Introduction
Despite the fact that camel milk composition shows wide variability in its constituents due to multiple physiological, genetic and environmental factors (Ayadi et al. Reference Ayadi, Hammadi, Khorchani, Barmat, Atigui and Caja2009; Konuspayeva et al. Reference Konuspayeva, Faye and Loiseau2009; Aljumaah et al. Reference Aljumaah, Almutairi, Ismail, Alshaikh, Sami and Ayadi2012; Mostafa et al. Reference Mostafa, El-Malky, Abd El-Salaam and Nabih2018), it has a unique composition compared to other ruminants. It contains higher fats, vitamins (such as A, E, B2 and C) and minerals levels (such as potassium, calcium, iron, magnesium, copper and zinc) but lower lactose level compared to cows’ milk (Konuspayeva et al. Reference Konuspayeva, Faye and Loiseau2009; Al-Humaid et al. Reference Al-Humaid, Mousa, El-Mergawi and Abdel-Salam2010; Yoganandi et al. Reference Yoganandi, Bhavbhuti, Wadhwani, Darji and Aparnathi2015). Additionally, camels’ milk contains several distinct proteins (such as immunoglobulins, lysozymes, lactoperoxidase, N-acetyl-§-glucosaminidase and lactoferrin) that exert numerous functions due to their antibacterial, antiviral and immunological properties (El-Agamy et al. Reference El-Agamy, Ruppanner, Ismail, Champagne and Assaf1992; Shabo et al. Reference Shabo, Barzel, Margoulis and Yagil2005). Camel milk has actually been proposed as a beneficial substitute for humans’ and cows’ milk for premature new-born and milk-allergic children because of the lack of β-lactoglobulin and beta casein, in addition to its comparably smaller nanobodies (Shabo et al. Reference Shabo, Barzel, Margoulis and Yagil2005; Zafra et al. Reference Zafra, Fraile, Gutiérrez, Haro, Páez-Espino, Jiménez and de Lorenzo2011). Furthermore, a considerable amount of research has been published indicating the potential therapeutic employment of camel milk for treating many dysfunctions and autoimmune diseases such as diabetes mellitus, hypercholesterolemia, obesity, atherosclerosis, oxidative stress, hepatitis B, Crohn's disease, autism and cancer (Corl et al. Reference Corl, Barbano, Bauman and Ip2003; Lock & Bauman, Reference Lock and Bauman2004; Agrawal et al. Reference Agrawal, Beniwal, Kochar, Tuteja, Ghorui, Sahani and Sharma2005, Sboui et al. Reference Sboui, Djegham, Khorchani, Hammadi, Barhoumi and Belhadj2010; Diaz-Medina et al. Reference Díaz-Medina, Caja, Rovai, Salama, Cabrera, Ayadi, Aljumaah and Alshaikh2016; Abrhaley and Leta, Reference Abrhaley and Leta2018), which reflects a growing interest in utilizing camels’ milk for human nutrition.
Due to the increasing global demand for value-added foods with potential health benefits for humans, a specific initiative has been promoted to produce dairy products enriched with conjugated linoleic acid (CLA), a frequently used term that represent a family of positional and geometrical isomers of octadecadienoic (C18) acids with a conjugated double bond system. Rumenic acid (RA; C18:2, cis-9, trans-11), also known as bovinic acid, is a major CLA isomer found in dairy and meat products of ruminant animals, accounting for 0.75–0.90 of the total CLA in milk fat (Kramer et al. Reference Kramer, Parodi, Jensen, Mossoba, Yurawecz and Adlof1998; Bauman et al. Reference Bauman, Corl, Baumgard, Griinari, Garnsworthy and Wiseman2001). This isomer is primarily a product of de novo synthesis in the tissues of ruminants, including the mammary gland, via the enzyme Δ9-desaturase. The substrate is vaccenic acid [VA; C18:1(n-7), trans-11], an intermediate substrate produced in rumen bacteria by biohydrogenation of dietary polyunsaturated fatty acids (PUFA), especially linoleic acid [C18:2(n-6), cis-9,12] and α-linolenic acid [C18:3(n-3), cis-9,12,15], using isomerases and reductases (Griinari et al. Reference Griinari, Corl, Lacy, Chouinard, Nurmela and Bauman2000; Bauman et al. Reference Bauman, Corl, Baumgard, Griinari, Garnsworthy and Wiseman2001; Lock and Garnsworthy, Reference Lock and Garnsworthy2002). Based on several biomedical studies, RA and other CLA isomers have been shown to possess a number of potential health benefits across a variety of animal and in vitro cell culture models, which include immune system enhancement as well as anti-atherogenic, anti-obesity, anti-diabetic and anti-carcinogenic effects (McGuire and McGuire, Reference McGuire and McGuire2000; Scharf et al. Reference Scharf, Demmer and Deboer2013; Jéssica et al. Reference Jéssica, Suellen, Josevan, Ana, de Figueirêdo, Rita and Marta2015). Therefore, enrichment of ruminant products with these CLA isomers is an active area of research.
Numerous studies, mainly in dairy cows, have demonstrated that such enrichment depends on several factors. Diet has a major influence on milk and meat fat RA level. In fact, this factor has been investigated extensively, with type of forage (Bradford and Allen, Reference Bradford and Allen2004; Cruz-Hernandez et al. Reference Cruz-Hernandez, Kramer, Kennelly, Glimm, Sorensen, Okine, Goonewardene and Weselake2007), forage-to-concentrate ratio (Bell et al. Reference Bell, Griinari and Kennelly2006; Roy et al. Reference Roy, Ferlay, Shingfield and Chilliard2006), starch source in the concentrate (Cruz-Hernandez et al. Reference Cruz-Hernandez, Kramer, Kennelly, Glimm, Sorensen, Okine, Goonewardene and Weselake2007; Al-Dobaib, Reference Al-Dobaib2009), plant oil used and its PUFA content and composition (Lock and Bauman, Reference Lock and Bauman2004; Chilliard and Ferlay, Reference Chilliard and Ferlay2004), as well as inclusion of marine oil, meal, or algae (Chilliard et al. Reference Chilliard, Ferlay and Doreau2001; AbuGhazaleh and Jenkins, Reference AbuGhazaleh and Jenkins2004; Cruz-Hernandez et al. Reference Cruz-Hernandez, Kramer, Kennelly, Glimm, Sorensen, Okine, Goonewardene and Weselake2007) all being used. However, the influence of other factors such as animal genetics (e.g. breed), parity and stage of lactation on the rate of biohydrogenation and level of CLA isomers have received little attention in ruminants (Kelsey et al. Reference Kelsey, Corl, Collier and Bauman2003; Kay et al. Reference Kay, Weber, Moore, Bauman, Hansen, Chester-Jones, Crooker and Baumgard2005). Moreover, limited data exists evaluating natural variations in the milk fatty acid (MFA) profile due to management conditions (Mohamed and Mustafa, Reference Mohamed and Mustafa2016) or throughout lactation (Konuspayeva et al. Reference Konuspayeva, Faye, Loiseau, Narmuratova, Ivashehenko, Meldebkova and Davletov2010) in camels, despite the fact that MFA composition in camels has already been documented (Abu-Lehia, Reference Abu-Lehia1989; Haddadin et al. Reference Haddadin, Gammoh and Robinson2008; Konuspayeva et al. Reference Konuspayeva, Faye and Mussaad2014). Consequently, further studies are warranted.
In the past two decades, the Tunisian camel husbandry system a distinct shift from the traditionally extensive grazing system to the modern intensive stabling system has been seen due to the increasing consumption of camels’ milk in the market (Hammadi et al. Reference Hammadi, Khorchani, Khaldi, Majdoub, Abdouli, Slimane, Portetelle and Renaville2001; El-Hatmi et al. Reference El-Hatmi, Khorchani, Abdennebi, Hammadi and Attia2004). Under current management, feeding and milking differ between those systems. In fact, before weaning, grazing camels are group-fed in pastures on several halophyte native species throughout the day and on farm concentrate mixture at night, while stabled camels are fed individually under sheltered pens on a forage mixture supplemented with a commercial concentrate. Both groups are hand-milked using a half-milking system during this period. However, after weaning both groups are occasionally combined to be reared under intensive stabling conditions and machine-milked for the rest of their lactation. The present study was conducted to evaluate such husbandry system by evaluating the effect of type of management (grazing vs. stabling) and stage of lactation (early- to late-lactation stages) on lactation performance and MFA profile in dairy dromedary camels. It was hypothesized that one or both of these factors can have an influence.
Materials and Methods
Animals and management
Eleven healthy Maghrebi dromedary camels were selected randomly, 8 weeks after calving, from two groups reared under different husbandry systems (grazing, n = 6, used as control; and stabled, n = 5). Camels were 7–13 yr old, had a mean body-mass of 422 ± 27.1 kg, and identified using ear-tags.
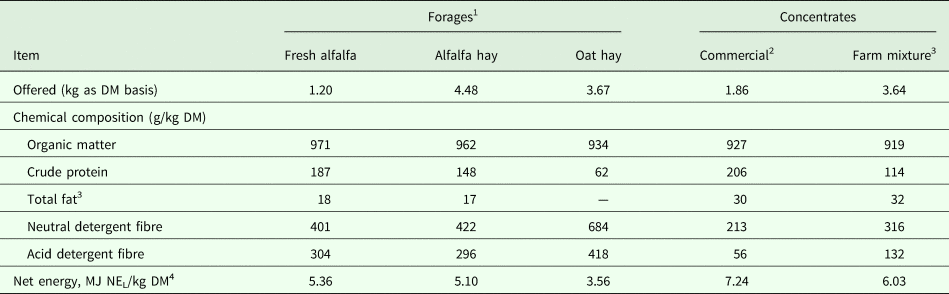
Before weaning (i.e. 9–15 weeks post-calving), grazing camels were fed during the day (08:00 to 16:00 h), at low stocking rate (approximately 1 camel/10 ha), on several halophyte native species (Arthrocnemum indicum Willd. Moq., Atriplex halimus L., Imperata cylindrica L. P. Beauv., Limoniastrum gyunianum L., Nitraria retusa Forsk., Reaumuria vermiculata L., Salicornia arabica L., Salsola tetragona Del., Tamarix gallica L., Zygophyllum album L.) that grew on saline rangelands (rain, 180 mm per year) at Fjé region, Medenine, Tunisia (Hammadi et al. Reference Hammadi, Khorchani, Khaldi, Majdoub, Abdouli, Slimane, Portetelle and Renaville2001). At sunset (16:00 h), the grazing camels and their calves returned to the shelters, where they were fed on farm concentrate mixture at a flat rate of 4 kg/d (as fed). Meanwhile, stabled camels were placed under intensive conditions, where they were fed individually and sheltered in sand-bedded pens (20 m2/camel). The daily ration of these camels consisted of a forage mixture supplemented with a commercial concentrate (Office Elevage et Paturage, Chenchou, Tunisia) at a flat rate of 2 kg/d (as fed). Batches of this diet were sampled throughout the study and analysed. The nutritive composition of forages and concentrate fed to dairy camels are shown in Table 1. During this period, both groups were milked in the morning (08:00 h) with a 14-h separation interval using a half-milking system. This system consisted of once-a-day hand milking of the front- and rear-left teats by a trained milker, while the calf sucked the right teats to induce an effective milk letdown. Milk recording and sampling were performed in duplicate on consecutive days during the last week of suckling (i.e. week 15), to evaluate lactation performance and MFA profile before weaning. All udder quarters were checked, once sampled, for sub-clinical mastitis using the California mastitis test (CMT, Hauptner, Solingen, Germany).
Table 1. Nutritive composition of forages and concentrate fed to dairy dromedary camels
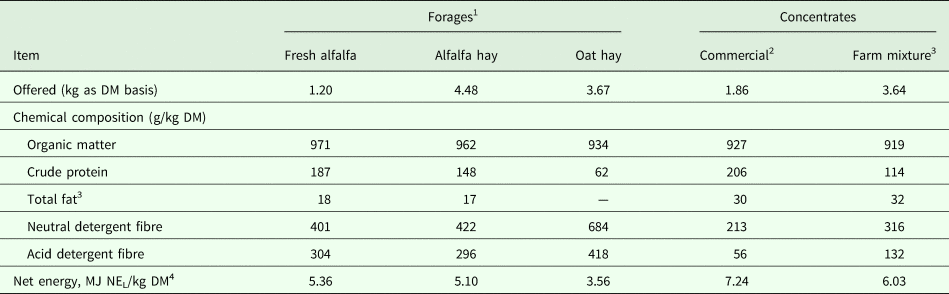
1 Fed to stabling camels.
2 Fed to stabling camels as a complement to the forages (Office d'Elevage et des Paturages, Chenchou, Tunisia).
3 Fed to grazing camels and composed of (g/kg as fed): 600 barley meal, 175 wheat bran, 175 olive cake meal, and 50 minerals and vitamins complex (IRA, Medenine, Tunisia).
4 Estimated from INRA tables using INRAtion PrévAlim software v3.23.
Weaning of the calves began at 4 months old (i.e. week 16), when they had approximately tripled their birth weight. Thereafter, both groups were combined and reared under the intensive stabling condition for the rest of lactation (i.e. from weeks 16–52). After weaning, camels were machine-milked twice daily (08:00 and 16:00 h), with a transition period of 2 weeks to allow adaptation to machine milking, using a restraining stall and a portable milking machine (Agromilk, Agro-service, Tunis, Tunisia) throughout lactation. Milking machine was set at 48 kPa, 60 pulses/min, and 60:40 pulsation ratio, while milking routine included teat washing, teat drying with paper towels, machine milking, machine stripping and finally teat dipping with Benzalkonium chloride (Polycide, Laboratoires Interchem, Tunis, Tunisia). Daily milk recording and sampling were performed in triplicate on consecutive days at the morning milking of early- (week 20), mid- (week 35) and late-lactation stages (week 47), in order to evaluate lactation performance and MFA profile after weaning. All udder quarters were checked using CMT, as described previously.
Sample analysis
For feed analysis, forage and concentrate composition was determined according to the standard procedures for feedstuffs analysis (AOAC, 1990; Van Soest et al. Reference Van Soest, Robertson and Lewis1991).
For milk analysis, three milk samples were collected from each animal. The first sample (~500 ml) was analysed immediately after milking to determine pH using a pH meter (Thermo Orion™, Model 420A+, Thermo Scientific, Breda, Netherlands), density (kg/l) using a thermo-lacto-densimeter (Funke-Gerber, Berlin, Germany), and titratable acidity (Dornic acidity, °D) by titrating 100 ml of fresh milk with N 1/9 sodium hydroxide (NaOH) using phenolphtalein as the indicator. The other two samples (~50 ml) were transported to the laboratory inside an ice box, and then preserved at 4°C for 24 h prior to analysis. Total milk solids and milk ash were analysed using gravimetry according to IDF (1987, IDF 21B and IDF 027). Milk true protein was determined using a spectrophotometer (CECIL CE2041, 2000 series, Cecil Instruments, Cambridge, UK) according to the Bradford method (Bradford, Reference Bradford1976; Kamizake et al. Reference Kamizake, Gonçalves, Zaia and Zaia2003), while milk fat was determined using a Gerber-butyrometer according to the Neusal method (Wangoh and Farah, Reference Wangoh, Farah, Farah and Fischer2004). Daily milk yield values were transformed according to fat-corrected-milk at 3% fat content (FCM3%) using the following equation: [FCM3% = daily milk yield × [0·197 × Fat (%) + 0·408], as recommended by Aljumaah et al. (Reference Aljumaah, Ayadi, Alshaikh, Caja, Simianer, Lindberg, Fourichon, Vestergaard, Bernués, Kuipers, Bodin, Knap, Miraglia and Pollott2013).
For MFA analysis, milk samples (~10 ml) were at first centrifuged at 9000 g at 4°C for 30 min. Then, fat samples (~500 mg) were collected after cooling at –20°C for 10 min and preserved at –80°C until analysis for MFA. Briefly, a fat sample (~70 mg) was first dissolved in 1 ml of benzene. Then, alkaline transesterification was completed using 2 ml of 0·5 M sodium methoxide in methanol (10 min at 50°C). A second methylation with 3 ml of 100 ml/l of methanolic hydrochloric acid (HCl; 10 min at 80°C) was also performed. After adding 1 ml of heptane and 7·5 ml of 60 g/l of potassium carbonate (K2CO3) and centrifuging at 6000 g and 4°C, the top solvent layers were transferred to a tube. Then, 1 g of sodium sulphate (Na2SO4) was added, and samples were centrifuged again at 6000 g and 4°C. Clear layers containing fatty acid methyl esters (FAME) were transferred to 1 ml autosampler vials and stored at −20°C until analysis. Separation and quantification of the FAME was carried out using a gas chromatograph (HP 6890, Agilent Technologies, Palo Alto, CA, USA) equipped with a flame ionization detector and capillary column (CP-Sil-88, 100 m × 0·25 mm internal diameter (i.d.) with a 0·20 µ capillary thickness; Varian Inc., Palo Alto, CA, USA). The initial temperature of 70°C (for 1 min) was increased to 225°C (for 15 min) at a rate of 1°C/min. In that way, MFA were converted into FAME without migration of conjugated double bonds of unsaturated fatty acids (Palmquist and Jenkins, Reference Palmquist and Jenkins2003). Individual fatty acids were identified by comparing the retention time with pure standards (Sigma-Aldrich Química, Madrid, Spain) and then expressed as grams per 100 gram of fatty acids. However, two standards were used for identifying RA and VA. The C18:1, cis-11 and C18:1, trans-11 isomers (Sigma-Aldrich Inc., St. Louis, MO, USA) were used for VA, while the C18:2, cis-9, trans-11 and C18:2, trans-10, cis-12 isomers (Matreya Inc., State College, PA, USA) were used for RA.
Furthermore, several indices were calculated: the Δ9-desaturase index was calculated for four pairs of fatty acids (C14:1,cis-9/C14:0, C16:1,cis-9/C16:0, C18:1,cis-9/C18:0 and RA/VA) that represent products and substrates for Δ9-desaturase enzyme, as carried out by Kelsey et al. (Reference Kelsey, Corl, Collier and Bauman2003), while the overall-desaturase index was calculated according to Kay et al. (Reference Kay, Weber, Moore, Bauman, Hansen, Chester-Jones, Crooker and Baumgard2005). Moreover, atherogenicity and health-promoting indices were calculated according to Ulbricht and Southgate (Reference Ulbricht and Southgate1991) and Chen et al. (Reference Chen, Bobe, Zimmerman, Hammond, Luhman, Boylston, Freeman and Beitz2004), respectively.
Statistical analysis
Data were analysed using SAS version 9.1 (SAS Inst., Inc., Cary NC, USA). For data obtained before weaning, the PROC GLM procedure was used to determine the effects of the management system, where the general mean, fixed effects of the management system (grazing vs. stabling) and the residual error were all included in the model. Meanwhile, PROC MIXED with repeated measurements was used for data obtained after weaning, where the model included the general mean, fixed effects of the management system (grazing vs. stabling) and stage of lactation (early-, mid- or late-lactation stages), their interactions and the residual error. However, no carry-over effect of the management was detected on any of the variables (P = 0·185–0·988, data not shown); therefore, this factor was removed from the model in this case. Mean differences, in all cases, were elaborated using the PDIFF option after having been subjected to ANOVA. Statistical significance was declared at P < 0·05, while trends were declared at P < 0·10. Moreover, the interrelationship analyses were attained using the PROC CORR and PROC REG procedures. Means and their SE are presented, unless otherwise indicated.
Results
Lactation performance
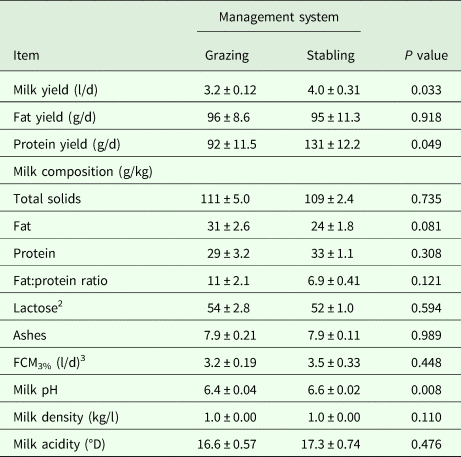
The influence of management system and stage of lactation on lactation performance are summarized in Tables 2 and 3, respectively. Results showed that overall means of milk and protein yields of stabled camels were greater (P < 0·05) than that of grazing camels during suckling (Table 2). Meanwhile, milk pH increased (P < 0·05) and milk fat tended to decrease (P < 0·10) in milk samples collected from stabled camels compared to grazing camels. No differences were observed for the remaining milk components and parameters (Table 2).
Table 2. Lactation performance (Means ± SE) as influenced by the management system in dairy dromedary camels during the suckling period (9–16 weeks of lactation)
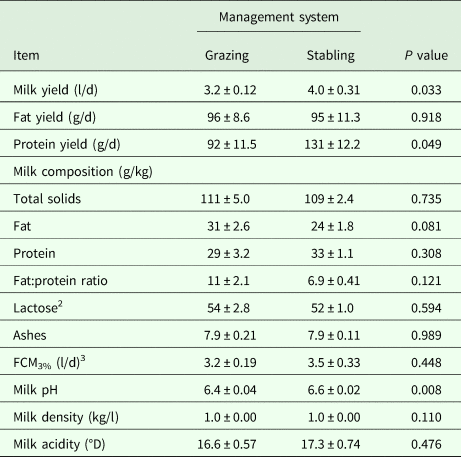
1Milk was collected in duplicate during consecutive days at week 15 of lactation using a half-milking system (Details are shown in the text).
2 Estimated by difference.
3 Fat-corrected-milk at 3% fat content (FCM3%) was calculated as recommended by Aljumaah et al. (Reference Aljumaah, Ayadi, Alshaikh, Caja, Simianer, Lindberg, Fourichon, Vestergaard, Bernués, Kuipers, Bodin, Knap, Miraglia and Pollott2013).
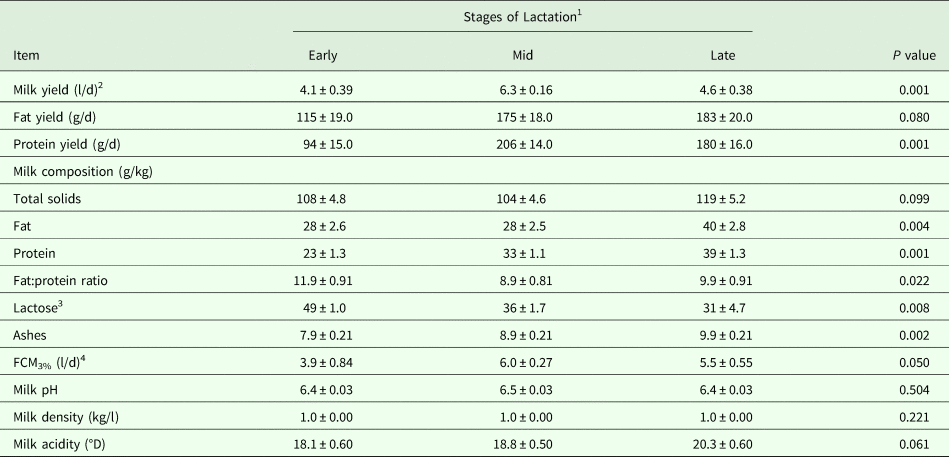
Table 3. Lactation performance (Means ± SE) as influenced by the stage of lactation in dairy dromedary camels during the milking period (16–47 weeks of lactation)
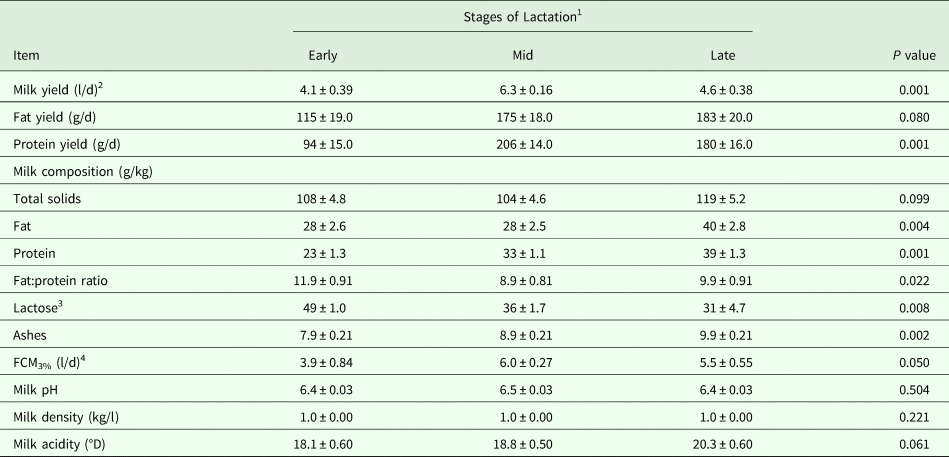
1 Samples were taken for early, mid and late lactation at 20, 35 and 47 weeks of lactation, respectively.
2 Milk was collected in triplicate using machine-milking system (Details are shown in the text).
3 Estimated by difference.
4 Fat-corrected-milk at 3% fat content (FCM3%) was calculated as recommended by Aljumaah et al. (Reference Aljumaah, Ayadi, Alshaikh, Caja, Simianer, Lindberg, Fourichon, Vestergaard, Bernués, Kuipers, Bodin, Knap, Miraglia and Pollott2013).
On the other hand, results demonstrated a convex quadratic trend (R2 = 0·72; P < 0·05) in daily milk yield as lactation advanced, where milk yield collected after weaning using the machine-milking system peaked (P < 0·05) at mid-lactation (Table 3). Daily milk yields of fat (P < 0·10) and protein (P < 0·05) also increased, although no differences were observed after mid-lactation. However, no difference was observed in milk fat content at mid-lactation, causing an inverted ratio of fat:protein, but was improved at late-lactation. As a consequence, milk fat and protein contents increased (P < 0·05) by 41·28 and 70·74%, respectively, from early- to late-lactation. Additionally, milk yield values expressed as FCM3% differed (P < 0·05) according to stage of lactation (Table 3). For other milk components and parameters, milk ash increased (P < 0·05), milk total solids and acidity tended to increase (P < 0·10), while milk lactose content decreased (P < 0·05) as lactation advanced. No differences were observed for the rest of the parameters (Table 3).
Milk fatty acid profile
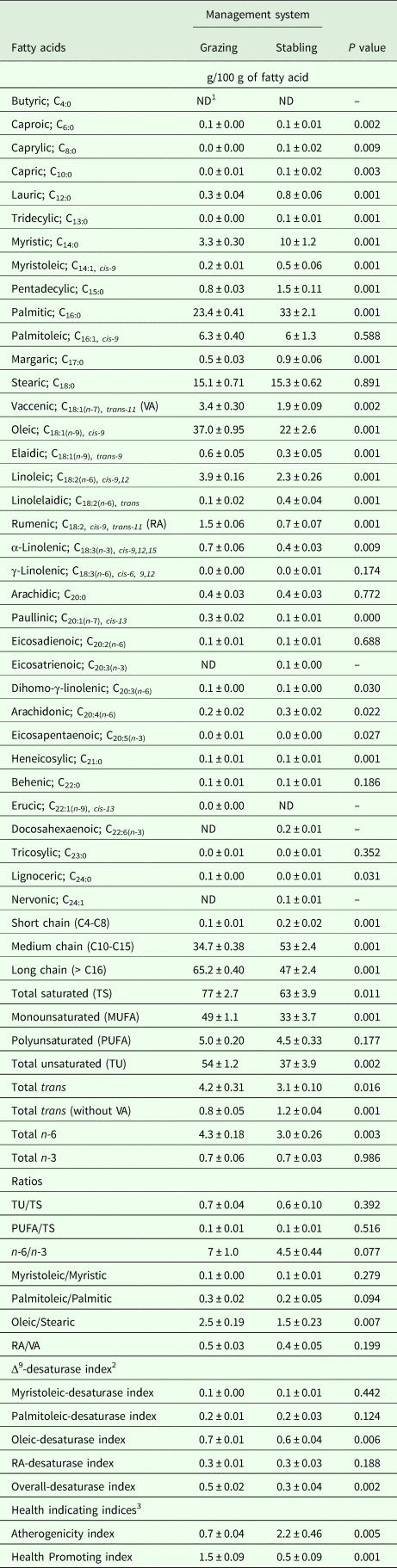
Table 4 displays the influences of management system on the MFA profile. During suckling, stabled camels produced milk richer (P < 0·05) in caproic acid (120%), caprylic acid (350%), capric acid (175%), lauric acid (152%), tridecylic acid (100%), myristic acid (220%), myristoleic acid (194%), pentadecylic acid (93%), palmitic acid (40%), margaric acid (76%), linolelaidic acid (186%), dihomo-γ-linolenic acid (20%), arachidonic acid (39%), eicosapentaenoic acid (100%), heneicosylic acid (133%), short- (C4-C8, 186%) and medium- (C10-C15, 52%) chains fatty acids and total trans fatty acids without VA (49%), but poorer (P < 0.05) in VA (–43%), oleic acid (–40%), elaidic acid (–52%), linoleic acid (–41%), RA (–53%), α-linolenic acid (–34%), paullinic acid (–57%), lignoceric acid (–60%), long-chain fatty acids (>C16, –28%), total saturated fatty acids (–19%), monounsaturated (MUFA, –34%), total unsaturated fatty acids (–32%), total trans fatty acids (–26%) and total n-6 fatty acids (–29%), as well as having a lower oleic/stearic ratio (2.53 vs. 1.50), when compared to grazing camels (Table 4). Total n-6 fatty acids in milk samples collected from stabled camels was approximately 0·41 times lower (P < 0·05) than grazing camels, while both ratios of n-6 fatty acids to n-3 fatty acids and palmitoleic acid to palmitic acid tended (P < 0·10) to decrease in milk samples collected from stabled camels. Moreover, atherogenicity index was higher (222%, P < 0·05), while oleic-desaturase, overall-Δ9-desaturase and health promoting indices were lower (–17%, –34% and –65%, respectively; P < 0·05) in stabled camels compared to grazing camels (Table 4). No differences were observed for the rest of the estimated and calculated parameters (Table 4).
Table 4. Milk fatty acids profile (Means ± SE) as influenced by the management system in dairy dromedary camels during the suckling period (9–16 weeks of lactation)
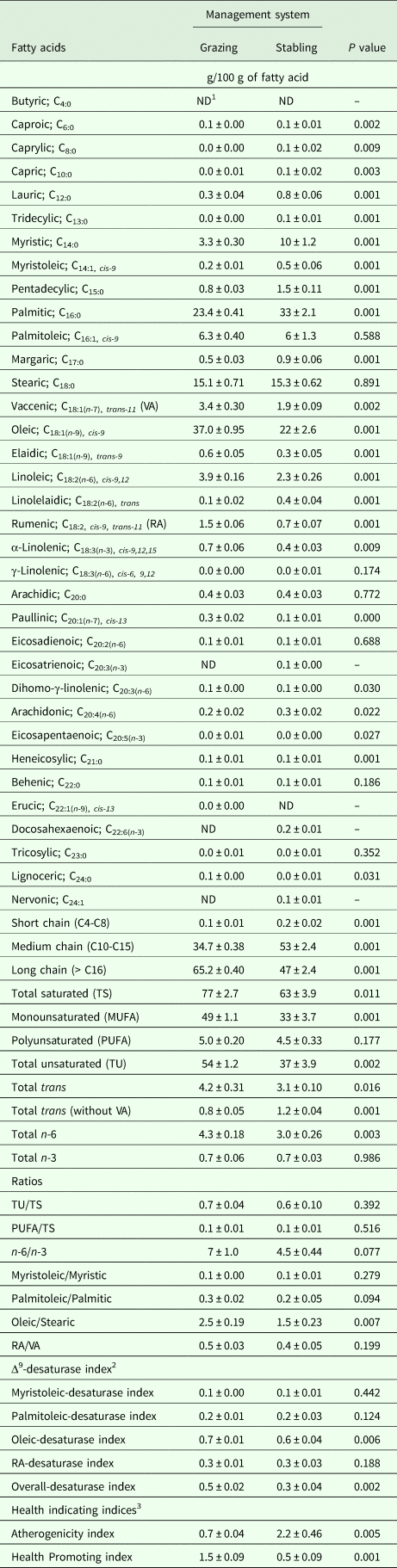
1 ND = not determined.
2 Δ9-desaturase index was calculated for four pairs of fatty acids (C14:1,cis-9/C14:0, C16:1,cis-9/C16:0, C18:1,cis-9/C18:0, and RA/VA) that represent products and substrates for Δ9-desaturase as carried out by Kelsey et al. (Reference Kelsey, Corl, Collier and Bauman2003), while the overall-desaturase index was calculated according to Kay et al. (Reference Kay, Weber, Moore, Bauman, Hansen, Chester-Jones, Crooker and Baumgard2005).
3 Health indicating indices like the atherogenicity and health promoting indices were calculated according to Ulbricht and Southgate (Reference Ulbricht and Southgate1991) and Chen et al. (Reference Chen, Bobe, Zimmerman, Hammond, Luhman, Boylston, Freeman and Beitz2004), respectively.
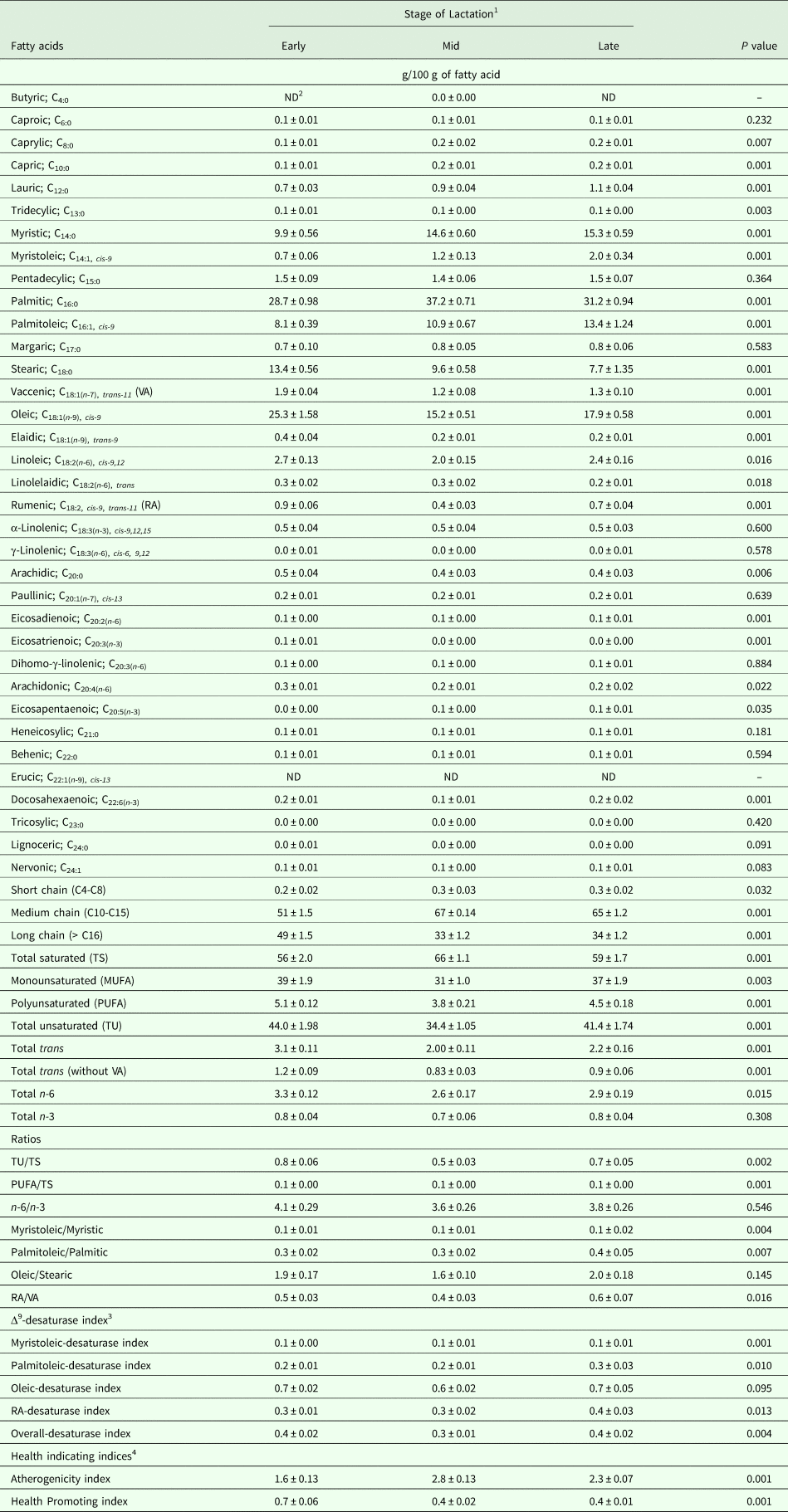
Results obtained after weaning and throughout lactation revealed that lactation stage affected the MFA profile markedly in dairy camels (Table 5). As a matter of fact, camels at mid-lactation produced milk richer (P < 0·05) in caprylic acid (78%), capric acid (64%), lauric acid (27%), myristic acid (47%), myristoleic acid (65%), palmitic acid (30%), palmitoleic acid (34%), eicosapentaenoic acid (25%), short- (47%) and medium- (33%) chains fatty acids and total saturated fatty acids (17%), but poorer (P < 0·05) in tridecylic acid (–40%), stearic acid (C18:0, –28%), VA (–38%), oleic acid (–40%), elaidic acid (-60%), linoleic acid (–24%), RA (–51%), arachidic acid (–29%), eicosadienoic acid (–50%), eicosatrienoic acid (–75%), arachidonic acid (–22%), long-chain fatty acids (–34%), MUFA (–21%), PUFA (–26%), total unsaturated fatty acids (–22%), total trans fatty acids (–36%), total trans fatty acids without VA (–32%), total n-6 fatty acids (–22%), as well as the ratios of total unsaturated to total saturated fatty acids (0·53 vs. 0·80), PUFA to total saturated fatty acids (0·06 vs. 0·09) and RA/VA (0·38 vs. 0·47), when compared to milk samples collected at the early stage of lactation (Table 5). Similar to the intensive stabling system, atherogenicity index was higher (76%, P < 0·05), while overall-Δ9-desaturase and health promoting indexes were lower (–21% and –45%, respectively; P < 0·05) at the mid-lactation compared to the early-lactation (Table 5).
Table 5. Milk fatty acids profile as influence by the stage of lactation in dairy dromedary camels (Means ± SE) during the milking period (16–47 weeks of lactation)
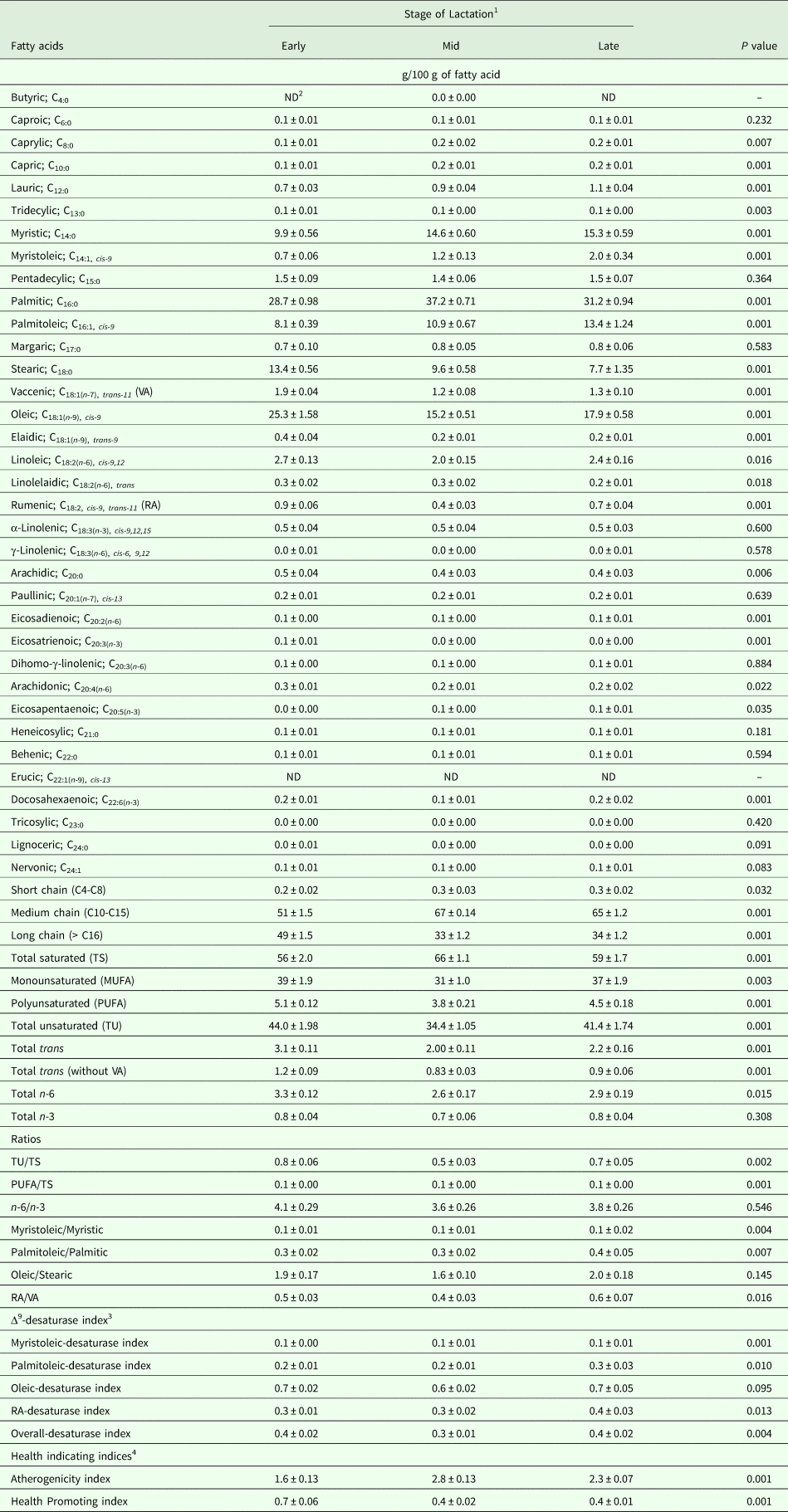
1 Samples were taken for early, mid and late lactation at 20, 35 and 47 weeks of lactation, respectively.
2 ND = not determined.
3 Δ9-desaturase index was calculated for four pairs of fatty acids (C14:1,cis-9/C14:0, C16:1,cis-9/C16:0, C18:1,cis-9/C18:0, and RA/VA) that represent products and substrates for Δ9-desaturase as carried out by Kelsey et al. (Reference Kelsey, Corl, Collier and Bauman2003), while the overall-desaturase index was calculated according to Kay et al. (Reference Kay, Weber, Moore, Bauman, Hansen, Chester-Jones, Crooker and Baumgard2005).
4 Health indicating indices like the atherogenicity and health promoting indices were calculated according to Ulbricht and Southgate (Reference Ulbricht and Southgate1991) and Chen et al. (Reference Chen, Bobe, Zimmerman, Hammond, Luhman, Boylston, Freeman and Beitz2004), respectively.
During late lactation, overall levels of palmitic acid, total saturated and total unsaturated fatty acids, MUFA, overall-Δ9-desaturase index and the ratios of total unsaturated to total saturated fatty acids and RA/VA returned to early lactation levels, while stearic, VA, oleic acid, elaidic acid, arachidic acid, eicosatrienoic acid, arachidonic acids, eicosapentaenoic acid, short-, medium- and long- chains fatty acids, total trans fatty acids, and total trans fatty acids without VA did not change compared to the mid-lactation levels (Table 5). In addition, linoleic acid, RA, eicosadienoic acid, PUFA, total n-6 fatty acids, atherogenicity and health promoting indexes, and the ratio of PUFA to total saturated fatty acids, although different (P < 0·05) from mid-lactation levels, did not return (P < 0·05) to their values at early lactation (Table 5). Meanwhile, camels produced milk richer (P < 0·05) in lauric acid (45% and 14%) and palmitoleic acid (64% and 23%), and had higher myristoleic acid to myristic acid and palmitoleic acid to palmitic acid ratios, as well as the myristoleic-desaturase (57% and 57%) and palmitoleic-desaturase indices (36% and 30%) compared to the early- and mid-lactation, respectively. Nevertheless, no differences were observed throughout lactation in caproic acid, pentadecylic acid, margaric acid, α-linolenic acid, γ-linolenic acid, paullinic acid, dihomo-γ-linolenic acid, heneicosylic acid, behenic acid, tricosylic acid, total n-3 fatty acids, or the n-6/n-3 and oleic/stearic ratios (Table 5).
It is worth mentioning that no sub-clinical mastitis cases were detected before or after weaning in any of the dairy camels based on CMT (data not shown), thereby indicating that the MFA profiles obtained herein were not affected by such factors.
Relationships among key milk fatty acids
The results obtained on the inter-relationships between RA and VA levels before weaning showed a strong positive correlation (r = 0.85; P < 0.001), and RA increased linearly as the VA score increased [RA = 0·383 × VA + 0·057; R2 = 0·72; P < 0·001], while after weaning a moderate positive correlation (r = 0.63; P < 0.001) was seen, and the linear increase of RA with increasing VA score had a lower coefficient of determination; [RA = 0·375 × VA + 0·131; R2 = 0·39; P < 0·001]. However, when all of the data collected before and after weaning is considered, the relationship between RA and VA becomes stronger [RA = 0·357 × VA + 0·147; r = 0.84; R2 = 0·71; P < 0·001]. Furthermore, the correlation coefficients calculated between pairs of Δ9-desaturase indices before weaning were as follows: Corr(myristoleic, palmitoleic) = 0.58, Corr(myristoleic, oleic) = 0.66, Corr(myristoleic, RA) = 0.87, Corr(palmitoleic, oleic) = 0.85, Corr(palmitoleic, RA) = 0.82 and Corr(oleic, RA) = 0.89. After weaning they were as follows: Corr(myristoleic, palmitoleic) = 0.98, Corr(myristoleic, oleic) = 0.81, Corr(myristoleic, RA) = 0.87, Corr(palmitoleic, oleic) = 0.85, Corr(palmitoleic, RA) = 0.92 and Corr(oleic, RA) = 0.89. Meanwhile, considering the whole data, the calculated correlation coefficients were as follows: Corr(myristoleic, palmitoleic) = 0.88, Corr(myristoleic, oleic) = 0.63, Corr(myristoleic, RA) = 0.82, Corr(palmitoleic, oleic) = 0.76, Corr(palmitoleic, RA) = 0.87 and Corr(oleic, RA) = 0.86.
Discussion
Influence of the management system
A quick look at the obtained data in Table 2 would imply that the management applied in the present study had no explicit impacts on lactation performance of dairy camels, thereby suggesting that diet and husbandry practice generally fail to demonstrate any influence on milk characteristics in camels. However, a more detailed examination of the obtained findings indicate otherwise. Notably, milk and protein yields of stabled camels were observed to be greater, while milk fat content was observed to be lower than that of grazing camels. There are two possible reasons for this: firstly, the high levels of dietary concentrate supplementation of the stabled camels compared to the grazing camels. This is consistent with previous reports on dairy cattle, where increasing the concentrate to roughage ratio was observed to increase milk production substantially (Kuoppala et al. Reference Kuoppala, Yrjanen, Jaakkola, Kangasniemi, Sariola and Khalili2004), DM and energy intake (Beyero et al. Reference Beyero, Kapoor and Tewatia2015), and apparent digestibilities of DM, OM, CF, NDF and ADF fractions (Beyero et al. Reference Beyero, Kapoor and Tewatia2015), while decreasing the growth of ruminal cellulolytic bacteria (Grant and Mertens, Reference Grant and Mertens1992) and milk fat percentage (Bauman et al. Reference Bauman, Perfield, Harvatine and Baumgard2008). The high level of concentrate in the diet gives rise to more propionic acid instead of acetic acid in the rumen, which in turn promotes partitioning of energy towards synthesis of body fat instead of milk fat, resulting ultimately in decreased milk fat (McDonald et al. Reference McDonald, Edwards, Greenhalgh, Morgan, Sinclair, Wilkinson, McDonald, Edwards, Greenhalgh, Morgan, Sinclair and Wilkinson2010). Indeed, Alhaj and Al-Kanhal (Reference Alhaj and Al Kanhal2010) and Aljumaah et al. (Reference Aljumaah, Almutairi, Ismail, Alshaikh, Sami and Ayadi2012) obtained the same results in camels. The second reason that could explain the findings obtained herein is the milking system. The half-milking system used before weaning in the current study might have contributed to decreased milk fat in that phase because of incomplete or lack of milk letdown, where the collected milk was mainly a cisternal milk known to be markedly lower in fat than alveolar milk (Ayadi et al. Reference Ayadi, Caja, Such, Rovai and Albanell2004). However, during this period both groups were milked using this system, which directs attention back to the first reason. In fact, a number of earlier reports support the lack of effect observed in the present study on FCM production, as well as protein and lactose percentages with the high levels of dietary concentrate (Sutton, Reference Sutton1989; Beyero et al. Reference Beyero, Kapoor and Tewatia2015).
Furthermore, it was evident that the management applied herein had prominent effects on the MFA profile in dairy camels (Table 4). According to several review articles, dairy ruminants generally derive fatty acids for MFA synthesis from multiple sources; about 100 g/kg from adipose tissues, about 400 g/kg from the diet and rumen microorganisms, and about 500 g/kg from the de novo biosynthesis in the mammary gland, where the relative contributions of these sources to MFA are highly dependent upon diet composition and feed intake (Walker et al. Reference Walker, Dunshea and Doyle2004; Kalač and Samkova, Reference Kalač and Samkova2010). In fact, a large number of articles have demonstrated previously the association of concentrate-based diets with a higher level of de novo synthesis resulting in more saturated milk fat, while higher intakes of dietary roughage resulted in more unsaturated milk fat (Schroeder et al. Reference Schroeder, Delahoy, Vidaurreta, Bargo, Gagliostro and Muller2003; AlZahal et al. Reference AlZahal, Or-Rashid, Greenwood, Douglas and McBride2008; Martini et al. Reference Martini, Liponi and Salari2010; Beyero et al. Reference Beyero, Kapoor and Tewatia2015). In the current study, stabled camels produced milk richer in short- and medium-chain fatty acids compared to grazing camels. In addition, the concentrations of long-chain fatty acids, MUFA and total unsaturated fatty acids were lower in the milk samples collected from stabled camels, thereby indicating a low availability of unsaturated fatty acids to be used by the mammary gland in the synthesis of milk lipids. As a matter of fact, the present data revealed that increasing the proportion of concentrate contents in the diet of stabled camels produced milk poorer in those fatty acids linked with possible health benefits such as oleic acid, VA, RA, linoleic acid and α-linolenic acid, as well as with lower palmitoleic acid to palmitic acid ratio. As stated earlier, most of the RA in milk fat is of endogenous origin, synthesized via the enzyme Δ9-desaturase with the substrate being VA, an intermediate substrate produced in rumen bacteria by biohydrogenation of PUFA, especially linoleic acid and α-linolenic acid (Bauman et al. Reference Bauman, Corl, Baumgard, Griinari, Garnsworthy and Wiseman2001). In the present study, the lower levels of linoleic and α-linolenic acid, precursors of milk RA and VA, detected in milk fat of stabled camels could indicate that these fatty acids were present at lower levels in the intensive diet than at grazing, which may therefore explain the positive relationship obtained between the RA and VA levels during this period (r = 0.85; R2 = 0.72; P < 0.001). Despite the fact that, unfortunately, feed fatty acid analysis were not performed in the current study to better support the results, these findings are coincident with other reports in dairy cattle (Kay et al. Reference Kay, Weber, Moore, Bauman, Hansen, Chester-Jones, Crooker and Baumgard2005; Beyero et al. Reference Beyero, Kapoor and Tewatia2015), ewes (Nudda et al. Reference Nudda, McGuire, Battacone and Pulina2005; Casals et al. Reference Casals, Caja, Pol, Such, Albanell, Gargouri and Casellas2006), goats (Chilliard et al. Reference Chilliard, Rouel, Ferlay, Bernard, Gaborit, Raynal-Ljutovac, Lauret, Leroux, Williams and Buttriss2006; Bouattour et al. Reference Bouattour, Casals, Albanell, Such and Caja2008) and camels (Ayadi and Casals, Reference Ayadi and Casals2009; Faye et al. Reference Faye, Konuspayeva, Narmuratova, Serikbaeva, Musaad and Mehri2013); thus, the avoidance of feeding high levels of dietary concentrate to dairy camels can be tentatively recommended.
On the other hand, the calculated atherogenicity and its inverse, health-promoting indices, in the present study were higher by 222% and lower by 65%, respectively, in stabled camels compared to grazing camels. These indices were proposed to characterize the atherogenic potential of different foods and diets on human health, where high values of the atherogenicity index, or low values of the health-promoting index, reflect the risk of lipid intake on coronary heart diseases due to the obstruction of coronary vessels by atherosclerosis (Wahle and Heys, Reference Wahle and Heys2002). According to Konuspayeva et al. (Reference Konuspayeva, Lemarie, Faye, Loiseau and Montet2008), camels’ milk is considered healthier for milk consumers than cows’ milk because of its low atherogenicity index values. However, under the stabling conditions of the present study, as the level of concentrate increased in the diet, the levels of oleic acid, VA, RA, linoleic acid, α-linolenic acid, long-chain fatty acids, MUFA and unsaturated fatty acids were decreased and the levels of lauric acid, myristic acid, palmitic acid, and short- and medium-chain fatty acids were increased, which in turn worsens both indices. In fact, several studies have shown that these short- and medium-chain fatty acids are considered to be cholesterol- and low-density lipoprotein-raising fatty acids (Berner, Reference Berner1993; Maijala, Reference Maijala2000), while others showed that using RA, linoleic acid, α-linolenic acid and long-chain fatty acids as a dietary supplement could have anti-atherosclerotic effects (Gavino et al. Reference Gavino, Gavino, Leblanc and Tuchweber2000). Therefore, from a dietary point of view, it is important to increase their levels in milk to improve the quality of camel milk and derived products, to subsequently decrease cardiovascular risk to consumers. Collectively, these findings suggest that management can influence the MFA profile in dairy camels.
This deduction was confirmed herein through the activity of the Δ9-desaturase enzyme in the mammary gland, which was estimated from four pairs of fatty acids that represent a product/substrate relationship for this enzyme. The current results revealed that the activity of this enzyme takes a part in fatty acid synthesis in the mammary gland of camels, despite the fact that factors affecting Δ9-desaturase activity are not yet well explained (Lock and Garnsworthy, Reference Lock and Garnsworthy2002; Tonhati et al. Reference Tonhati, Lima, Lanna, de Camargo, Baldi, de Albuquerque and Montrezor2011). In fact, it is evident that the activity of oleic-desaturase and overall-Δ9-desaturase were the only indices decreased in stabled camels compared to grazing camels. However, the calculated correlation coefficients among all indices were observed to range from 0.58 to 0.89, thereby indicating that all indices run in the same direction. Low Δ9-desaturase activity in stabled camels can be explained by the low levels of PUFA in their diets, and/or by the inclusion of olive cake meal (containing about 40 g/kg fat, rich in oleic) in the diet of grazing camels, which could be taken into account to justify the higher content of oleic in the milk of these animals compared with the intensively managed camels. In fact, data from several studies have previously illustrated that animals receiving olive cake showed higher levels of oleic acid and more unsaturated to saturated fatty acids (Caparra et al. Reference Caparra, Foti, Scerra, Postorino, Vottari, Cilione, Scerra and Sinatra2005; Faye et al. Reference Faye, Konuspayeva, Narmuratova, Serikbaeva, Musaad and Mehri2013). Moreover, Lock and Bauman (Reference Lock and Bauman2004) showed that the Δ9-desaturase enzyme in mammary gland acts on saturated fatty acids and converts them to unsaturated fatty acids, which raises the possibility that the lower ∆9-desaturase activity is responsible for milk fat depression as well as the low level of unsaturated fatty acids observed in milk samples collected from stabled camels. Therefore, these outcomes clearly bear substantial evidence that management can impact the lactation performance and MFA profile of dairy dromedary camels.
Influence of the lactation stage
Weaning of the calves and full milking of the camels using a machine milking did not instantly produce an increase in their daily milk yield (4.09 l/d vs. 3.95 l/d) but it increased as lactation advanced. Other studies in sheep have demonstrated a drop in milk yield after weaning (Ayadi et al. Reference Ayadi, Matar, Aljumaah, Alshaikh and Abouheif2014), where such a drop was explained by incomplete milk letdown at milking, when the stimulus from the lamb was suppressed by weaning. However, the resemblance of milk drop after weaning found in the present study to the findings of Ayadi et al. (Reference Ayadi, Matar, Aljumaah, Alshaikh and Abouheif2014) might go back to the adaptation of camels to the 2 weeks of machine milking. Although milk fat and protein yields, as well as FCM3%, were all observed to increase at mid-lactation, no difference was observed in milk fat content, causing an inverted ratio of fat:protein. At late lactation, the low galactopoiesis and metabolizable energy intake can both interact to reduce the daily milk yield, which in turn demonstrated an increase in milk fat content and improvement of the fat:protein ratio, while the observed increase in milk protein content might be due to the elevation of milk whey fraction instead of the casein fraction (Chilliard et al. Reference Chilliard, Ferlay and Doreau2001). Furthermore, the observed decrease in milk lactose content and the tendency to increase milk acidity throughout lactation might be a consequence of the seasonal increase in ambient temperature as lactation advanced (Farah and Bachmann, Reference Farah and Bachmann1987; Musaad et al. Reference Musaad, Faye and Abu-Nikhela2013; Aziz et al. Reference Aziz, Faye, Al-Eknah and Musaad2016).
In a similar way, the results obtained herein revealed that the stage of lactation can affect the MFA profile in dairy camels, where the data acquired at mid-lactation herein are similar to the data acquired from the intensive stabling system. As a matter of fact, saturation of milk fatty acids followed the lactation curve, where saturated fatty acids peaked at mid-lactation. The reason for such observations might go back mainly to the fact that the pre-formed fatty acids, such as oleic and others, coming from feed or from body mobilization, seemed to be responsible for higher levels of long-chain and unsaturated fatty acids in milk at the early stage of lactation, while short- and medium-chain fatty acids produced from de novo synthesis become more important at mid-lactation, leading to reductions in the concentration of VA, RA and the unsaturated to saturated fatty acids ratio at this stage (Chilliard et al. Reference Chilliard, Ferlay and Doreau2001; Casals et al. Reference Casals, Caja, Pol, Such, Albanell, Gargouri and Casellas2006). This was also confirmed by the lower activity of the ∆9-desaturase enzyme observed in the current study. As a consequence, atherogenicity and health-promoting indices were also observed to be worst at mid-lactation when compared to early- and late-lactation. This is in accord with some studies in other ruminants (Kelsey et al. Reference Kelsey, Corl, Collier and Bauman2003; Kay et al. Reference Kay, Weber, Moore, Bauman, Hansen, Chester-Jones, Crooker and Baumgard2005), but disagrees with others (Stanton et al. Reference Stanton, Lawless, Kjellmer, Harrington, Devery, Connolly and Murphy1997; MacGibbon et al. Reference MacGibbon, van der Does, Fong, Robinson and Thomson2001; Auldist et al. Reference Auldist, Kay, Thomson, Napper and Kolver2002).
The current results appear to tentatively support the avoidance of using camel milk produced at mid-lactation for human consumption. Nevertheless, the findings of several authors (Mensink et al. Reference Mensink, Zock, Kester and Katan2003; Knopp and Retzlaff, Reference Knopp and Retzlaff2004) indicate that there is little evidence of an atherogenic effect of lauric acid, myristic acid or palmitic acid, where these indices could be of interest only when there is an excessive fat consumption. The observed depression of milk fat at mid-lactation herein consequently contradicts such a tentative recommendation. In fact, camels’ milk appears to be healthier for human consumption in terms of levels of VA, RA, unsaturated fatty acids and health-promoting indices, when compared to those observed in cows’, ewes’ or goats’ milk produced under similar circumstances (McGuire and McGuire, Reference McGuire and McGuire2000; Agrawal et al. Reference Agrawal, Beniwal, Kochar, Tuteja, Ghorui, Sahani and Sharma2005, Sboui et al. Reference Sboui, Djegham, Khorchani, Hammadi, Barhoumi and Belhadj2010; Abrhaley and Leta, Reference Abrhaley and Leta2018).
In conclusion, it is becoming clear that the natural variations of lactation performance and MFA profile in dairy dromedary camels involve not only balancing the dietary forage-to-concentrate ratio and selecting carbohydrate sources, but also considering the variation of other factors. In fact, the present study sheds some basic light on the influence of management system and the stage of lactation in dairy camels, where it demonstrated that intensive stabling system and mid-lactation stage can alter lactation performance and MFA profile.
Author ORCIDs
Emad M. Samara, 0000-0002-3231-9193
Financial Support
The authors would like to express their sincere appreciation to the Deanship of Scientific Research at King Saud University for funding this work through research group No RGP-VPP-171.
Conflict of interest
We declare that no competing interests exist that are of influence on this work.
Ethical Standards
The whole protocols were carried out at Institut des Régions Arides (IRA), Médenine, Tunisia (33°N, 10°E) in accordance with the Tunisian laws on wellbeing and good practices of livestock animals.