1. Introduction
Hyoliths are among the first biomineralized bilaterians to occur in the Cambrian Period, living throughout the entire Palaeozoic Era until their Permian extinction. These enigmatic animals, subdivided into the morphologically distinct orders Orthothecida and Hyolithida, comprise a conical shell associated with a cap-like operculum and, in hyolithids, an additional pair of logarithmically curving lateral helens and a ventral ligula on the conch (Martí Mus et al. Reference Martí Mus, Jeppsson and Malinky2014). However, hyoliths are usually preserved as simple conical shells, and the lack of other significant skeletal and anatomical information tends to render speculative their systematics, phylogeny, biomechanics, palaeoecology and other aspects. The biological relationships of hyoliths have long been debated, and the group has variously been treated as a lineage close to molluscs (Malinky & Yochelson, Reference Malinky and Yochelson2007), sipunculans or a separate phylum (Runnegar et al. Reference Runnegar, Pojeta, Morris, Taylor, Taylor and McClung1975; Sun et al. Reference Sun, Babcock, Peng and Zhao2016). Recently, the preserved remains of soft tissue associated with hyoliths likely support the notion that these animals may have been lophophorates (Moysiuk et al. Reference Moysiuk, Smith and Caron2017; Sun et al. Reference Sun, Smith, Zeng, Zhao, Li and Zhu2018 a). However, this idea has been challenged by later work on skeletal microstructures and soft anatomy (Li et al. Reference Li, Zhang, Skovsted, Yun, Pan and Li2019, Reference Li, Skovsted, Yun, Betts and Zhang2020; Liu et al. Reference Liu, Skovsted, Topper, Zhang and Shu2020 b).
Burgess Shale-type biotas offer deep insights into Cambrian life. Exceptionally preserved hyoliths have been commonly documented in the Konservat-Lagerstätten of South China, mainly those from the lower Cambrian (Chen, Reference Chen2004; Sun et al. Reference Sun, Babcock, Peng and Kastigar2017, Reference Sun, Smith, Zeng, Zhao, Li and Zhu2018 a; Liu et al. Reference Liu, Skovsted, Topper and Zhang2020 a,b, Reference Liu, Skovsted, Topper and Zhang2021), with the exceptions of the Cambrian Wuliuan Kaili Biota (Mao et al. Reference Mao, Zhao, Yu and Qian1992; Chen et al. Reference Chen, Zhao and Wang2003), Guzhangian Fulu Biota (Peng et al. Reference Peng, Yang, Liu, Zhu, Sun, Zamora, Mao and Zhang2020) and Jiangshanian Guole Biota (Zhu et al. Reference Zhu, Peng, Zamora, Lefebvre and Chen2016); however, little is known from North China, despite abundant hyolith skeletons occurring as part of the small shelly fauna that has been documented from the lower Cambrian strata of this block (Pan et al. Reference Pan, Skovsted, Sun and Li2019). Here, we report a new finding of exceptionally well-preserved hyoliths, Novakotheca weifangensis sp. nov., from the Mantou (alternatively ‘Manto’) Formation (Cambrian Miaolingian, Wuliuan) in the Longgang section of Weifang City in Shandong Province, North China. The exceptionally well-preserved soft parts of this new taxon provide important new data for reconstructing the complete digestive system of hyolithids. The different taphonomic modes of the soft organs and conical shells of the same species highlight the taphonomic bias and their effect on taxonomy. The biological association of hyoliths and brachiopod epibionts provides insights into their ecological interactions and the adaptability of early metazoans during Cambrian time.
2. Material and methods
The material described in this study was collected from the Upper Shale Member of the Mantou Formation in the Longgang section, Linqu County, Shandong Province, China. Trilobites from this interval are indicative of the Bailiella lantenoisi Zone (uppermost Wuliuan, Cambrian Miaolingian) (Sun, Z. X. et al. Reference Sun, Zeng and Zhao2020 a,b). The hyolith-bearing interval also yields brachiopods, vermiform animals, diverse arthropods, sponges, chancelloriids, algae and other problematic fossils of uncertain affinity (Sun, Z. X. et al. Reference Sun, Wang and Yuan2015, Reference Sun, Zeng and Zhao2020 a,b). For detailed information on geography, stratigraphy and sedimentology of this section see Sun, Z. X. et al. (Reference Sun, Zeng and Zhao2020 a,b). All the specimens in this paper are housed in the Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences (NIGPAS).
Specimens were examined and imaged by standard light microscopy, scanning electron microscopy (SEM; instrument model SU3500) with energy dispersive X-ray (EDX) spectroscopy and a Carl Zeiss SteREO Discovery V12 microscope linked to an AxioCam HR3 digital microscope CCD camera. Specimen NIGPAS173866 was scanned by a three-dimensional X-ray microscope (Zeiss Xradia 520 Versa) in the Micro-CT Lab of the Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences. The volume data were processed using VGStudio MAX software.
3. Systematic palaeontology
Class HYOLITHA Marek, Reference Marek1963
Order HYOLITHIDA Sysoyev, 1957
Family Hyolithidae Sysoyev, 1958
Genus Novakotheca Malinky, Reference Malinky1990
Novakotheca weifangensis sp. nov.

Fig. 1. Novakotheca weifangensis sp. nov. from the Mantou Formation in the Longgang section, North China. (a) NIGPAS173867, ventral view, almost complete conical shell with an operculum, showing a sinuous U-shaped gut and brachiopod epibionts near the apex. (b) NIGPAS173868c, dorsal view, a hyolith with an articulated operculum and right-side helen and preserved partial intestine. (c) NIGPAS173869a, dorsal view, incomplete conical shell with an operculum and a pair of helens. (d) NIGPAS173870, ventral view, conical shell with an operculum, a preserved U-shaped gut and other soft parts. (e) NIGPAS173871, ventral view, a hyolith shell with a helen, showing secondarily mineralized but unrecognizable soft parts. (f) NIGPAS173866, ventral view, a conical shell with a partial operculum. (g) NIGPAS173872, dorsal view, a hyolith with an operculum preserved in association with brachiopods. (h) NIGPAS173873a, dorsal view, a conical shell with an operculum, a left-side helen and a possible gut, preserved in association with a brachiopod valve. (i) Close-up of the boxed area in (h). (j) NIGPAS173874, ventral view, a deformed shell with an articulated operculum and one helen. (k) NIGPAS173875, the interior of an operculum showing clavicles and cardinal processes. Scale bars are 1 mm. Abbreviations: br – brachiopod; cl – clavicle; cp – cardinal process; ct – connective tissue; g – gut; he – helen; op – operculum; vo – visceral organ.
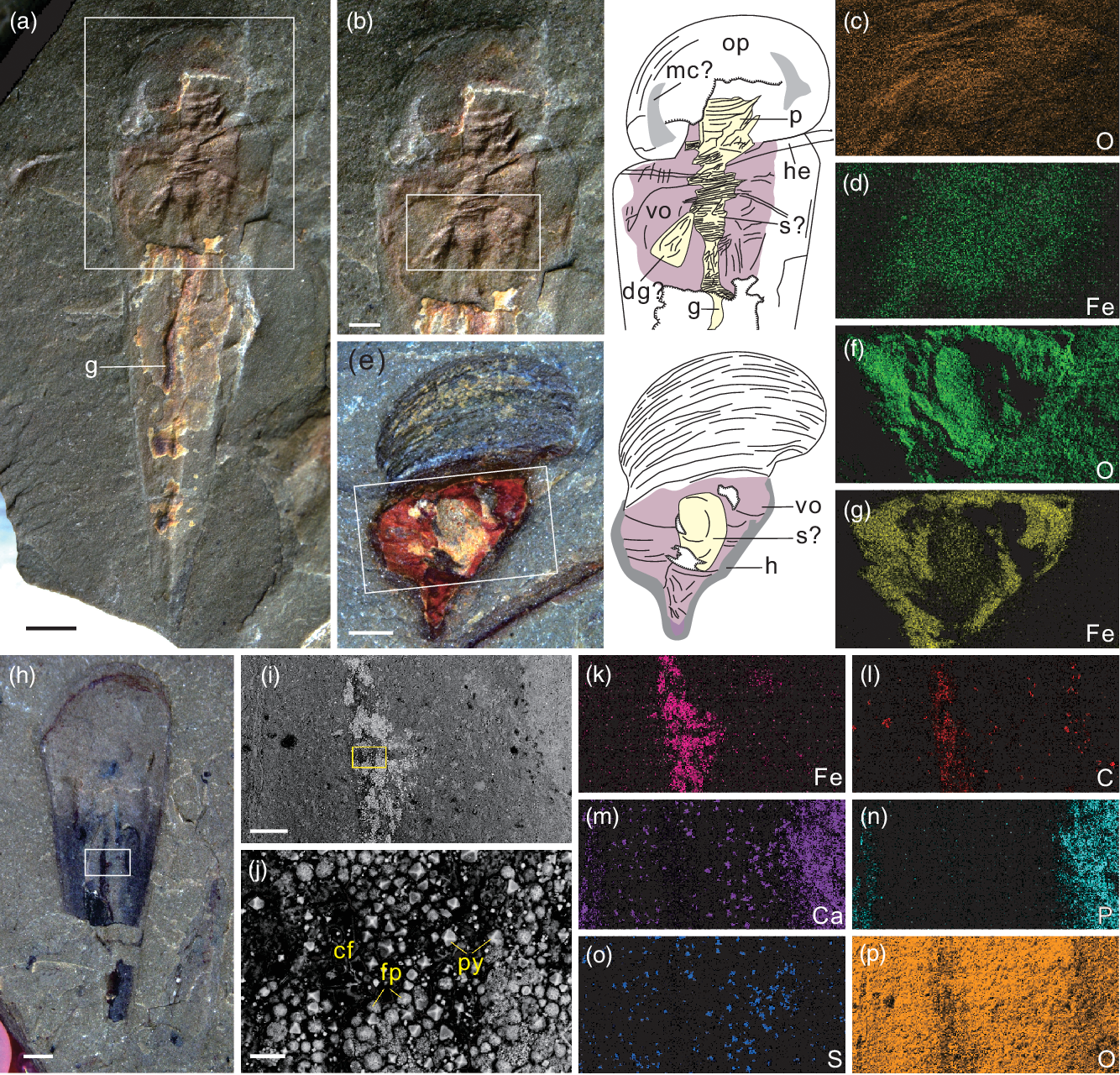
Fig. 2. Novakotheca weifangensis sp. nov. from the Mantou Formation in the Longgang section, North China. (a) NIGPAS173876, ventral view, a conical shell with an operculum and a helen, showing a curving gut and other soft organs. (b) Magnified image and interpretive drawing of the boxed area in (a). (c, d) Elemental distribution of the boxed area in (b). (e) NIGPAS173877, ventral view, an incomplete shell with secondarily mineralized soft organs and an interpretive drawing on the right side. (f, g) Elemental composition of the boxed area in (e). (h) NIGPAS173878, ventral view, an incomplete shell preserved with part of the intestine. (i) SEM image of the boxed area in (h). (j) Close-up of the boxed area in (i), showing the dark carbonaceous film, bright euhedral pyrite crystals and framboidal pyrites in the gut. (k–p) Elemental distribution of the area in (i). Scale bars are 1 mm for (a, h), 500 μm for (b, e), 200 μm for (i), and 20 μm for (j). Abbreviations: cf – carbonaceous film; dg – digestive organ; g – gut; h – halo; he – helen; mc – muscle scar; op – operculum; p – pharynx; py – euhedral pyrite; fp – framboidal pyrite; s – stomach; vo – visceral organ.

Fig. 3. Micro-CT images of Novakotheca weifangensis sp. nov. from the Mantou Formation in the Longgang section, North China, NIGPAS173866. (a) Dorsal view. (b) Ventral view. (c) Lateral view. (d) Posterior view showing the cross-section. (e) Slicing positions. The yellow line and red plane indicate the slicing sites of (f) and (g), respectively. (f) Cross-section of the shell. (g) Longitudinal section of the shell. Scale bars are 1 mm for (a–c) and 500 μm for (d, f, g). Abbreviations: bc – body cavity; bw – body wall; do – dorsum; es – oesophagus; lf – lateral furrow; li – ligula; lm – lateral margin; op – operculum; s – stomach; ve – venter.

Fig. 4. SEM images of three-dimensionally preserved Novakotheca weifangensis sp. nov. from the Mantou Formation in the Longgang section, North China. (a) NIGPAS173879, dorsal view. (b) NIGPAS173880, dorsal view. (c) NIGPAS173881, anterior-lateral view. (d) NIGPAS173882, cross-section. (e) NIGPAS173883, anterior view showing the cross-section. Scale bars are 1 mm for (a), 600 μm for (b), 300 μm for (c, e), and 200 μm for (d).

Fig. 5. Novakotheca weifangensis sp. nov. from the Mantou Formation in the Longgang section, North China. (a) NIGPAS173884, a cluster of hyolith shells and trilobite fragments. (b) Line drawing of (a). (c) NIGPAS173875, a cluster of hyoliths with opercula and shells; conical shells display the combination of compression and three-dimensional phosphatization (indicated by the white arrowheads). (d) NIGPAS173885, dorsal view, an incomplete shell showing compressed wrinkles. (e) Sketch of (d). Scale bars are 2 mm for (a, b) and 1 mm for (c–e). Abbreviations: hf – undistinguishable hyolith fragments; hy – hyolith shell; ma – matrix; op – operculum; tr – trilobite fragments.
Etymology. Named for Weifang City, Shandong Province, China, where the species occurs.
Type material. Holotype: NIGPAS173868c (Fig. 1b); paratypes: NIGPAS173866 (Figs 1f, 3), NIGPAS173867 (Fig. 1a), NIGPAS173870 (Fig. 1d), NIGPAS173875 (Fig. 1k), NIGPAS173876 (Fig. 2a), NIGPAS173877 (Fig. 2e), NIGPAS173879–NIGPAS173883 (Fig. 4).
Additional material. 118 specimens in total, including seven slabs with three-dimensional phosphatized conical shells in a shale matrix; the rest are highly compressed specimens in shale. Most of them are incompletely preserved.
Occurrence. Cambrian Miaolingian Wuliuan, the Upper Shale Member of the Mantou Formation (Bailiella lantenoisi Zone) in Weifang, Shandong Province, North China.
Diagnosis. Hyolithid with subtriangular cross-section; aperture orthogonal; dorsum of intermediate height and narrowly rounded in the centre; a pair of shallow longitudinal furrows along each margin of dorsum; lateral margins gently rounded. Operculum roughly ovate and platyclaviculate.
Description. Conical shell small with thin body wall; the dorsal length of complete specimens ranges from 6.7 to 16.2 mm (mean = 9.8 mm, n = 6), and the ventral length varies from 9 to 19 mm (mean = 11.6 mm, n = 7). Shell surface covered with faint growth lines, slightly arched on the venter. Cross-section subtriangular (Figs 3d, f, 4d, e). Aperture orthogonal, dorsal apertural rim unflared; apertural width ranges from 2.2 to 5.8 mm (mean = 3.3 mm, n = 12). Dorsum of intermediate height and narrowly rounded in the centre; adjacent slopes nearly flat (Figs 3a, d, f, 4d, e), terminating in a pair of lateral furrows (Fig. 3c, d, f) and transitioning into adjacent convex flanks (Figs 3f, 4e); lateral margins broadly rounded (Fig. 4e). Venter gently inflated (Fig. 4e); ventral ligula short with broadly rounded anterior edge (Figs 1d–f, 2h); the ligula length varies from 1.6 to 2.4 mm (mean = 1.8 mm, n = 7), which is approximately 54 % of the apertural width and 16 % of the ventral shell length. Apex pointed; apical termination slightly turned to the left; apical angle averages 21.5° (n = 13), ranging from 19.3° to 25.5°.
Operculum roughly ovate (Figs 1b, d, g, h, i, k, 2a, b); width of operculum ranges from 2.4 to 4.2 mm (mean = 3.5 mm, n = 4). Clavicles platyclaviculate, covered with longitudinal ridges (Fig. 1b, g, i, k), divergence averaging 97.4° (n = 5, min. = 87.7°, max. = 102.3°); length of clavicles ranges from 1 to 1.6 mm (mean = 1.4 mm, n = 5). Summit bluntly pointed. Conical shield large, subovate to fan-shaped, lateral sides straight. Cardinal shield small and subtriangular. Cardinal processes subconical, small and projecting divergently.
Helens tapering distally with pointed tips of variable curvature, protruding forward (Figs 1b, c, h, i, 2a, b), laterally (Fig. 1c, e) or backward (Fig. 1j).
Remarks. Compression can be a significant factor secondarily shaping shell morphology, which complicates the precise taxonomy of specimens preserved in shale. The cross-section, medial axis, inflation of dorsum and venter and lateral margins, etc. of N. weifangensis vary with the degree of compression. Sharper and more distinct dorsal medial axes (e.g. Figs 1b, c, g, 3a, d, 4a, b, 5d), less inflated venters (Figs 3f, 4d), lower dorsa (Figs 3d, f, 4d) and sharper lateral margins (Figs 3f, 4d) are observed in the compressed specimens compared to those in rocks that exhibit little or no compression (e.g. Fig. 4e). The specimens described here exhibit a range of shell deformations and provide enough information for the recognition of their biological features.
The fossils described here are assigned to Novakotheca based on its diagnostic characters including an orthogonal aperture and dorsum with flattened, steeply dipping slopes, terminating in a pair of longitudinal sulci near the lateral margins (Malinky, Reference Malinky1990). The specimens from the Mantou Formation possess the first known examples of an operculum and helens for Novakotheca. The general morphology of the new species is quite similar to that of N. crebescens, but the latter has sharper lateral margins and more prominent lateral furrows than in N. weifangensis (Malinky, Reference Malinky1990, figs 1.2, 1.7, 2.5, 2.14). Specimens of N. crebescens preserved in limestone expose enough detail of its original characteristics (Malinky, Reference Malinky1990) to ensure the validity of comparison between these two species.
A pair of horn-shaped structures are present on the lateral sides of one operculum of N. weifangensis (Fig. 2a, b), which possibly represent muscle scars. Hyolithid hyoliths are suggested to share a common skeleto-muscular system with similar arrangements of muscle scars (Martí Mus & Bergström, Reference Martí Mus and Bergström2005). The morphology of the scars in this study is different from that known from other species, but their lateral occurrence and location partially overlap with the clavicles, indicating a similarity with the ‘d’ scars described in the conical shield of Gompholites and Maxilites (Martí Mus & Bergström, Reference Martí Mus and Bergström2005), even though their precise correspondence in their relative position remains speculative (Martí Mus & Bergström, Reference Martí Mus and Bergström2005). Although the horn-shaped structures of N. weifangensis merely appear on a single specimen, the possibility of muscle scars cannot be ruled out.
4. Discussion
4.a. Taphonomy
The hyoliths were discovered as exceptionally well-preserved articulated specimens with soft parts (Figs 1a–j, 2, 3) and as clusters of shell fragments (Fig. 4a–c), which represent event-induced rapid burial of the assemblage and time-averaging background accumulation, respectively (Zhao et al. Reference Zhao, Hu, Caron, Zhu, Yin and Lu2012). Two taphonomic modes of the hyoliths from the Mantou Formation of the Longgang section have been recognized:
-
(1) Pyritization of nonmineralized tissue. Soft parts of the hyoliths, mostly alimentary canals, show preservation as a dark carbon film associated with pyrite (Figs 1a, h, i, 2a, h–l) or yellow to reddish imprints enriched in iron oxides (Figs 1d, e, 2a–d, e–g). Pyrites are present as both euhedral crystals and framboids (Fig. 2j), possibly indicating oxygenated bottom waters but anoxic or dysoxic conditions after burial or during late diagenesis, as in the Guanshan Biota (Forchielli et al. Reference Forchielli, Steiner, Kasbohm, Hu and Keupp2014; Liu et al. Reference Liu, Skovsted, Topper and Zhang2021). The EDX analysis reveals iron minerals with little to no sulfur (Fig. 2o), representing pseudomorphic alteration of pyrites during weathering processes (Zhu et al. Reference Zhu, Babcock and Steiner2005; Forchielli et al. Reference Forchielli, Steiner, Kasbohm, Hu and Keupp2014). Pyritization is a common pathway for the replication of soft tissues of organisms throughout the Phanerozoic Eon (Szczepanik & Sawłowicz, Reference Szczepanik and Sawłowicz2005; Farrell et al. Reference Farrell, Briggs, Hammarlund, Sperling and Gaines2013; Farrell, Reference Farrell2014; Schiffbauer et al. Reference Schiffbauer, Xiao, Cai, Wallace, Hua, Hunter, Xu, Peng and Kaufman2014), and the pyritization process revealed herein is a characteristic preservational mode in the Cambrian Burgess Shale-type biotas of China (Zhu et al. Reference Zhu, Babcock and Steiner2005; Gaines et al. Reference Gaines, Briggs and Zhao2008; Forchielli et al. Reference Forchielli, Steiner, Kasbohm, Hu and Keupp2014).
-
(2) Phosphatization of conical shells. Small shelly fossils (SSFs) dominated the early Cambrian diversity, but witnessed a decline from the middle Cambrian onwards (Porter, Reference Porter2004). The phosphatic replacement of skeletons usually occurs in shallow water carbonates (Li et al. Reference Li, Zhu, Steiner and Qian2004; Creveling et al. Reference Creveling, Knoll and Johnston2014; Yang et al. Reference Yang, Steiner and Keupp2015; Skovsted et al. Reference Skovsted, Balthasar, Vinther, Sperling and Álvaro2021), with scattered records from deeper shelf environments (Steiner et al. Reference Steiner, Li, Qian, Zhu and Erdtmann2003; Yang et al. Reference Yang, Zhang, Danelian, Feng and Steiner2014). Little is known about phosphatized skeletal fossils from siliciclastic facies (Sun, H. J. et al. Reference Sun, Zhao, Steiner, Li, Na, Pan, Yin, Zeng, Van Iten and Zhu2020). The phosphatized internal moulds of hyolith shells herein (Fig. 4) are preserved in three dimensions in shale of the Mantou Formation in the Longgang section (Fig. 5c). Organic matter and iron minerals are two vital reactive phosphorus fluxes to sediments in both modern and ancient marine environments (Delaney, Reference Delaney1998; Dornbos et al. Reference Dornbos, Bottjer, Chen, Gao, Oliveri and Li2006; März et al. Reference März, Poulton, Beckmann, Küster, Wagner and Kasten2008; Creveling et al. Reference Creveling, Knoll and Johnston2014). Decayed soft tissues or phosphatic skeletons possibly provided additional dissolved phosphorus in pore water. The formation of phosphatized hyoliths possibly underwent the following process (Creveling et al. Reference Creveling, Knoll and Johnston2014): phosphorus was delivered to the sediments by organic and iron-bound shuttles, and then hyolith skeletons trapped P-laden anoxic pore waters and facilitated the nucleation and precipitation of phosphate minerals via microbial metabolic pathways.
Taphonomic processes can significantly impact on taxonomy and our understanding of the fossil record (Hughes, Reference Hughes1995; Allison & Bottjer, Reference Allison and Bottjer2011; Kammerer et al. Reference Kammerer, Deutsch, Lungmus and Angielczyk2020), but this issue has received little attention in hyoliths. Taphonomic deformation creates abiotic changes in biological structures, obscuring original diagnostic morphological characteristics, including the cross-section, height of the dorsum, inflation of the venter, sharpness of the median ridge and divergence angle, etc. To improve the resolution of hyolith systematics for broader research on other palaeobiological aspects, the following approaches might be considered: (1) assess the taphonomic condition of specimens; (2) evaluate the taphonomic distortion of the morphology and introduce quantitative analysis (e.g. geometric morphometrics) to correct deformation in fossils (Kammerer et al. Reference Kammerer, Deutsch, Lungmus and Angielczyk2020), if the quality and quantity of material available is sufficient; and (3) learn more about morphological variations at the species level across different facies and taphonomic windows.
4.b. Soft anatomy of hyolithids
In addition to muscle scars (Martí Mus & Bergström, Reference Martí Mus and Bergström2005), soft parts of hyoliths have been extensively documented but are mainly restricted to their guts. The digestive tracts of orthothecids are zigzag-folded and sediment-filled (Thoral, Reference Thoral1935; Runnegar et al. Reference Runnegar, Pojeta, Morris, Taylor, Taylor and McClung1975; Malinky, Reference Malinky2003; Devaere et al. Reference Devaere, Clausen, Alvaro, Peel and Vachard2014), while simpler U-shaped guts seem to be characteristic of hyolithids (Meshkova & Sysoev, Reference Meshkova, Sysoev and Sokolov1981; Babcock & Robison, Reference Babcock and Robison1988; Butterfield, Reference Butterfield2003; Moysiuk et al. Reference Moysiuk, Smith and Caron2017; Liu et al. Reference Liu, Skovsted, Topper and Zhang2021) with a few exceptions displaying a variety of gut configurations (Chen, Reference Chen2004; Martí Mus, Reference Martí Mus2016; Sun et al. Reference Sun, Babcock, Peng and Zhao2016). Soft organs other than the intestines of hyolithids have rarely been described. Martí Mus (Reference Martí Mus2016) reported a hyolithid specimen preserving a relatively complete gut including a possible oesophagus and a tentacled mouth, as well as other soft parts associated with the operculum from the Ordovician Fezouata Konservat-Lagerstätte, Morocco. Recently, the hyolithid Haplophrentis described from the Burgess Shale and Spence Shale was illustrated with a pharynx that expands anteriorly and is connected to a tentacled gullwing-shaped band encircling a mouth, connected posteriorly to a U-shaped gut surrounded by plausible visceral organs (Moysiuk et al. Reference Moysiuk, Smith and Caron2017). Other reports of hyolithid soft organs (Houbrick et al. Reference Houbrick, Stürmer and Yochelson1988; Mao et al. Reference Mao, Zhao, Yu and Qian1992) are questionable (Martí Mus, Reference Martí Mus2016).
Soft tissues of N. weifangensis include a pharynx with a wrinkled muscular wall protruding forward towards the anterior of the operculum (Fig. 2a, b), and likely connected with tentacles as described in the hyolithids Haplophrentis reesei and H. carinatus (Moysiuk et al. Reference Moysiuk, Smith and Caron2017) and the orthothecid Triplicatella opimus (Liu et al. Reference Liu, Skovsted, Topper and Zhang2020 a,b). The pharyngeal organ extends posteriorly, connected to a relatively narrow tube, possibly the oesophagus (Fig. 3g); this is joined with a swollen structure (Figs 2a–g, 3f, g), which is the assumed stomach with wrinkled muscular walls (Fig. 2a–c), and the stomach narrows downward to a U-shaped gut (Figs 1d, 2a). A triangular structure connected to the lower lateral side of the stomach may represent a digestive gland (Fig. 2a, b). The digestive system is encompassed by visceral organs (Figs 1d, 2a, b, e).
The stomach and digestive gland are essential digestive organs of lophotrochozoans (Williams et al. Reference Williams, James, Emig, Mackay, Rhodes and Kaesler1997; Lobo-da-Cunha, Reference Lobo-da-Cunha2019); however, knowledge of these organs in hyoliths is very limited. The speculative stomach of N. weifangensis possibly served as an area that sorted food particles captured by the tentacles, as in brachiopods and some molluscs (Reid, Reference Reid1965; Williams et al. Reference Williams, James, Emig, Mackay, Rhodes and Kaesler1997; Lobo-da-Cunha, Reference Lobo-da-Cunha2019). Once the nutrient substances entered the stomach, suspended particles were rotated under the effect of the stomach fluid and mechanical action through relaxation and contraction of muscles. Then, fine partially digested grains flowed towards the digestive gland, and the U-shaped intestine received the residues coming from the stomach for further digestion, which is inferred from the smaller aperture between the digestive gland and the stomach than that between the intestine and the stomach (Fig. 2b). The digestive gland may have performed multiple functions, including secretion of digestive enzymes, nutrient absorption and energy storage, as suggested in molluscs (Lobo-da-Cunha, Reference Lobo-da-Cunha2019). The digestive system of hyolithids seems more complicated and efficient than previously thought. Although hyolithids have been commonly reconstructed as suspension or filter feeders (Marek et al. Reference Marek, Parsley and Galle1997; Butterfield, Reference Butterfield, Briggs and Crowther2001; Moysiuk et al. Reference Moysiuk, Smith and Caron2017), supported by the absence of sediments in the guts of the individuals described here, the effective digestive system likely adapted to a wide range of food resources (Martí Mus, Reference Martí Mus2016; Sun et al. Reference Sun, Zhao, Wen, Zeng and Peng2018 b), such as organic particles in the water column, microorganisms, animal corpses, detritus and deposits, which, as passive prey, were especially beneficial in nutrient-stressed environments. Although the new material does not resolve the precise phylogeny of hyoliths, it provides significant additional information for reconstructing the complete digestive system of hyolithids.
4.c. Epibionts and their palaeoecological implications
Biomineralized skeletons serve as hard substrates for sessile invertebrates throughout the Phanerozoic Eon (Topper et al. Reference Topper, Strotz, Holmer and Caron2015; Taylor, Reference Taylor2016; Zhang et al. Reference Zhang, Strotz, Topper, Chen, Chen, Liang, Zhang, Skovsted and Brock2020; Zicha et al. Reference Zicha, Bruthansová and Kraft2020), and the host/epibiont association provides direct evidence for species interactions and ecological strategies (Topper et al. Reference Topper, Strotz, Holmer and Caron2015). Hyoliths were one of the main preferred hosts from the Cambrian to the Devonian (Marek & Galle, Reference Marek and Galle1976; Galle & Plusquellec, Reference Galle and Plusquellec2002; Galle & Parsley, Reference Galle and Parsley2005; Malinky, Reference Malinky2006; Sun et al. Reference Sun, Babcock, Peng and Kastigar2017; Zicha et al. Reference Zicha, Bruthansová and Kraft2020). Epibionts living on hyoliths included echinoderms (Sun et al. Reference Sun, Babcock, Peng and Kastigar2017; Zicha et al. Reference Zicha, Bruthansová and Kraft2020), molluscs (Zicha et al. Reference Zicha, Bruthansová and Kraft2020), brachiopods (Topper et al. Reference Topper, Strotz, Holmer and Caron2015; Moysiuk et al. Reference Moysiuk, Smith and Caron2017), encrusting bryozoans and tabulate corals (Marek & Galle, Reference Marek and Galle1976; Galle & Plusquellec, Reference Galle and Plusquellec2002; Galle & Parsley, Reference Galle and Parsley2005; Malinky, Reference Malinky2006).
Some specimens of articulated hyoliths from North China are preserved with brachiopods (Fig. 1g–i). These specimens show no sign of attachment, suggesting chance superimposition during burial. However, one example displays two brachiopod specimens fixed to the hyolith, close to the apex, and the latter shows articulation with soft parts (Fig. 1a), indicating a live association. Previous work suggests that hyolithids had the ability to actively orient themselves to currents (Marek et al. Reference Marek, Parsley and Galle1997) and to be movable on or partly in substrates (Sun et al. Reference Sun, Zhao, Wen, Zeng and Peng2018 b). The sessile–mobile association benefitted brachiopods by increased nutrient availability, stabilization from benthic turbidity and possible anti-predation (Topper et al. Reference Topper, Holmer and Caron2014). However, the increasing body mass of brachiopods could have become burdensome to the hyolithids; thus, their relationship might have shifted from commensalism to resulting in a negative impact on the host during ontogeny.
5. Conclusions
The specimens from the Cambrian (Wuliuan) Mantou Formation, Longgang section, North China described herein are exceptionally well preserved with soft parts including a U-shaped gut, a possible pharynx, an oesophagus, a stomach and a digestive gland, providing important information for the reconstruction of the complete digestive system of hyolithids, which may have contributed to the effective digestion and absorption of food particles. Hyolithids possibly benefitted from this effective digestive system to adapt to a variety of feeding strategies on the suspension–detritus–deposit–scavenging spectrum and to survive in a nutrient-poor environment. Pyritization and phosphatization are recognized taphonomic modes for the preservation of soft tissues and shells in three dimensions, respectively. The different preservational pathways highlight the taphonomic impact on the morphological variations of hyoliths, thus affecting the taxonomic evaluation. Assessing taphonomic conditions, degrees of deformation and morphological variations at the species level across different facies is necessary to achieve high-resolution hyolith systematics. Brachiopods attached to hyoliths in live associations provide direct evidence for species interactions, indicating the complexity of Cambrian marine ecosystems and the ecological adaption of animals.
Acknowledgements
We appreciate Dr Martin Valent for his beneficial discussion and Dr Lanyun Miao (NIGPAS) for her assistance in the fossil collection. We thank Ms Suping Wu (NIGPAS) for her help in micro-CT scanning and volume data processing. We are grateful to the reviewers Dr John Malinky and Dr Christian B. Skovsted for their valuable comments. This research was supported by the Strategic Priority Research Programme (B) of the Chinese Academy of Sciences (XDB26000000), the National Natural Science Foundation of China (41602002), the State Key Laboratory of Palaeobiology and Stratigraphy (SKLPS, NIGPAS) (193125) and China Postdoctoral Science Foundation (2020M682935).







