Introduction
Dongxiang wild rice (Oryza rufipogon Griff., DXWR) is a common wild rice, with its northernmost habitat located at 28°14′N latitude in Dongxiang county, Jiangxi province, China (Mao et al., Reference Mao, Yu, Chen, Li, Zhu, Xiao, Zhang and Chen2015). DXWR has abundant genetic diversity and many valuable agronomic traits (Zhang et al., Reference Zhang, Zhou, Fu, Su, Wang and Sun2006). However, the introgression of favourable genes from DXWR to cultivated rice through traditional breeding could be associated with complications due to the substantial amount of linkage drag from DXWR.
Molecular marker assisted selection (MAS) can greatly improve the efficiency of rice breeding through precise transfer of genomic regions of interest (Kaur et al., Reference Kaur, Panesar, Bera and Kaur2015). The most frequently used molecular markers include simple sequence repeat (SSR), insertion-deletion (InDel), and single nucleotide polymorphism (SNP) markers. These markers reflect specific sites in the genome with polymorphisms between individuals from one to hundreds of nucleotide bases (Hayashi et al., Reference Hayashi, Hashimoto, Daigen and Ashikawa2004; Yadav et al., Reference Yadav, Aravindan, Ngangkham, Shubudhi, Bag, Adak, Munda, Samantaray and Jena2017). Additionally, with the rapid development of high-throughput sequencing, structural variation (SV) is receiving increasing attention for molecular marker development (Davey et al., Reference Davey, Hohenlohe, Etter, Boone, Catchen and Blaxter2011). SV markers are genomic structural variation markers that represent deletions, insertions, duplications, inversions, and translocations of DNA segments in the genome (Xu et al., Reference Xu, Liu, Ge, Jensen, Hu, Li, Dong, Gutenkunst, Fang, Huang, Li, He, Zhang, Zheng, Zhang, Li, Yu, Kristiansen, Zhang, Wang, Wright, McCouch, Nielsen, Wang and Wang2011). SV markers can not only detect structural variations of up to several thousand nucleotide bases, but also have the advantages of co-dominance, repeatability, stability, and simplicity of use (Wang et al., Reference Wang, Chen, Zhang, Yan, Lu, Zhang, Jiang, Wu, van Peer, Li and Xie2015). Although SVs had been studied in DXWR (Zhang et al., Reference Zhang, Xu, Mao, Yan, Chen, Wu, Chen, Luo, Xie and Gao2016), the development of SV markers in this valuable genetic resource has not been reported. Therefore, the main objectives of this study were to: (i) develop a set of genome-wide SV markers for DXWR; and (ii) test their stabilities and polymorphisms in DXWR and cultivated rice.
Experimental
Whole-genome sequencing, reads filtering, and clean reads mapping to the reference genome were performed as previously (Zhang et al., Reference Zhang, Zhang, Cui, Luo, Zhou and Xie2015). Based on the paired-end sequence method, one read should be mapped to the forward strand, and the other to the reverse strand. The distance between the two locations should be in accordance with insert size (Eren et al., Reference Eren, Vineis, Morrison and Sogin2013). Therefore, the alignment of the two paired reads to the genome is regarded to be of normal direction and appropriate distance. If the direction or distance is abnormal, then the region might has SVs. Abnormal pair-end alignments were further analysed by clustering and compared with previously defined SVs (Wang et al., Reference Wang, Chen, Zhang, Yan, Lu, Zhang, Jiang, Wu, van Peer, Li and Xie2015). The SVs were detected using the SOAPsv program as described by Li et al. (Reference Li, Zheng, Luo, Wu, Zhu, Li, Cao, Wu, Huang, Shao, Ma, Zhang, Feng, Zhang, Du, Tian, Li, Zhang, Li, Bolund, Kristiansen, de Smith, Blakemore, Coin, Yang, Wang and Wang2011), with support from at least three abnormal paired-end reads.
Primer pairs were designed using Primer3 (v. 0.4.0) software based on the region including flanking sequences on both sides of SV loci (Untergasser et al., Reference Untergasser, Cutcutache, Koressaar, Ye, Faircloth, Remm and Rozen2012). Genomic DNA was extracted from fresh leaves using the CTAB method (Abdel-Latif and Osman, Reference Abdel-Latif and Osman2017). Polymerase chain reaction (PCR) amplification reactions were performed in a mixture, including 1 µl DNA (200 ng/μl), 0.2 µl dNTP (10 mmol/l), 0.2 µl primers (10 µmol/μl), 1.5 µl 10 × buffer (with Mg2+), 0.2 µl Taq DNA polymerase (5 U/μl), and 11.9 µl ddH2O. The reaction parameters were set as follows: initial denaturation at 95°C for 5 min, followed by 35 cycles at 95°C for 30 s, 56°C for 30 s, 72°C for 2 min, and a 10 min final extension step at 72°C. PCR products were separated on agarose gels and stained with GelRed nucleic acid staining dye. A total of 21 accessions of rice germplasm, including DXWR and 20 rice cultivars (online Supplementary Table S1), were collected in our laboratory.
Discussion
In our previous study, SVs between DXWR and Nipponbare (Oryza sativa L. ssp. japonica) had been detected to investigate lost/acquired genes during rice domestication (Zhang et al., Reference Zhang, Xu, Mao, Yan, Chen, Wu, Chen, Luo, Xie and Gao2016). However, the development of SV markers in DXWR has not been reported. To enrich the number of molecular markers available for DXWR, we detected SVs at a genome-wide level based on the results from the comparative genomics analysis. Totally, 17,893 SVs were detected between DXWR and Nipponbare using the SOAPsv program (Table 1). The genome-wide average SV density was one SV per 19.97 kb, whereas the density of SVs was varied among the 12 rice chromosomes (Table 1). The most abundant chromosomes for SVs were chromosomes 1, 12, 10, and 6, with one SV per 18.24, 18.67, 18.81, and 18.99 kb, respectively (Table 1). Of these, chromosomes 1, 6, and 10 also have a high density of InDels and SNPs (Zhang et al., Reference Zhang, Zhang, Cui, Luo, Zhou and Xie2015, Reference Zhang, Zhou, Zhang, Luo and Xie2017), implying that these chromosomes had more abundant genetic variations than the other chromosomes.
Table 1. Densities of SVs on 12 chromosomes detected between Dongxiang common wild rice (DXWR) and Nipponbare genomes
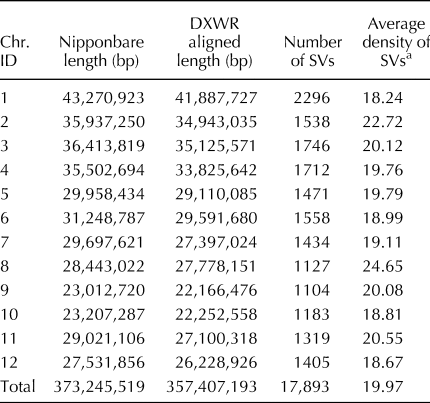
a Refers to a SV density in DXWR, and equals to aligned length (bp)/number of SVs/1000.
With the rapid development of high-throughput sequencing, SVs are receiving increasing attention for molecular marker development (Davey et al., Reference Davey, Hohenlohe, Etter, Boone, Catchen and Blaxter2011). For example, Ren et al. (Reference Ren, Zhao, Kou, Jiang, Guo, Zhang, Hou, Zou, Sun, Gong, Levi and Xu2012) constructed a high-resolution genetic map for watermelon with a total of 953 molecular markers, including 36 SV markers. Wang et al. (Reference Wang, Chen, Zhang, Yan, Lu, Zhang, Jiang, Wu, van Peer, Li and Xie2015) developed 104 SV markers and constructed a linkage map for the basidiomycete Volvariella volvacea. In this study, we developed 195 SV markers for DXWR. These molecular markers were mostly derived from the type of insertion and evenly distributed across the 12 chromosomes (online Supplementary Table S2). To validate their stabilities and polymorphisms, we synthesized these molecular markers for PCR amplification. The results of agarose gel electrophoresis showed that 147 (75.4%) of the 195 markers can be successfully amplified and showed abundant polymorphisms between DXWR and Nipponbare (Fig. 1 and online Supplementary Table S3). Furthermore, to test these SV markers if they can be applied in the analysis of cultivated rice, we amplified the genome DNA fragments from 20 rice cultivars using the polymorphic SV markers. As a result, abundant polymorphisms were detected among these rice varieties (online Supplementary Fig. S1), which suggested that these polymorphic SV markers have a wide application prospect.
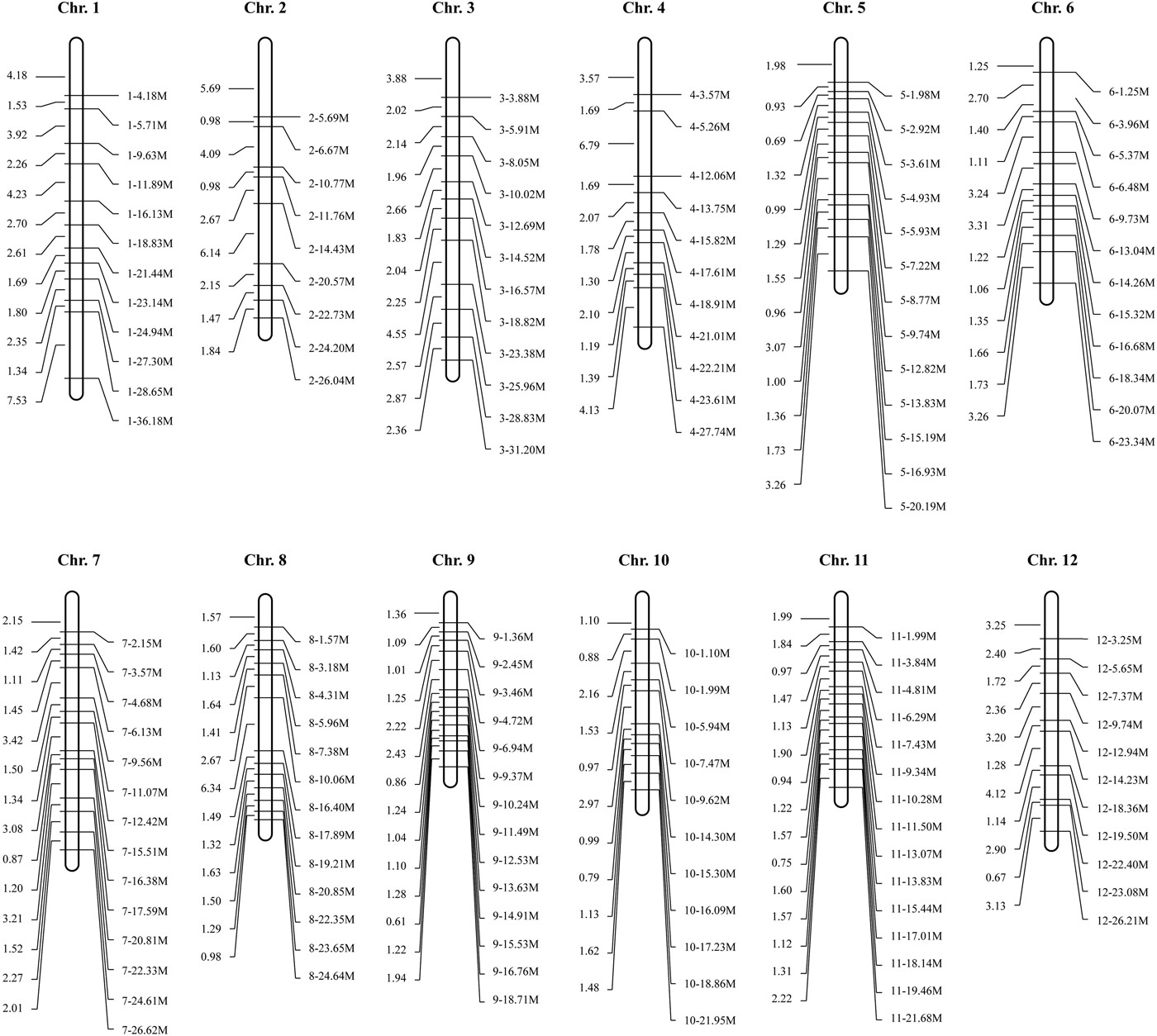
Fig. 1. Distribution of polymorphic SV markers on 12 chromosomes. The numbers to the left of each chromosome indicate the physical locations in Mb.
In conclusion, these SV markers greatly enrich the number of markers available for DXWR, which can serve as additional resources for assessing genetic diversity, constructing genetic maps, and identifying valuable genes from this important germplasm resource in the future.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S1479262119000145
Acknowledgements
This research was partially supported by the National Natural Science Foundation of China (31660386), the Natural Science Foundation of Jiangxi Province for Distinguished Young Scholars (20171BCB23040), the Natural Science Foundation of Jiangxi Province, China (20181BAB204010), the Foundation of Jiangxi Educational Committee (GJJ170193), and the Sponsored Program for Distinguished Young Scholars in Jiangxi Normal University.