INTRODUCTION
The increase of human activities in coastal and ocean areas around the world has put enormous pressure on marine biodiversity and ecosystems in general, particularly in coastal areas and the continental shelf (see Halpern et al., Reference Halpern, Walbridge, Selkoe, Kappel, Micheli, D'Agrosa, Bruno, Casey, Ebert, Fox, Fujita, Heinemann, Lenihan, Madin, Perry, Selig, Spalding, Steneck and Watson2012). Marine mammals are particularly susceptible to human activities such as fishing interactions, vessel strikes and pollution in all forms (Reeves et al., Reference Reeves, Smith, Crespo and di Sciara2003). Although the interaction with fisheries has been identified as the main problem for marine mammals around the world (Read et al., Reference Read, Drinker and Northridge2006; Reeves et al., Reference Reeves, McClellan and Werner2013), there is increasing concern about the impact of vessel strikes for both large and small cetaceans as well (Laist et al., Reference Laist, Knowlton, Mead, Collet and Podesta2001; Van Waerebeek et al., Reference Van Waerebeek, Baker, Félix, Gedamke, Iñiguez, Sanino, Secchi, Sutaria, Van Helden and Wang2007; Luksenburg, Reference Luksenburg2014). The impact of these activities may be severe and compromises the recovery of endangered species such as right whales (Eubalaena glacialis) in the western North Atlantic (Knowlton & Kraus, Reference Knowlton and Kraus2001) and blue whales (Balaenoptera musculus) in the eastern Pacific (Irvine et al., Reference Irvine, Mate, Winsor, Palacios, Bograd, Costa and Bailey2014). They cause significant reduction in populations as observed in the case of the vaquita (Phocaena sinus) from the Gulf of California Jaramillo-Legorreta et al. (Reference Jaramillo-Legorreta, Rojas-Bracho, Brownell, Read, Reeves, Ralls and Taylor2007) and in extreme cases the extinction of species as occurred with the baiji (Lipotes vexillifer) from the Yangtze River (Turvey et al., Reference Turvey, Pitman, Taylor, Barlow, Akamatsu, Barrett, Zhao, Reeves, Stewart, Wang, Wei, Zhang, Pusser, Richlen, Brandon and Wand2007).
Interactions with fishing gear and vessel collisions are not always fatal or provoke a later mortality that is difficult to quantify (Van Waerebeek et al., Reference Van Waerebeek, Baker, Félix, Gedamke, Iñiguez, Sanino, Secchi, Sutaria, Van Helden and Wang2007; Bechdel et al., Reference Bechdel, Mazzoil, Murdoch, Howells, Reif, McCulloch, Schaefer and Bossart2009). The interaction may result in mutilated appendages, disfigured fins and cutting wounds that penetrate the muscle and sometimes reach bone (e.g. Bloom & Jager, Reference Bloom and Jager1994; Fertl, Reference Fertl1994a; Wells & Scott, Reference Wells and Scott1997; Parsons & Jefferson, Reference Parsons and Jefferson2000; Baird & Gorgone, Reference Baird and Gorgone2005; Van Waerebeek et al., Reference Van Waerebeek, Baker, Félix, Gedamke, Iñiguez, Sanino, Secchi, Sutaria, Van Helden and Wang2007; Freitas et al., Reference Freitas Nery, Espécie and Marino Simão2008; Kiszka et al., Reference Kiszka, Pelourdeau and Ridoux2008; Bechdel et al., Reference Bechdel, Mazzoil, Murdoch, Howells, Reif, McCulloch, Schaefer and Bossart2009; Elwen & Leeney, Reference Elwen and Leeney2010; Byard et al., Reference Byard, Winskog, Machado and Boardman2012). Parallel and regularly spaced wounds most probably are caused by propellers, but in other cases the evidence is less clear and it is difficult to determine the origin. Photographic evidence of animals with large healed wounds on different parts of the body shows that cetaceans are highly resilient to those injuries (Zasloff, Reference Zasloff2011; Bossley & Woolfall, Reference Bossley and Woolfall2014). Such wounds may take several months to heal or may cause pain for weeks until the animals die (Corkeron et al., Reference Corkeron, Morris and Bryden1987; Dwyer et al., Reference Dwyer, Kozmian-Ledward and Stockin2014); consequently, this is an animal welfare issue as well.
For small and discrete populations as in the case of some coastal cetaceans, interactions with fishing gear and vessel strikes may have a devastating impact (Parsons & Jefferson, Reference Parsons and Jefferson2000), particularly in developing countries where interaction with fisheries appears to have worsened over time because small-scale fisheries are widely dispersed and not sufficiently monitored or regulated (Reeves et al., Reference Reeves, McClellan and Werner2013). According to the authors, the problem is of such magnitude that 61 of 74 (82%) of the odontocete species recognized and 13 of the 14 mysticete species (93%) have been reported as by-catch in some kind of fishing gear. In the case of vessel strikes, at least 18 species of small cetaceans have been observed as injured by different types of vessels around the world, the most affected being the bottlenose dolphin (Tursiops truncatus), the Indo-Pacific humpback dolphin (Sousa chinensis) and the Irrawaddy dolphin (Orcaella brevirostris) (Van Waerebeek et al., Reference Van Waerebeek, Baker, Félix, Gedamke, Iñiguez, Sanino, Secchi, Sutaria, Van Helden and Wang2007).
A population of coastal bottlenose dolphins inhabiting the inner estuary of the Gulf of Guayaquil, in the south-western part of Ecuador, has been studied for nearly three decades (Félix, Reference Félix1994, Reference Félix1997; Félix et al., Reference Félix, Calderón, Vintimilla and Bayas-Rea2017). This population, estimated by the early 1990s at 637 (95% CI 541–733) individuals, is organized in several partially discrete sub-units referred to as communities (sensu Wells et al., Reference Wells, Scott, Irvine and Genoways1987) that occupy home ranges extending between 20 and 30 km of coastline (Félix, Reference Félix1994). Recent population estimates indicate that some communities in the western part of the inner estuary have decreased about 50% on average over the past 25 years (Félix et al., Reference Félix, Calderón, Vintimilla and Bayas-Rea2017). By-catch and vessel strikes have been suggested among possible causes for population decline (Jiménez & Alava, Reference Jiménez, Alava and Samuels2014; Félix et al., Reference Félix, Calderón, Vintimilla and Bayas-Rea2017), however their effect has yet to be evaluated in depth. A preliminary assessment of the problem in the Gulf of Guayaquil based on information from the early 1990s mainly on the analysis of wounds and deformed dorsal fins, demonstrated a 2% incidence of animals affected (Van Waerebeek et al., Reference Van Waerebeek, Baker, Félix, Gedamke, Iñiguez, Sanino, Secchi, Sutaria, Van Helden and Wang2007). New information is now available allowing a better understanding of the extent of the issue.
This study aims to address in more detail the impact of some human activities on coastal bottlenose dolphin communities inhabiting the inner estuary of the Gulf of Guayaquil. The high incidence of scars and wounds upon free-ranging dolphins, as well as confirmed cases of dolphins entangled in fishing gear, demonstrate that dolphins have a high probability of suffering vessel strikes and interactions with fishing gear throughout their life. Thus, it seems that concerns about human activities as potential causes of population decline are well founded.
METHODOLOGY
The study area
This study was focused in the Gulf of Guayaquil, the largest estuary on the west coast of South America covering around 12,000 km2. It is located in the south-western part of Ecuador (centred on 3°S 80°55′W) (Figure 1). Geographically, the gulf may be described by an outer estuary west of Puna Island between 80°15′W and 81°W, and an inner estuary that extends nearly 130 km inland to the east and north-east of Puna Island (Stevenson, Reference Stevenson1981). The coast has a steady climate pattern characterized by a rainy and warm season (December–April) and a dry and cold season (May–November), with seawater temperatures fluctuating between 22 and 28°C. The northern inner estuary of the Gulf of Guayaquil is shaped by two large water bodies: the Estero Salado and the Guayas River, the main contributor of fresh water to the estuary. Both, the Estero Salado and the Guayas River run parallel in a north-easterly direction to Guayaquil City. The Estero Salado starts at the strait formed by the western side of Puna Island and the continent where a small fishing village named Posorja is located. Along the Estero Salado there are channels and numerous islands covered by mangroves. Main activities within the inner estuary are fishing, aquaculture and maritime transportation.

Fig. 1. The study areas located on the western side of the inner estuary of the Gulf of Guayaquil (Posorja-Puerto el Morro) and northern side (Salinas).
The northern gulf is drier and characterized by sandy beaches and low cliffs. On the northernmost tip is Salinas, the most important tourist centre in south-west Ecuador. Two small-scale fishing ports are located at Salinas, Santa Rosa and Anconcito, the former is the second largest small-scale fishing fleet of the country with 1400 boats, and the latter includes 600 boats and fishing vessels (Herrera et al., Reference Herrera, Castro, Coello, Saa and Elías2013).
Surveys
The information presented in this work was generated during a long-term study of coastal bottlenose dolphins in the inner estuary of the Gulf of Guayaquil. The study aims to assess their population status in terms of structure, and trends, as well as include socio-ecological aspects. A total of 117 trips were undertaken within the inner estuary (~4500 km2) between March 2011 and January 2017. In this period more than 6281 km were sampled and 373 hs spent in the field, including 91 h with direct observations of dolphins. Trips were made aboard either tourist (N = 63) or research dedicated boats (N = 54) between 6 and 10 m in length. Although sampling effort was concentrated on the western side of the estuary (Posorja, Puerto El Morro and Estero Salado), the north and east sides of Puna Island and the western side of the inner estuary around Bajoalto were also surveyed (Figure 1). Typical routes of tourist boats from Posorja consisted of short 10–20 km trips north-east along the coast where most fishing docks are located. Tourist boats from Puerto El Morro navigated along the El Morro channel and sometimes further reaching Posorja over longer trips (25–30 km). Research dedicated trips departed either from Posorja or Puerto el Morro and extended 94 km on average (45.5–153 km) along the Estero Salado, north-western side of Puna Island and west of Posorja.
Additionally, nine trips were conducted in 2016 at Salinas (northern part of the gulf), where a small community of dolphins has been intermittently monitored since 2005. From Salinas, trips extended for 587 km and 47.5 h, including both sides of the peninsula tip (Zavala, Reference Zavala2017). Finally, the sample includes an opportunistic sighting of a group of coastal bottlenose dolphins at Valdivia in June 2015, located 40 km north of Salinas.
During the surveys, sighting information about group size, group composition and dolphin behaviour was recorded. Digital cameras equipped with 70–300 mm and 100–400 mm lenses and HD video were used as part of standard field methodology. Some 15,000 photographs of sufficient quality (focus and appropriate perpendicular angle) were analysed. Photographs of dorsal fins were used for individual identification (see Würsig & Würsig, Reference Würsig and Würsig1977) and catalogues of identified dolphins was created. Currently the catalogues contain 196 individuals.
Dolphin population structure
Previous studies have demonstrated that coastal bottlenose dolphins have a continuous distribution along the coast of Ecuador and are organized in semi-discrete communities as observed elsewhere (Félix, Reference Félix1997). We identified seven different dolphin communities in the study area, five within the inner estuary (Bajoalto, East Puna Island, Estero Salado, Playas and Posorja), one in the northern border of the gulf (Salinas) and one at Valdivia. Population estimates for three of them have been recently obtained (Posorja, Estero Salado and Salinas) (Félix et al., Reference Félix, Calderón, Vintimilla and Bayas-Rea2017; Zavala, Reference Zavala2017) but for the other five only the total number of identified animals is provided (Table 1). Membership to a particular dolphin community was assigned taking into consideration both site fidelity and individuals’ association pattern (see Félix, Reference Félix1997; Félix et al., Reference Félix, Calderón, Vintimilla and Bayas-Rea2017).
Table 1. Scarring prevalence in five sites in the inner estuary of the Gulf of Guayaquil and in Valdivia on the south-western coast of Ecuador. Period 2011–2017.

Evaluation criteria of scars and wounds
Good quality photographs were used to assess missing dorsal fin tips, bent over dorsal fins, scars, wounds and any other form of trauma that dolphins had suffered in the dorsal area, following the criteria used previously in similar studies (e.g. Wells & Scott, Reference Wells and Scott1997; Visser, Reference Visser1999; Fertl, Reference Fertl1994b; Van Waerebeek et al., Reference Van Waerebeek, Baker, Félix, Gedamke, Iñiguez, Sanino, Secchi, Sutaria, Van Helden and Wang2007). Location of scars along the dorsal area, whether parallel or perpendicular to the animal axis, as a result of wounds caused by a severe trauma that penetrated the animal's body were labelled according to Figure 2. Visible scars were estimated to measure 20–60 cm in length; nonetheless, animals were able to recover completely. The scarring prevalence was estimated independently for each dolphin community as the proportion of animals with this type of wounds/scars.

Fig. 2. Body areas used to label where scars and wounds were located.
RESULTS
Scarring prevalence
Twenty-five of 189 catalogued animals (13.2%) showed some type of scar on the dorsal area consistent with injuries caused probably by either interaction with fishing gear or vessel strike (Table 1). Prevalence varied among sites, being very high in East Puna Island, Salinas and Valdivia (25–44.4%), high in Estero Salado and Posroja (14–15%) and mid in Bajoalto (7.1%). Scars were not evident in only one of the seven dolphin communities (Table 1).
Scars were classified in four types: dorsal fin tip missing, v-shaped, sawed edge and caudal deformity (Figures 3–7). There was one individual at Salinas and another one at Estero Salado with two different types of scars probably having originated from two different events (Table 2). The scarring location analysis showed differences among sites as well. At Posorja scars were located with similar frequency along the entire dorsal area, at Estero Salado scars were concentrated from the posterior lumbar to the posterior caudal region, and at Salinas they were located mainly in the posterior caudal area (Table 2).

Fig. 3. Scars and wounds recorded in bottlenose dolphins from Posorja community. Scars location: (A) thoracic area, (B, C) lower lumbar, (D) upper caudal region, (E) caudal area, (F) dorsal fin tip missing.
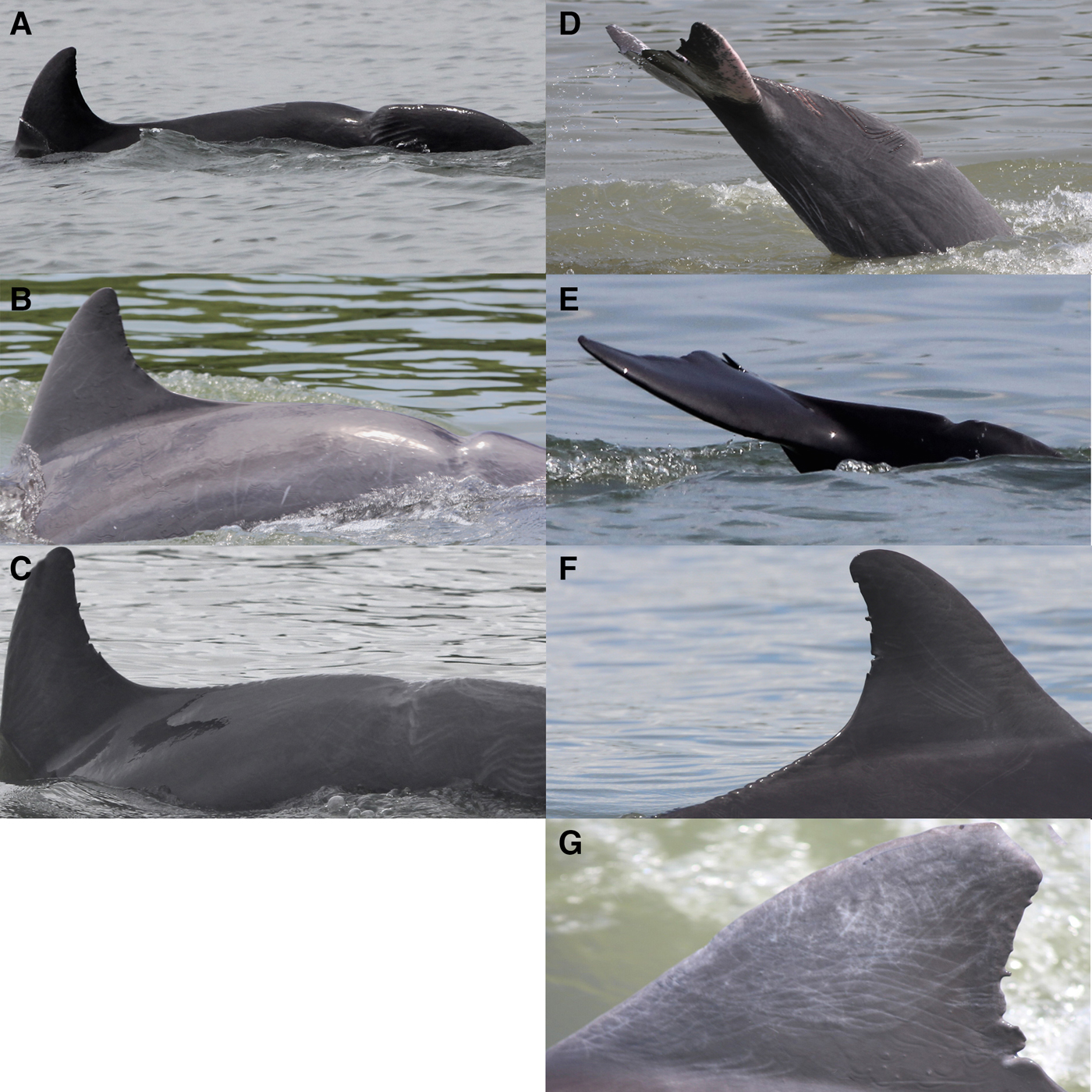
Fig. 4. Scars and wounds recorded in bottlenose dolphin from the Estero Salado community. Period 2011–2016. Scars location: (A–C) upper caudal, (D, E) lower caudal region, (F) sawed edge, and (G) dorsal fin tip missing.

Fig. 5. Scars recorded in four bottlenose dolphins photographed at Salinas in July 2016. Scars location: A thoracic, B–E lower caudal region.

Fig. 6. Dorsal fins with different deformities in dolphins found at Bajoalto.

Fig. 7. Dorsal fins of dolphins photographed from the beach at Valdivia.
Table 2. Location of scars and wounds (N = 16) in different parts of dolphins’ body in two bottlenose dolphin communities in the inner estuary of the Gulf of Guayaquil (Estero Salado and Posorja) and Salinas. Period 2011–2017.

a Includes two different types of scars in the same animal.
POSORJA
Four animals with large scars, one dorsal fin tip missing and one fresh wound in the peduncle/flukes area were recorded at Posorja (Figure 3) (Table 1). All animals were adults and some of them have been followed for more than 10 years. The individual in Figure 3A shows a j-shaped scar behind the head extending from the dorsal area down to the left flank. The other three animals show single v-shaped healed scars in transversal position to the animal axis behind the dorsal fin, two in the lower lumbar region and one in the upper caudal region (Figure 3B, C, D). The individual in Figure 3E shows multiple fresh parallel wounds on the anterior border of the right fluke (also present in the left one but not seen in the photograph) and a large wound along the upper part of the lower lumbar region.
ESTERO SALADO
Five animals with single v-shaped scars in the caudal region and one with dorsal tip missing were recorded in this dolphin community (Figure 4) (Table 1). All but one animal were adults (the one in Figure 4E is an immature). The scars suggest different original wounds; four are deep and wide scars penetrating several centimetres into the animal's body and it seems there was tissue lost with the trauma that caused the depression (Figure 4A, B, C, D), and one was less severe and shallower (Figure 4E). One individual had a v-shaped scar in the anterior caudal area and a sawed edge in the posterior lumbar area starting in the base of the dorsal fin (Figure 4C, F). Finally, a sixth animal showed a dorsal tip missing (Figure 3G).
SALINAS
Four bottlenose dolphins at Salinas showed scars along the dorsal area (Figure 5) (Table 1). One animal has two types of scars; one in the thoracic area between the blowhole and the dorsal fin and two shallow v-shaped scars in the posterior caudal region (Figure 5A, B). Two small depressions are also evident forward (four in total). The individual in Figure 5C shows a scarring area on the left lower caudal area where a swollen zone is visible and the spine shows a curvature. It is possible that a trauma caused fracture of bone in this area and a tumour-like bump grew or a type of spondylitis developed. A sawed edge is present in the lower caudal region of the individual in Figure 5D. Finally, a v-shaped scar is present in the posterior caudal region of the individual in Figure 5E.
BAJOALTO
Three individuals with different traumas in the dorsal fin were found at Bajoalto (Figure 6). One individual showed a cut in the anterior border of the dorsal fin and the dorsal fin tip bent over the right side (Figure 6A). A second individual had a small depression on the anterior insertion of the dorsal fin (Figure 6B). The third individual missed the dorsal fin tip (Figure 6C).
VALDIVIA
Five individuals photographed from the beach at Valdivia showed a dorsal fin tip missing. Mutilations in all cases were similar in appearance and resemble those found at Posorja, Estero Salado and Bajoalto. Most probably all of them were produced by the same cause. Three of Valdivia's animals have visible pink-coloured patches over the mutilated area.
Entanglements
Three dolphins entangled in fishing gear were recorded in January 2017 at Estero El Morro, a secondary branch of the Estero Salado (Figure 8). In the first case, an immature animal was towing the remains of a 5.5 inch monofilament gillnet tied to a polypropylene bag, probably used as ballast. The animal was exhausted when encountered almost motionless, in an inclined position with the head and anterior dorsal area above the water. As a consequence, the skin was severely burnt by the sun on the top and the right side. The animal missed the tip of one flipper and most probably acquired several scars in the peduncle because of the interaction (Figure 8A, B).
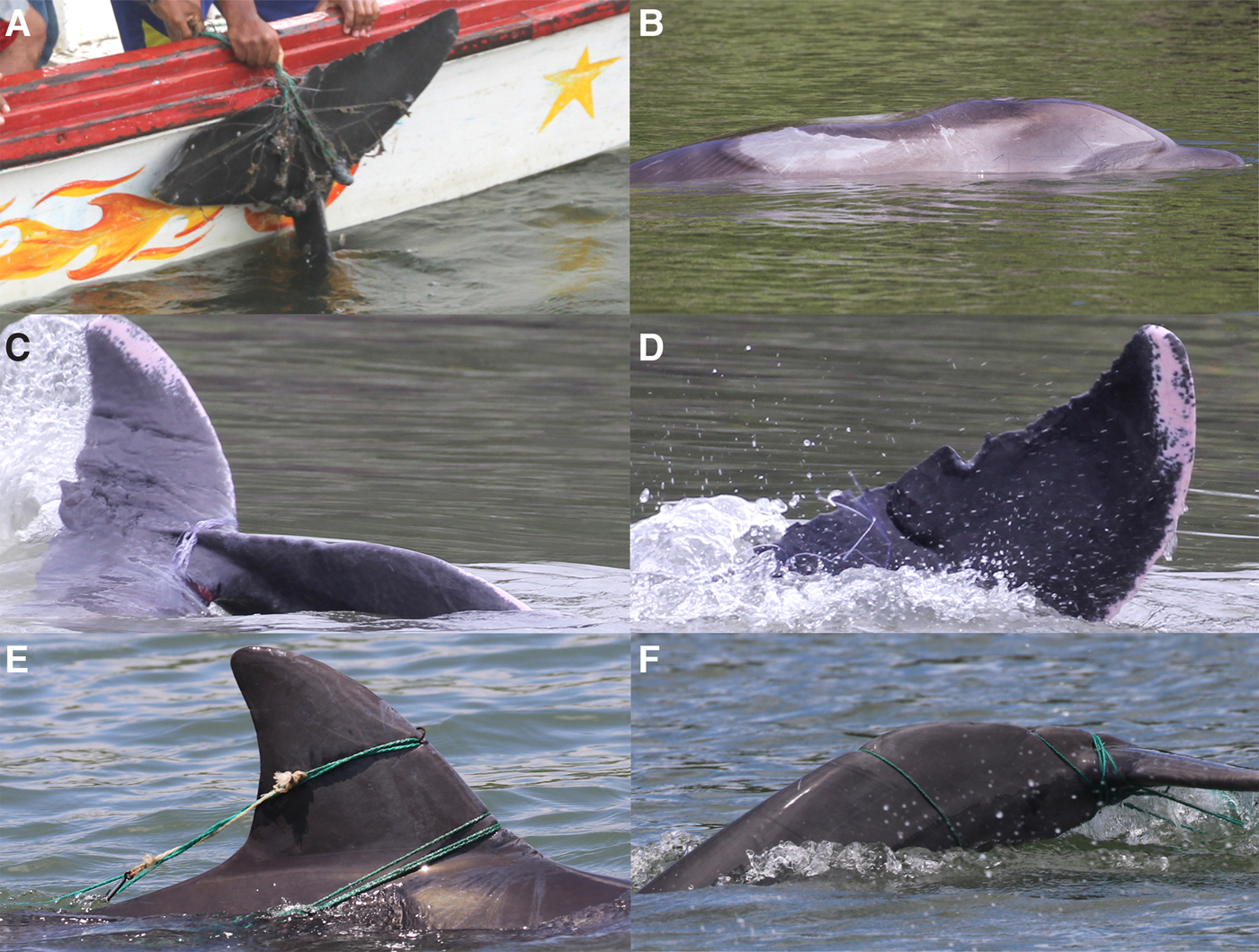
Fig. 8. (A) Moments in which an immature dolphin was raised near a boat to remove entangled fishing gear; (B) burnt and lost skin of the entangled dolphin; (C, D) remains of gear around the tail of an adult dolphin; (E, F) an immature dolphin entangled in a long-line. Note the tense line and a hook in the anterior border of the dorsal fin, as well as more tight rope around the lumbar region and tail.
The second case involved an adult animal (Figure 8C, D). It was caught in a gillnet set in one of the branches of El Morro Channel. A tourist who witnessed the event took pictures and made a video of the animal fighting while entangled until one of the fishermen cut the net with a machete. After 30 min only remains of the tail were evident, apparently the animals managed to escape the net. However, at least one bleeding wound in the base of the tail was visible as well as a cut on the left fluke border.
The third case involved another immature individual entangled with ropes and hooks of a long-line made of polypropylene rope (Figure 8D, E). One hook was lodged in the anterior border of the dorsal fin and several ropes were caught around the caudal region and peduncle. Bleeding cuts were visible in the anterior border of the dorsal fin and around the peduncle where ropes were tense. The gear could not be removed.
DICUSSION
The high rate of scarring in coastal bottlenose dolphins indicates that interactions with human activities, including fishing gear and vessel collision is a common phenomenon along the south-western coast of Ecuador. It is acknowledged, however, that only photographic evidence from free-ranging animals was used in this assessment, thus uncertainty persists surrounding the circumstances behind most of the observed scarring. In the case of fishing interactions, the problem seems to be the result of an over-sized small-scale fishing effort. Currently, the national small-scale fishing fleet includes 20,000 boats of different types (Herrera et al., Reference Herrera, Castro, Coello, Saa and Elías2013). Ecuador has been identified among the countries in Latin America with the highest small-scale fishing effort (Steward et al., Reference Steward, Lewison, Dunn, Bjorkland, Kelez, Halpin and Crowder2010) and as a by-catch hotspot given the high level of megafauna mortality in fishing gear (Lewison et al., Reference Lewison, Crowder, Wallace, Moore, Cox, Zydelis, MacDonald, DiMatteo, Dunn, Kot, Bjorkland, Kelez, Soykan, Steward, Sims, Boustany, Read, Halpin, Nichols and Safina2014). Collision with vessels would be related with the small-scale fishing fleet, as well as with the industrial fishing fleet established mainly at Posorja, as well as within those intensely trafficked channels in the inner estuary where activities such as aquaculture, tourism and shipping are carried out.
Type of wounds
Basically four types of wounds were found in coastal bottlenose dolphins in this study: dorsal fin damage, v-shaped scars, sawed edge and caudal deformity. Dorsal fin damage in cetaceans has been attributed to fishing gear, mainly lines and gillnets (e.g. Baird & Gorgone, Reference Baird and Gorgone2005; Freitas et al., Reference Freitas Nery, Espécie and Marino Simão2008; Kiszka et al., Reference Kiszka, Pelourdeau and Ridoux2008; Azevedo et al., Reference Azevedo, Lailson-Brito, Dorneles, van Sluys, Cunha and Fragoso2009) but also to vessel strikes (Van Waerebeek et al., Reference Van Waerebeek, Baker, Félix, Gedamke, Iñiguez, Sanino, Secchi, Sutaria, Van Helden and Wang2007). The high incidence of missing dorsal fin tips found at Valdivia (26.3%) is probably related to the intense use of nylon monofilament gillnets of 2–3 inch mesh wide in coastal areas to capture live shrimp for hatcheries (Herrera et al., Reference Herrera, Castro, Coello, Saa and Elías2013). Nylon monofilament gillnets are also utilized within the inner estuary of the Gulf of Guayaquil (Herrera et al., Reference Herrera, Castro, Coello, Saa and Elías2013), even inside small channels regularly frequented by dolphins, as shown by two cases of entanglement at Estero El Morro in 2017 (Figure 8). Nylon monofilament gillnets are used in the eastern Puna Island and may be the main reason why this area also has a high scarring prevalence.
In the case of v-shaped scars, individuals show different degrees of severity; shallow scars as in Figures 3B, 3D and 4E and deep scars as in Figures 3C, 4A, 4B, 4C and 4D. In the first cases, scars could be caused by fishing gear that penetrated the skin and blubber layer. But in the second cases wounds penetrated deeper into the muscle and most probably were caused by vessel propellers. Dolphins at Posorja and Estero Salado with high prevalence of deeper v-shaped scars are continuously exposed to fishing vessels propellers as well as to dolphin watching boats. Similar v-shaped scars behind the dorsal fin and in the caudal peduncle have been reported to occur in bottlenose dolphins in areas of heavy traffic associated with trawling vessels in Texas, USA (Fertl, Reference Fertl1994a, Reference Fertlb), in other species such as the Atlantic spotted dolphins (Stenella frontalis) in the Southern Caribbean (Luksenburg, Reference Luksenburg2014) and in killer whales (Orcinus orca) in New Zealand where they were also attributed to fishing vessel propellers (Visser, Reference Visser1999). Sawed edge wounds are too shallow to be produced by propellers, most probably being produced by gillnets given that the irregularities observed are evenly spaced (see Figure 4F). Scars extending on the thoracic area behind the heads of individuals in Figures 3A and 5A resemble those produced by shark attacks as reported to occur in Australia (e.g. Corkeron et al., Reference Corkeron, Morris and Bryden1987; Orams & Deakin, Reference Orams, Deakin, Hindell and Kemper1997). This could be the case of the individual in Figure 5A from Salinas, but unlikely in the other two because no large sharks that could cause such huge wounds have been reported in the inner estuary of the Gulf of Guayaquil. Most likely they were caused by low speed propellers from trawlers or purse seiners. The deformity showed by the individual in Figure 5C in the caudal region would be the result of strong trauma as a result of a collision with the hull or skeg of an outboard engine. Most scars found in bottlenose dolphins in Ecuador were located in the posterior lumbar and caudal regions, which may be related with a sudden diving manoeuvre to avoid boats approaching.
Level of prevalence
The prevalence of injuries attributed to fishing gear or vessel collisions found in Ecuadorian dolphins (0–44.4%) is higher than similar evaluations carried out elsewhere. For example, in the Brazilian marine tucuxi (Sotalia guianensis), wounds attributed to fishing interactions ranged between 5 and 9% (Freitas et al., Reference Freitas Nery, Espécie and Marino Simão2008; Azevedo et al., Reference Azevedo, Lailson-Brito, Dorneles, van Sluys, Cunha and Fragoso2009); 3.75% in false killer whales (Pseudorca crassidens) in Hawaii (Baird & Gorgone, Reference Baird and Gorgone2005); and between 1 and 15% in several species at Mayote (Kiszka et al., Reference Kiszka, Pelourdeau and Ridoux2008). Likewise, the prevalence of scars presumably caused primarily by collisions reported in other bottlenose dolphin populations ranged from 3 to 6% in different parts of the USA (Fertl, Reference Fertl1994a; Wells & Scott, Reference Wells and Scott1997; Bechdel et al., Reference Bechdel, Mazzoil, Murdoch, Howells, Reif, McCulloch, Schaefer and Bossart2009) and 4.3% in several cetacean species in the Southern Caribbean (Luksenburg, Reference Luksenburg2014). Evidence of vessel collision in 11.1% of stranded bottlenose dolphins has been reported in Salinas, Ecuador during the period 1996–2009 (Félix et al., Reference Félix, Haase, Denkinger and Falconí2011). Similar incidences were found in carcasses of Indo-Pacific humpback dolphins (Sousa chinensis) and finless porpoise (Neophocaena phocaenoides) examined in Hong Kong with wounds consistent with boat collisions (prevalence of 10.7 and 9.3% of the total stranded animals, respectively), suggesting that mortality due to collisions may have an impact on the viability of small resident populations in areas with heavy maritime traffic (Parsons & Jefferson, Reference Parsons and Jefferson2000).
Because the prevalence of scarring estimated in this study is based on animals that survived a strike, the true occurrence as well as direct and secondary associated mortality are unknown. Deeper wounds than those shown in Figure 4A and B are probably lethal because they would cut important arteries or reach bone. The low proportion of young animals in the whole sample could be explained given that severe wounds for juveniles are more likely to be fatal (e.g. Parsons & Jefferson, Reference Parsons and Jefferson2000; Byard et al., Reference Byard, Winskog, Machado and Boardman2012). Thus in severe cases as shown in Figure 4A and B the strike most probably occurred when the animals were adults. Direct evidence of fishing interaction was found in three cases (Figure 5). Two of them were immature, which suggest that curiosity and inexperience would increase the risk of entanglement as reported in Florida bottlenose dolphin by Wells et al. (Reference Wells, Hofmann and Moors1989) and in Australia (Mann et al., Reference Mann, Scott and Smuts1995). In general, it is difficult to estimate mortality caused by fishing interaction or propeller strikes in wild marine mammals because cases are under reported or carcasses cannot be recovered for examination (Van Waerebeek et al., Reference Van Waerebeek, Baker, Félix, Gedamke, Iñiguez, Sanino, Secchi, Sutaria, Van Helden and Wang2007; Byard et al., Reference Byard, Winskog, Machado and Boardman2012).
A previous evaluation of similar marks on bottlenose dolphins from the inner estuary of the Gulf of Guayaquil during the early 1990s, showed an average prevalence of 2.2%, attributed either to vessel strikes or fishing gear (Van Waerebeek et al., Reference Van Waerebeek, Baker, Félix, Gedamke, Iñiguez, Sanino, Secchi, Sutaria, Van Helden and Wang2007). When the new data available for five communities from the inner Gulf of Guayaquil are pooled the current scarring prevalence is 11.1% (18 of 161 individuals) or five times higher. It has been demonstrated recently that members of coastal bottlenose dolphin communities in the inner estuary of the Gulf of Guayaquil have decreased in number by 51% on average over the last 25 years (Félix et al., Reference Félix, Calderón, Vintimilla and Bayas-Rea2017). Therefore, there seems to be a correlation between the increased risk of damaging interactions with human activities and population decrease in the bottlenose dolphins resident to the inner estuary of the Gulf of Guayaquil. Given the difficulties inherent in obtaining reliable data to quantify mortality of cetaceans in fishing gear (see Reeves et al., Reference Reeves, McClellan and Werner2013), the scarring prevalence in free-ranging cetaceans remains a meaningful proxy for the impact of such human activities, and is probably implicated in the recent and alarming observed reduction in local populations.
Management implications
Management measures that address the problem caused by fishing activities and vessel collisions on coastal bottlenose dolphins and other marine megafauna such as sea turtles and mantas in Ecuador are required. However, we recognize this is a daunting task, particularly in the inner estuary of the Gulf of Guayaquil given that the area comprises hundreds of kilometres of channels and many small fishing villages settled in the middle of the mangroves, where control of maritime activities is as yet non-existent. The inventory of the small-scale fishing sector in Ecuador conducted by Herrera et al. (Reference Herrera, Castro, Coello, Saa and Elías2013) is a first step to identify possible problem ‘hot spots’ and understand the extent of gillnet and other fishing gear use with potential to affect coastal bottlenose dolphins. In that sense, the third case of a dolphin entangled in a long line at El Morro in 2017 indicates that the problem is not limited to gillnets in this part of the country but also long-lines are sources of dolphin entanglement. Although in the second case at El Morro the dolphins were released from an actively used net, it is possible that in the other two cases dolphins were entangled in discarded gear, which increases the complexity when addressing this problem.
An important aspect to be considered in Ecuador is the need to strengthen institutional competencies and commitments regarding marine mammal interactions with fisheries. Because this problem involves the fishing sector, marine mammal interactions are not considered by public agencies as conservation but rather fishing issues. Despite the use of still snoods in long lines to reduce the capture of sharks and other measures such as minimum size and closure seasons for some bentonic resources (clams and crabs), there are no limits or measures as yet on fishing effort designed to help reduce cetacean mortalities. With such a jurisdiction issue unresolved, it is difficult to encourage fishing authorities to adopt measures to address something unregulated, underestimated and with evident associated political implications given the scale of the small-scale fishing sector.
The coastal bottlenose dolphin is particularly vulnerable to fishing interactions and other anthropogenic activities because of its late sexual maturation, low reproduction rate and low recruitment as a consequence of an extended nursing period (3–4 years) (Wells et al., Reference Wells, Scott, Irvine and Genoways1987). Effective measures need to be implemented to protect the species in Ecuador in the short term to revert the current decreasing population trend, taking into consideration those biological constraints inherent to the species. Measures may involve, among others: (1) reduction of the fishing effort in known areas of bottlenose dolphin distribution, particularly mouths of large channels and inner branches where gillnets and long-lines should be forbidden; (2) implementing an area-based planning approach to strengthen local governance and regulate the different activities in coastal areas; (3) extend or create new coastal Marine Protected Areas for better control of activities that are harmful to dolphins; (4) promotion of research on the species, with emphasis on habitat use and interactions with fisheries. For this last issue, working with fishers is crucial to implement more sustainable fishing practices.
ACKNOWLEDGEMENTS
The authors thank Francisco Parrales and other skippers and tourist operators at Posorja and El Morro for their collaboration. Several wardens of the El Morro Wildlife Refuge collaborated and supported the authors' research. Moira Brown, Stuart Banks and three anonymous reviewers made valuable comments to improve an earlier version of the manuscript.
FINANCIAL SUPPORT
This research was conducted under research authorizations No. 004-IC-FAU-DPG/MAE, No. 008-IC-FLO/FAU-DPG/MAE and No. 005-16 IC-FAU-DPSE-MA issued by the Ministry of the Environment. Field work of MZ was financed by the University of Guayaquil.












