Introduction
Stressful experiences can produce extinction-resistant memory traces through ‘over-consolidation’ and subsequent strengthening via repeated retrieval-reconsolidation (de Quervain et al., Reference de Quervain, Schwabe and Roozendaal2017). However, overly-consolidated aversive memories can be maladaptive: their unintended, cue-driven retrieval in non-threatening contexts confers no survival advantage, but instead limits behavioural flexibility by promoting additional symptoms, such as avoidance and distress (Brewin and Holmes, Reference Brewin and Holmes2003). Such involuntary (‘intrusive’) memories are a canonical symptom of posttraumatic stress disorder (PTSD) (Brewin and Holmes, Reference Brewin and Holmes2003; Brewin, Reference Brewin2011). Their formation putatively relies on synaptic and systems-level neuronal adaptations interacting with psychological processes (e.g. rehearsal; emotion arousal). The time-course of ‘early’ (synaptic) memory consolidation (Shadmehr and Holcomb, Reference Shadmehr and Holcomb1997; Dudai, Reference Dudai2004) provides a window of opportunity to interfere with this process behaviourally, and this has been proposed as a means to protect trauma victims against developing re-experiencing symptoms in PTSD (Holmes et al., Reference Holmes, James, Kilford and Deeprose2010; Horsch et al., Reference Horsch, Vial, Favrod, Harari, Blackwell, Watson, Iyadurai, Bonsall and Holmes2017; Iyadurai et al., Reference Iyadurai, Blackwell, Meiser-Stedman, Watson, Bonsall, Geddes, Nobre and Holmes2018). The reliance of memory consolidation on protein synthesis also suggests pharmacological routes to such secondary prevention (e.g. using drugs that have indirect, downstream protein synthesis inhibiting properties; Sijbrandij et al., Reference Sijbrandij, Kleiboer, Bisson, Barbui and Cuijpers2015), especially if treatment is delivered soon after trauma. For example, propranolol, a non-selective β-adrenoceptor antagonist, which inhibits protein-synthesis dependent ‘long-term potentiation’ (a cellular/molecular mechanism proposed to underlie memory formation) and selectively impairs emotional memory (McGaugh, Reference McGaugh2004), showed promise as a PTSD-preventive agent, in an early pilot study in emergency room trauma victims (Pitman et al., Reference Pitman, Sanders, Zusman, Healy, Cheema, Lasko, Cahill and Orr2002). Early observational studies also suggested that the anti-inflammatory drug, hydrocortisone, lowered the incidence of intensive care treatment related PTSD (Schelling et al., Reference Schelling, Stoll, Kapfhammer, Rothenhäusler, Krauseneck, Durst, Haller and Briegel1999; Schelling et al., Reference Schelling, Briegel, Roozendaal, Stoll, Rothenhäusler and Kapfhammer2001). This prompted several clinical trials examining hydrocortisone's potential as a preventive agent for PTSD, with promising results from these small-scale studies (Sijbrandij et al., Reference Sijbrandij, Kleiboer, Bisson, Barbui and Cuijpers2015). However, such clinical findings are difficult to reconcile with the well-established consolidation enhancing effects of glucocorticoids on emotional memory (de Quervain et al., Reference de Quervain, Schwabe and Roozendaal2017) and the therapeutic mechanism of action of hydrocortisone in PTSD prevention therefore remains unclear.
Despite promising clinical evidence for hydrocortisone in secondary prevention of PTSD, and at least a theoretically compelling rationale for propranolol's use in secondary prevention, progress in translating these discoveries into highly effective secondary preventive treatments has been slow. This may be because the conditions that dictate the preventive efficacy of these drugs remain poorly understood. Clinical trials are not ideally suited to studying such conditions, especially given the highly variable treatment regimes common in the medical (often emergency) settings in which relevant trials have been conducted. Such variability might explain disappointing results with propranolol, which, on the basis of several extant trials, shows no overall preventive efficacy (incident rate ratio: IRR = 0.95; see Sijbrandij et al., Reference Sijbrandij, Kleiboer, Bisson, Barbui and Cuijpers2015).
These observations call for translational research that allows the effects of propranolol, hydrocortisone, and related drugs to be examined cleanly, and in the absence of clinical confounds (Iyadurai et al., Reference Iyadurai, Visser, Lau-Zhu, Porcheret, Horsch, Holmes and James2019). The sources of variability present in clinical settings are readily avoided or controlled in laboratory studies that model (mild) psychological ‘trauma’. In addition, despite a number of clinical studies examining propranolol's preventive effects on general PTSD symptomatology, we are not aware of any existing experimental research examining the effects of a β-blocker specifically on intrusive memories. While such purely experimental studies do exist for hydrocortisone, these studies have involved either administering hydrocortisone before an analogue trauma (Rombold et al., Reference Rombold, Wingenfeld, Renneberg, Schwarzkopf, Hellmann-Regen, Otte and Roepke2016) or after a long (~24 h) delay (Graebener et al., Reference Graebener, Michael, Holz and Lass-Hennemann2017). These conditions may be suboptimal for revealing preventive effects of hydrocortisone on involuntary memory formation and do not allow effects on encoding (which is potentially enhanced by endogenous cortisol; van Ast et al., Reference van Ast, Cornelisse, Marin, Ackermann, Garfinkel and Abercrombie2013), consolidation, and retrieval to be parsed. As such, further experimental studies are essential for improving our understanding of the specific PTSD symptoms affected by, and the parameters that govern the efficacy of, noradrenergic and glucocorticoid drugs as potential secondary preventive agents. In the current study, we therefore carry out the first head-to-head comparison between single doses of propranolol and hydrocortisone on intrusive (and voluntary) emotional memories using the ‘trauma-film’ model of PTSD (Holmes and Bourne, Reference Holmes and Bourne2008). Comparing these drugs under identical experimental conditions is critical to understanding their apparently divergent effects in extant clinical trials (Sijbrandij et al., Reference Sijbrandij, Kleiboer, Bisson, Barbui and Cuijpers2015). To maximise the clinical relevance of our findings, we tested the effects of ‘post-trauma’ drug administration. Post-trauma treatment is important because it is usually not possible to prospectively treat potential trauma victims. In addition, treatments for PTSD should ideally selectively reduce the occurrence of ‘decontextualised’, sensory, involuntary memories, while sparing voluntarily-accessible memories for spatial and temporal contextual and narrative details. The latter are thought to inhibit the occurrence of cue-driven sensory memories, and hence are required for recovery from psychological trauma (Brewin, Reference Brewin2001). As such, we also assessed whether voluntary trauma memory was spared after administration of these drugs.
Methods
Participants
Medically and psychiatrically healthy women (n = 88; 18–35 years old) received oral propranolol (80 mg), hydrocortisone (30 mg) or placebo in a randomised, double-blind manner. All procedures were approved by University College London ethics committee and conducted in accordance with the Declaration of Helsinki. Participants provided written informed consent and received a £25 honorarium.
Procedure
Participants were telephone-screened to determine eligibility (see online Supplement). Testing commenced between 2 and 5 pm on both testing sessions (days 1 and 8). After ECG electrodes were attached, baseline questionnaires were completed in the following order: Beck Depression Inventory-II (BDI), State-Trait Anxiety Inventory (STAI) and Dissociative Experiences Scale-II (DES). These were followed by the first blood pressure (BP) reading and saliva (cortisol) sample at the baseline (T1) timepoint (Fig. 1). Heart rate (HR) was assessed continuously with the 5-min pre-film period forming the T1 HR measure. Baseline (T1) state measures – Positive-Negative Affect Schedule (PANAS), then the Bodily Symptoms Scale (BSS) – were then taken. See online Supplement for further details on self-report and physiological measures.
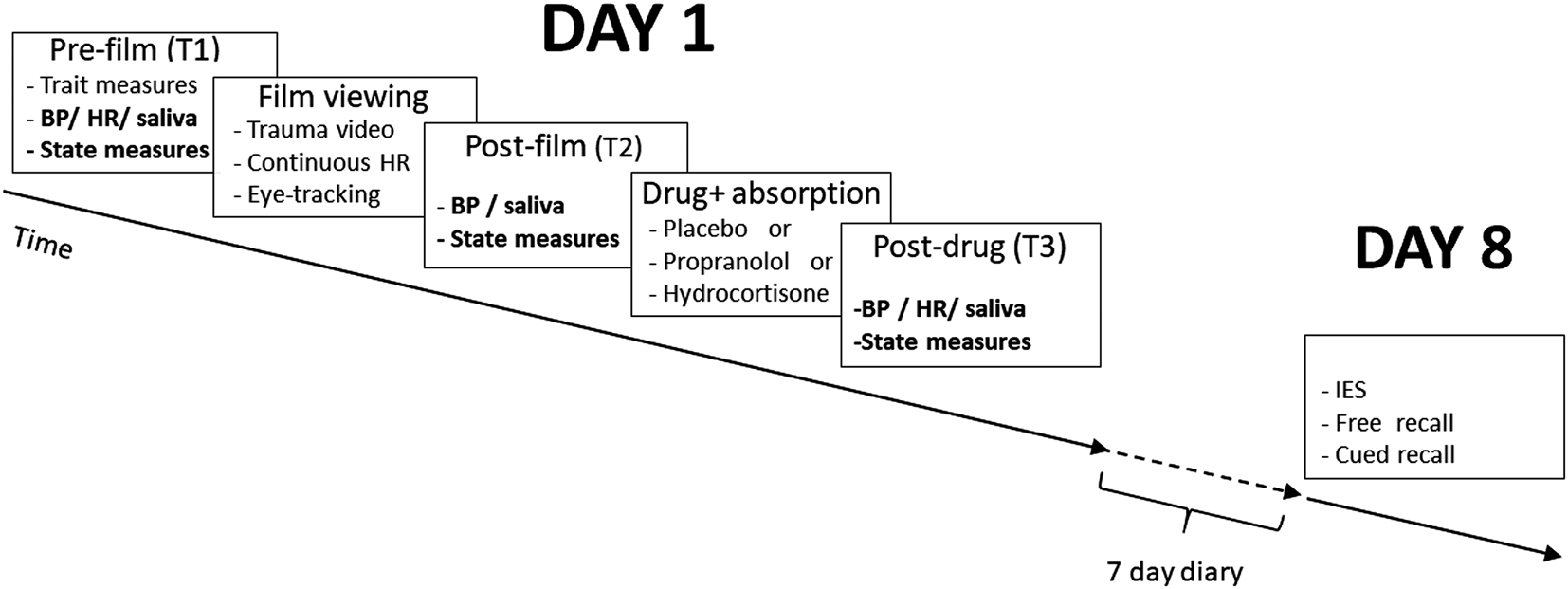
Fig. 1. Procedure. On day 1, pre-film measures was taken at T1; T2 is post-film measures (T2 HR was measured continuously during the film) and a final set of post-drug assessments were made at T3. Participants then completed a 7-day online memory monitoring procedure and returned to complete a general PTSD-symptom measure (adapted Impact of Events Scale; IES) and voluntary declarative memory assessments on day 8.
The trauma-film (formed of two scenes from the commercial film ‘Irreversible’, Studio Canal lasting approximately 15 min, including audio descriptions that introduced and linked the scenes) is described in detail elsewhere (Das et al., Reference Das, Tamman, Nikolova, Freeman, Bisby, Lazzarino and Kamboj2016). Participants were seated comfortably in front of a computer monitor and head movements were minimised using a chin rest while eye-tracker calibration was performed. While remaining in the chin rest, participants then viewed the trauma-film in a darkened room via a 15-in. laptop monitor with audio presented through headphones. The exact start time of the film was event-marked.
Eye-tracking was performed throughout the film viewing period to determine whether engagement during film viewing differed between groups at ‘baseline’ (i.e. before drug administration) with the aim of ruling this out as a potential explanation for any observed group effects on intrusion. Eye-movements metrics (dwell-time and number of fixations) were recorded continuously during film viewing over 44 pre-defined areas of interest (GP3 eyetracker, Gazepoint, Vancouver, Canada). Analysis was conducted offline using Gazepoint software.
HR during the entirety of the film served as the T2 measure of cardiac activity and the other T2 measures/samples were taken immediately after the film in the following order: BP, saliva, subjective measures (PANAS, BSS). Note: given the duration of the film and time-course of salivary cortisol changes, the cortisol sample taken immediately after the film (T2) was considered to reflect peri-film cortisol levels.
Drug capsules containing propranolol, hydrocortisone or placebo were then administered and participants sat quietly while completing a ‘filler’ task for 1 h. This involved rating 25 clips of classical music for pleasantness. A 10 min rest break occurred midway through the filler task, during which participants were discouraged from using electronic devices. After the 1 h period, participants and experimenters made independent guesses on treatment followed by final BP, saliva, PANAS and BSS measures at T3 (T3 HR was taken during the final 5-min of the filler task).
Detailed instructions on remote recording of intrusive memories were provided before participants left the laboratory on day 1 and participants received daily reminders at 8 pm to record (as close to bedtime as possible) the number of intrusions (with brief descriptions to allow verification of relevance to the trauma film contents). Vividness and distress were also recorded, using a 5-point rating scale (1 = ‘not at all’; 5 = ‘extremely’) and averaged across intrusions for each participant on each day. All intrusion variables were recorded using the online/smart device-ready survey tool, Qualtrics (Provo, UT). On day 2, participants also completed online questions on sleep quality (rated very good, fairly good, fairly bad, very bad) and quantity (hours of sleep) relating to the previous night.
Participants returned to the study centre on day 8 (at approximately the same time as day 1) and completed an adapted version of the Impact of Events Scale (IES), followed by free-, then cued recall. Upon completion, participants were debriefed and compensated.
Statistical analysis
One-way ANOVAs were used to analyse baseline variables, voluntary memory and (log transformed) IES scores. Day 1 physiological and subjective data were analysed using 3 (timepoint: T1, T2, T3) × 3 (group: placebo, propranolol, hydrocortisone) repeated measures ANOVAs. Mixed effects and generalised linear mixed models were used to analyse intrusion data (counts, vividness and distress), with participant and day as random factors, and model-fit assessed using Akaike's Information Criterion (AIC). Peri-film (T2) HR and cortisol were included as covariates in the analyses of intrusions and IES, based on previous research demonstrating their association with trauma-related symptoms (Chou et al., Reference Chou, La Marca, Steptoe and Brewin2014a; Chou et al., Reference Chou, Marca, Steptoe and Brewin2014b). The main outcome of interest was intrusion frequency; secondarily we examined intrusion vividness and distress, IES scores and voluntary memory recall. The threshold for statistical significance was p = 0.05 and post-hoc p values were Bonferroni-corrected. Two-tailed tests are reported throughout. See online Supplement for further details on statistical analyses.
Results
Baseline characteristics
Groups were well matched on demographic and psychological variables (Table 1; see online Supplementary Results).
Table 1. Baseline variables (mean ± s.d.)

The p values relate to the outcome of one-way ANOVAs.
Subjective and physiological response to film and drugs
PANAS-negative scores only showed a main effect of time (F 1.47,124.56 = 205.062, p < 0.001, η p2 = 0.707), reflecting an increase in negative affect from T1 to T2, followed by return to baseline at T3. PANAS-positive also only showed a main effect of time (F 2,170 = 93.566, p < 0.001, η p2 = 0.524), with deterioration in positive affect between T1 and T2, maintained at T3. No group or time effects were observed on BSS items, which were generally at floor level (i.e. <10 on a 0–100 scale) across time-points. The absence of reported changes in bodily symptoms on the BSS was consistent with chance level correct guessing on treatment by participants [29.54%; χ2(4) = 2.513, p = 0.642]. Experimenters also guessed at chance level [37.5%; χ2(4) = 3.206, p = 0.524], suggesting that blinding was successful.
A time × group interaction on HR (F 3.20,132.71 = 7.730, p < 0.001, η p2 = 0.157; Fig. 2a) was driven by a drop between T2 and T3 only after propranolol (p < 0.001). Systolic BP also showed a time × group interaction (F 4,168 = 3.766, p = 0.006) driven by a reduction in systolic-BP only in the propranolol group between T2 and T3 (p < 0.001). Baseline salivary cortisol levels (0.144 µg/dl) were in the expected range (Miller et al., Reference Miller, Stalder, Jarczok, Almeida, Badrick, Bartels, Boomsma, Coe, Dekker, Donzella, Fischer, Gunnar, Kumari, Lederbogen, Power, Ryff, Subramanian, Tiemeier, Watamura and Kirschbaum2016). A time × group interaction on cortisol levels (F 2.01,80.37 = 16.78, p < 0.001, η p2 = 0.296), reflected a T2 to T3 increase only after hydrocortisone (p < 0.001; Fig. 2b).

Fig. 2. (a) Time by group effects on (mean ± s.e.m.) heart rate (beats/min) and (b) salivary cortisol (μg/dl) on day 1. T1 = pre-film; T2 = post-film; T3 = 1 h post drug.
Involuntary memory
As shown in Fig. 3a, there was a steady decline in predicted intrusions across days (IRR = 0.636, s.e. = 0.024, z = 12.02, p < 0.001; T2 HR and cortisol were covariates in all intrusion analyses). The effect of group was also significant [χ2(2) = 9.81, p = 0.007]: relative to placebo, the propranolol group experienced fewer intrusions (IRR = 0.580, s.e. = 0.147, z = 2.16, p = 0.031), as did the hydrocortisone group (IRR = 0.452, s.e. = 0.118, z = 3.04, p = 0.002). The IRR values correspond to ~42% and ~55% fewer intrusions in the propranolol and hydrocortisone groups respectively (see online Supplement for full details on model building and testing).
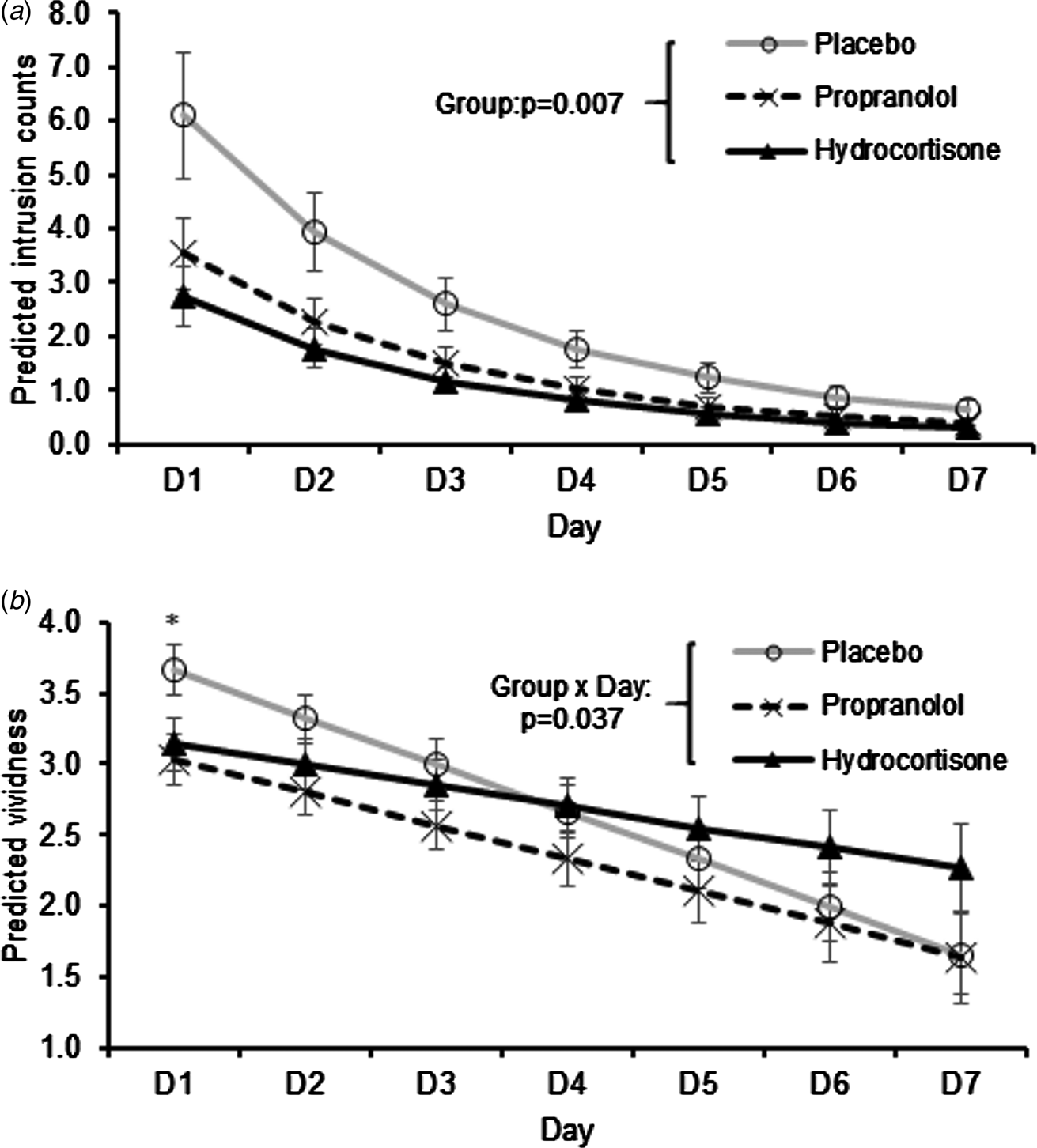
Fig. 3. (a) Intrusion counts. Predicted mean ± s.e.m. number of intrusion based on negative binomial regression results. The main effect of group reflects fewer intrusions relative to placebo in the propranolol (p = 0.031) and hydrocortisone (p = 0.002) groups. (b) Vividness of intrusions. Predicted mean ± s.e.m. vividness. *p = 0.04 for post-hoc Bonferroni corrected pairwise comparison on day 1. HR and salivary cortisol at T2 were covariates in both models.
Descriptively, the differences between placebo and the drug groups were most evident on days 1–2, whereas the number of intrusions was close to 0 in all groups by day 7 (Fig. 3a). However, whereas intrusions were abolished by day 7 in 87% and 76% of participants in the propranolol and hydrocortisone groups respectively, only 55% of participants in the placebo group had no intrusions on day 7 [χ2(2) = 7.59, p = 0.023].
Since drug effects were already evident on day 1, it was of interest to determine whether biomarkers of drug response were associated with sub-acute (day 1) intrusion counts. Correlations were therefore conducted between log-transformed intrusion frequency and (i) T3 salivary cortisol, with a primary focus on the hydrocortisone group, and (ii) T3 HR, focusing on the propranolol group. There was a significant negative correlation between T3 cortisol and day 1 intrusion frequency in the hydrocortisone group (r(28) = −0.480, p = 0.01; see online Supplementary Fig. S1), but no correlation between T3 HR and intrusion frequency in the propranolol group (r(24) = 0.128, p = 0.499).
For vividness of intrusions, a day × group interaction [χ2(2) = 6.62, p = 0.037] reflected a shallower slope in the hydrocortisone relative to placebo condition (p = 0.011; Fig. 3b), but not propranolol relative to placebo (p = 0.181). Bonferroni corrected post-hoc pairwise comparisons of vividness ratings showed that on day 1, only the propranolol v. placebo comparison was significant (p = 0.04). In contrast, groups did not differ significantly on vividness by day 7 (p’s>0.1). Distress ratings showed a similar declining pattern across days (B = −0.311, s.e. = 0.046, z = 6.76, p < 0.001). However, based on the same model specifications as used to analyse vividness (which also produced the best fitting model of distress), the effect of group did not reach statistical significance [χ2(2) = 5.25, p = 0.072] and neither did the day × group interaction [χ2(2) = 4.86, p = 0.088; see online Supplementary Results; Fig. S2]. It is important to note that average distress ratings on day 1 were 3.0 on a 1–5 scale (1 = ‘not at all’; 5 = ‘extremely’), suggesting at least moderate sub-acute levels of intrusion-related distress. It should also be noted that dwell-time and number of fixations on pre-specified areas of interest during film viewing did not differ between groups (online Supplementary Results) and therefore attentional differences are unlikely to explain the reported drug effects.
Voluntary memory: free and cued recall
In contrast to the effects on intrusions, voluntary memory tested on day 8 was unaffected by group. Participants in the three groups performed similarly on cued recall (mean ± s.d.): placebo: 7.76 ± 1.91; propranolol: 8.17 ± 1.96; hydrocortisone, 7.86 ± 2.61, free recall-gist: placebo: 13.09 ± 5.24; propranolol: 14.47 ± 4.65; hydrocortisone: 13.29 ± 4.72, and free recall-detail: placebo: 16.91 ± 8.60; propranolol: 18.33 ± 5.87; hydrocortisone: 17.19 ± 7.44 (F values ⩽0.692, p values ⩾0.503).
Adapted impact of event scale
The effect of group, co-varying T2 HR and cortisol, on IES scores was marginally significant (F 2,78 = 3.129, p = 0.049, η 2 = 0.074). Although both propranolol and hydrocortisone showed lower scores relative to placebo, only the pairwise Bonferroni-corrected comparison between placebo and propranolol was significant (p = 0.044; Fig. 4).

Fig. 4. Participant (filled circles) and group-level (solid black lines) scores on the adapted Impact of Events Scale (IES). For clarity, values are untransformed. Solid black horizontal lines represent estimated marginal means (T2 HR and cortisol levels as covariates).
Discussion
The current study is the first that we are aware of to directly compare effects of hydrocortisone and propranolol on voluntary and involuntary memory for emotional material intended to simulate re-experiencing symptoms (intrusive memories) in PTSD. We showed that both propranolol and hydrocortisone administered immediately after an analogue ‘trauma’ produced similar substantial reductions (by 42% and 55% respectively) in intrusive memories, starting on the day of the trauma-film. Drug effects on intrusions did not reflect generalised memory impairment, as long-term voluntary memory was intact in both drug groups.
The effects of propranolol reported here are consistent with a large body of neurobiological and behavioural research on the role of the noradrenergic system in long-term emotional memory in rodents and humans (Van Stegeren, Reference Van Stegeren2008; Lonergan et al., Reference Lonergan, Olivera-Figueroa, Pitman and Brunet2013) and extend this to involuntary (episodic) emotional memory. Although not often reported, impairing effects of propranolol on pre-sleep, short-term memory performance – as seen here – have also been reported (Maheu et al., Reference Maheu, Joober, Beaulieu and Lupien2004). The propranolol group also showed lower general trauma-like symptoms (IES scores) after 1 week.
Although our findings support the idea that propranolol can reduce the occurrence of intrusive memories, meta-analysis of randomised clinical trials of propranolol in trauma victims have not shown reduced incidence/severity of symptoms of PTSD (Sijbrandij et al., Reference Sijbrandij, Kleiboer, Bisson, Barbui and Cuijpers2015), and therefore the clinical evidence does not currently support its use in secondary prevention. Clearly, lab studies of simulated trauma, and clinical studies involving actual trauma, are not directly comparable. It is possible, for example, that the severity of the stress reaction in real-life traumas results in a level of noradrenergically-mediated hyper-consolidation that is not readily constrained by propranolol. This might suggest that larger acute (rather than cumulative) doses of propranolol might need to be tested in clinical studies, although this must be balanced against tolerability.
In studies reviewed by Sijbrandij et al. (Reference Sijbrandij, Kleiboer, Bisson, Barbui and Cuijpers2015), the interval between trauma and propranolol treatment varied between 6 and 48 h with 4/6 studies administering propranolol >6 h after trauma. If rapid treatment is critical, future clinical research with β-blockers may need to consider the clinical context in which such research is conducted. For example, first-line medical settings (e.g. ambulance or emergency triage) may be more appropriate treatment contexts than later in the healthcare chain.
The effects observed here with hydrocortisone are consistent with a number of small-scale clinical studies in recently traumatised individuals. In contrast to studies with propranolol, it is noteworthy that four out of five studies of hydrocortisone in Sijbrandij et al.'s meta-analysis administered the drug within the putative period of synaptic consolidation (<6 h after trauma; overall IRR = 0.38). Again, while this might suggest that rapid treatment is critical, the effects of endogenous/exogenous cortisol on memory are complex, with opposing effects on working memory v. consolidation (van Ast et al., Reference van Ast, Cornelisse, Marin, Ackermann, Garfinkel and Abercrombie2013) and retrieval v. (re)consolidation (de Quervain et al., Reference de Quervain, Schwabe and Roozendaal2017). The effects reported here are not readily reconciled with existing experimental behavioural studies showing enhancement of consolidation of emotional memories by endogenous and exogenous glucocorticoids (Roozendaal, Reference Roozendaal2000; Buchanan and Lovallo, Reference Buchanan and Lovallo2001). Such enhancing effects are usually most evident when there is a long delay (⩾24 h) between training (and drug administration) and the retention test (Roozendaal, Reference Roozendaal2002). However, after showing lower intrusion counts than placebo on day 1, there was no sign of a rebound increase in intrusive memories on day 2 in the hydrocortisone group, suggesting that there was a minimal effect of hydrocortisone on consolidation of sensory memories underlying intrusion.
Although the mechanism of hydrocortisone's protective effects against PTSD remains unclear, we speculate on two indirect routes to reduced intrusions. One explanation for our findings (and potentially, previous clinical studies with hydrocortisone as well) is that they reflect an impairment of involuntary retrieval which indirectly affects early consolidation due to limited retrieval-rehearsal. This explanation requires that such acute retrieval impairment (and consequent, indirect reductions in consolidation) precedes and/or exceeds any concurrent consolidation enhancing effects of hydrocortisone. An alternative explanation, drawing on dual representation theories of PTSD, is that voluntarily-retrievable, contextually-based memories – which ordinarily down-regulate the expression of sensory-based involuntary memories (Brewin, Reference Brewin2001; Reference Brewin2014; Brewin and Holmes, Reference Brewin and Holmes2003) – might be selectively over-consolidated by hydrocortisone, and hence exert increased top-down control over cue-driven retrieval of sensory representations. In contrast to the retrieval/indirect consolidation-impairment explanation, this would require that such effects of hydrocortisone exceed its retrieval impairing effects, and are relatively selective for declarative, contextual (rather than sensory-) representations of the traumatic event. However, as we found no evidence for improved voluntary memory for narrative aspects of the trauma film on day 8 in the hydrocortisone group, this latter explanation is not supported by our data. In addition, as with propranolol, hydrocortisone's effects were observed before sleep. This therefore also raises some problems for the former explanation, as the ‘retrieval as a route to memory consolidation’ account (Antony et al., Reference Antony, Ferreira, Norman and Wimber2017, p. 573) proposes a major role for memory reactivations during slow-wave sleep (Antony et al., Reference Antony, Ferreira, Norman and Wimber2017). Nonetheless, the idea that pre-sleep replay of events shortly after encoding could also contribute to consolidation, and be disrupted by hydrocortisone, has not, to our knowledge, been tested empirically. Regardless, other studies have also shown memory performance decrements due to endogenous/exogenous cortisol before sleep (Diamond et al., Reference Diamond, Fleshner, Ingersoll and Rose1996; Kirschbaum et al., Reference Kirschbaum, Wolf, May, Wippich and Hellhammer1996; Kuhlmann et al., Reference Kuhlmann, Kirschbaum and Wolf2005). Future studies should seek to test these ideas by, for example, testing declarative memory and behaviourally manipulating retrieval/rehearsal on day 1.
While the hydrocortisone group showed fewer intrusions, this group also showed a slower rate of decline in vividness of intrusions relative to placebo. The reasons for this are unclear but may reflect enhanced sensory processing, as previously demonstrated with hydrocortisone (Born et al., Reference Born, Hitzler, Pietrowsky, Pauschinger and Fehm1988). However, no drug was present during film viewing, and initial (day 1) vividness was (non-significantly) lower in the hydrocortisone relative to the placebo group. As such, any effects on sensory processing would have to have occurred after day 1 (e.g. through interaction between working memory and consolidation processes). It should also be noted that while vividness of residual intrusions on day 7 appeared to be higher in the hydrocortisone group, group differences were not statistically significant. As such, and in the absence of any relevant outcomes directly related to sensory processing performance following drug, we are unable to comment further on this finding at this stage.
A previous experimental study that examined the effects of hydrocortisone on intrusive memories in humans showed different effects to those described here (Rombold et al., Reference Rombold, Wingenfeld, Renneberg, Schwarzkopf, Hellmann-Regen, Otte and Roepke2016). That study had a number of similarities with the current study, including the use of the same trauma stimuli and participants with similar baseline demographic and mood characteristics. However, that study failed to show differences relative to placebo. Despite some methodological similarities, it is important to consider divergent procedures in the two studies, as these could point to critical boundary conditions that determine hydrocortisone's effects on intrusive memories. Three methodological differences stand out. Firstly, we administered hydrocortisone immediately after the trauma-film, whereas drug administration preceded film presentation by 1 h in the study by Rombold et al. (Reference Rombold, Wingenfeld, Renneberg, Schwarzkopf, Hellmann-Regen, Otte and Roepke2016). Secondly, our hydrocortisone dose (30 mg) was higher than used by Rombold et al. (Reference Rombold, Wingenfeld, Renneberg, Schwarzkopf, Hellmann-Regen, Otte and Roepke2016; 20 mg). Given the relatively short t 1/2 of hydrocortisone, these differences might have been particularly consequential in terms of the presence or persistence of adequate levels of cortisol during critical plasticity-related processes. Finally, although both studies tested healthy young women, participants differed in the use of contraceptive medication. Only 40% of participants in Rombold et al.'s (Reference Rombold, Wingenfeld, Renneberg, Schwarzkopf, Hellmann-Regen, Otte and Roepke2016) study were using oral contraceptives (compared to 100% in the current study), and such participants might show a distinct response to the trauma film (Roche et al., Reference Roche, King, Cohoon and Lovallo2013) and hydrocortisone (Gaffey et al., Reference Gaffey, Wirth, Hoks, Jahn and Abercrombie2014) relative to non-users.
In another study, hydrocortisone was administered on three consecutive days (10 mg twice a day), starting ~24-h after the trauma film (Graebener et al., Reference Graebener, Michael, Holz and Lass-Hennemann2017). Given this delayed treatment, the latter study might have less direct relevance for experimental investigation of secondary prevention. However, by starting treatment outside of the putative early consolidation period, that study (Graebener et al., Reference Graebener, Michael, Holz and Lass-Hennemann2017) does address the specific effects of hydrocortisone on retrieval, rather than encoding and/or consolidation. Regardless, no difference between hydrocortisone and placebo was found in the latter study either. Comparison of that study with the current one suggests that timing (immediacy) of treatment after trauma exposure might be a critical boundary condition for hydrocortisone's effects on intrusive memories.
A number of limitations of the current study must be acknowledged. Firstly, given the continued presence of circulating propranolol (t 1/2 = 4–6 h) and hydrocortisone (t 1/2 ~ 100 min) during the interval between the film and intrusion recording on day 1, we cannot rule out additional non-memory related drug effects on day 1 (e.g. changes in peripheral arousal, interoception, meta-cognition). Since the memory-based explanations proposed here were not tested mechanistically, they must remain speculative, but should be the subject of future research. The effects were also only tested in young women taking hormone-based contraceptives, raising questions about generalisability. However, it should be noted that use of the hormone-based contraceptive pill is common in the United States and many European countries, with approximately a quarter of women using it (Enewold et al., Reference Enewold, Brinton, McGlynn, Zahm, Potter and Zhu2010). The alternative approach of testing non-contraceptive using, regularly cycling women at a specific phase in their menstrual cycle (Soni et al., Reference Soni, Curran and Kamboj2013; Kamboj et al., Reference Kamboj, Krol and Curran2015) is cumbersome, and does not improve generalisability. Furthermore, studies limited to men are also problematic, especially given that there is a >2-fold higher lifetime prevalence of PTSD among women (Kessler et al., Reference Kessler, Sonnega, Bromet, Hughes and Nelson1995).
Another issue, which is common to most studies of the trauma-film paradigm, is that recording of memory events did not occur ‘online’ or very shortly after the memory events occurred, but rather, was retrospective. Although recent evidence suggests minimal differences between ecological momentary assessments using frequent probes (trauma reminders) on smartphones v. continuous recording (i.e. as and when intrusions occur), v. single episode recording (as used here) (Rattel et al., Reference Rattel, Grünberger, Reichenberger, Liedlgruber, Miedl, Blechert and Wilhelm2019), it is important to recognise that retrospectively recorded intrusion frequency must reflect, at least in part, metacognitive (meta-memory) processes. Although the role of noradrenergic and glucocorticoid pathways in modulating meta-memory remains poorly understood, it is worth noting that propranolol seems to enhance some aspects of metacognition (Hauser et al., Reference Hauser, Allen, Purg, Moutoussis, Rees and Dolan2017). On the other hand, emotional arousal is associated with increased memory confidence (Talarico and Rubin, Reference Talarico and Rubin2003), an aspect of meta-memory that could conceivably be down-regulated by arousal reducing drugs. This may therefore be a partial explanation for our observations with propranolol, but seems less likely for hydrocortisone.
As with any non-clinical, lab-based model of traumatic stress, we can never ethically simulate actual psychological trauma. The extent to which such experimental models reflect actual vulnerability to PTSD has yet to be determined, and as such, extending our conclusions to clinical treatment would be premature. However, clinical researchers might benefit from considering some of the methodological characteristics (dose, timing, participant characteristics) of this study in future testing of propranolol and hydrocortisone for secondary prevention. Finally, to enhance the credibility of future experimental studies, hypotheses, methods and statistical plans should be pre-specified and published on a suitable open science platform.
In summary, the current study is the first to demonstrate rapid reductions in involuntary memories with propranolol or hydrocortisone. The findings are consistent with previous clinical findings with hydrocortisone and also support continued investigation of propranolol in secondary prevention, especially if treatment can be delivered rapidly.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291719001028
Author ORCIDs
Sunjeev K. Kamboj, 0000-0003-2197-0826.
Acknowledgements
We thank Professor Chris Brewin and Tom Freeman for their valuable comments on the manuscript.
Financial support
This work was funded by a grant from Find a Better Way, UK Registered Charity 1140911 to SKK and RKD.
Conflict of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.







