “definite rheumatic heart disease”, “borderline rheumatic heart disease”, and “normal”. In addition, the morphological features of rheumatic heart disease and the criteria for pathological mitral and aortic regurgitation are defined.Reference Reményi, Wilson and Steer24
In studies in the incidence of acute rheumatic fever, the rate of carditis is between 44.4 and 65% in the acute period.Reference Özer, Olgu Hallıoğlu, Özkutlu, Çeliker, Alehan and Karagöz18–Reference Karaaslan, Oran, Reisli and Erkul22 It was determined that the rate of carditis was higher in our study, which suggests that this situation may have resulted from the fact that the patients with carditis findings were referred to our institution as the reference hospital in the region. In a review evaluating the results of 23 studies in which children diagnosed with acute rheumatic fever were evaluated, it was reported that subclinical carditis was found in 11.9–21.6% of children with acute rheumatic fever.Reference Tubridy-Clark and Carapetis25 Our study pointed out that subclinical carditis develops in a significant portion (10.8%) of the patients diagnosed with acute rheumatic fever. These rates show the importance of performing echocardiographic examination in patients without clinical signs of carditis.
The valves most frequently affected by acute rheumatic fever are mitral and aortic valves, respectively. Tricuspid and pulmonary valves are rarely affected. In most cases, the mitral valve is affected together with one or more of the other cardiac valves.Reference Khaled, Yosef, Petty, Laxer, Lindsley and Wedderburn9 The distribution and frequency of valve involvement we found in our study were consistent with the reported studies.Reference Özer, Olgu Hallıoğlu, Özkutlu, Çeliker, Alehan and Karagöz18–Reference Karaaslan, Oran, Reisli and Erkul22 In most patients with carditis in acute rheumatic fever, valvular insufficiency is mild or moderate, as in our study. These patients are usually treated with angiotensin-converting enzyme inhibitors and diuretics. Approximately 10–30% of patients have severe mitral insufficiency and 10% of them have heart failure.Reference Karthikeyan and Guilherme16
Salicylates (aspirin) have traditionally been recommended as first-line treatment, because of the extensive historical experience with their use in acute rheumatic fever and an established evidence base.Reference (ARF/RHD writing group)26 Non-steroidal anti-inflammatory drugs are recommended ahead of aspirin in children requiring anti-inflammatory treatment by rheumatic heart disease the 2020 Australian guideline (RHDAustralia).Reference (ARF/RHD writing group)26 Due to the rare possibility of Reye’s syndrome in children, aspirin may need to be ceased during intercurrent acute viral illness. Paracetamol may be used to relieve pain in children in the interim, or tramadol for more severe pain.Reference (ARF/RHD writing group)26 In our study (conducted between 2008 and 2018), anti-inflammatory treatment (acetylsalicylic acid or methylprednisolone) was initiated for all patients in accordance with their clinical and laboratory features (acetylsalicylic acid for arthritis and mild carditis, methylprednisolone for moderate and severe carditis). Four patient who was started acetylsalicylic acid treatment for mild carditis developed toxic hepatitis during treatment, which changed treatment to methylprednisolone. Based on this finding, it was noted that patients who started acetylsalicylic acid therapy should be carefully monitored clinically and laboratory for signs of acetylsalicylic acid toxicity.
Studies examining the risk factors affecting the development of carditis and arthritis in children with acute rheumatic fever have reported that gender and age distribution do not have a significant effect.Reference Özer, Olgu Hallıoğlu, Özkutlu, Çeliker, Alehan and Karagöz18,Reference Çağatay, Yıldız, Temel, Arslan and İnalhan20,Reference Pirinççioğlu, Alyan and Kanğın21 In our study, no significant relationship was found between gender and the incidence of arthritis. The incidence of chorea is reported to be between 2.2 and 17.9% in the diagnosis of acute rheumatic fever.Reference Özer, Olgu Hallıoğlu, Özkutlu, Çeliker, Alehan and Karagöz18–Reference Karaaslan, Oran, Reisli and Erkul22 In our study, the incidence of chorea was in the lower limits compared to the reported rates. The reason for this was that only inpatients were included in the study, and many chorea cases were followed up by outpatient treatment. Carditis was also present in all patients with chorea in our study. Although the high coexistence of chorea and carditis is consistent with general literature knowledge, number of our cases was insufficient for comparative analysis.
Study limitations
The fact that only one paediatric cardiologist evaluated all patients during the study increases the power of the study. On the other hand, the study was planned as a single centre, and it is not possible to generalise the data obtained to the whole country. Since our study was retrospective, complete information on known risk factors for acute rheumatic fever (socio-economic status, household overcrowding and background group A β-haemolytic streptococci levels) could not be reached. The study does not include long-term follow-up of patients causes insufficiency of data on recurrence frequency.
Conclusion
In a 10-year period, we evaluated 102 patients with diagnosed acute rheumatic fever. In our study, detection of subclinical carditis in 10.8% of all cases diagnosed with acute rheumatic fever supported that echocardiography should be performed as a standard method of diagnosis. It was also found that the frequency of erythrocyte sedimentation rate ≥30 mm/hour was 90.2% and that of C-reactive protein value ≥3 mg/dl was 96.1%, which were among the new minor criteria recommended for acute rheumatic fever diagnosis. In addition, patients should be followed closely in terms of hepatic toxicity due to acetylsalicylic acid used in the treatment.
Acknowledgements
None.
Authors’ contributions
Conceptualisation: Lida Bulbul and Canan Hasbal Akkus; Methodology: Mehmet Bedir Akyol and Canan Hasbal Akkus; Formal analysis and investigation: Mehmet Bedir Akyol and Hasret Ayyıldız Civan; Writing – original draft preparation: Gizem Kara Elitok and Lida Bulbul; Writing – review and editing: Hasret Ayyıldız Civan and Sami Hatipoglu; Resources: Sami Hatipoglu and Ali Bulbul; Supervision: Sami Hatipoglu and Ali Bulbul.
Financial support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflicts of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards and with the Helsinki Declaration of 1975, as revised in 2008, and have been approved by the institutional committees (Dr Sadi Konuk Training and Research Hospital ethic committee, with 2018/104 number).
Ethics approval
Ethics committee approval was obtained from the Dr Sadi Konuk Training and Research Hospital with the protocol number of 2018/104.







