Introduction
The search to isolate genes associated with psychiatric illnesses has been difficult. Psychiatric linkage and association studies have often reported contradictory results despite relatively large sample sizes and similar methodologies (Riley & McGuffin, Reference Riley and McGuffin2000; Faraone et al. Reference Faraone, Perlis, Doyle, Smoller, Goralnick, Holmgren and Sklar2005; Levinson, Reference Levinson2006). This may have arisen because of multiple genes with small effect sizes and interactions with environmental factors (Risch, Reference Risch1990). There also exists significant heterogeneity in the population and thus even larger sample sizes are needed for reliable findings (Crow, Reference Crow2007). One approach attempts to circumvent some of these issues by dissecting complex disorders into endophenotypes such as working memory (WM) (Gottesman & Gould, Reference Gottesman and Gould2003). This allows the identification of susceptibility genes by linking alleles to discrete behavioral traits that make up these disorders (Flint & Munafo, Reference Flint and Munafo2007). Impairments in WM occur across several psychiatric illnesses including attention-deficit hyperactivity disorder (ADHD), schizophrenia and depression (Goldman-Rakic, Reference Goldman-Rakic1994; Barkley, Reference Barkley1997; Chamberlain et al. Reference Chamberlain, Del Campo, Dowson, Müller, Clark, Robbins and Sahakian2007; Taylor Tavares et al. Reference Taylor Tavares, Clark, Cannon, Erickson, Drevets and Sahakian2007). The dopamine transporter gene (DAT1 or SLC6A3) and the catechol-O-methyltransferase gene (COMT) code for proteins that affect neurochemical clearance and have been implicated in the manifestation of these illnesses and WM (Castellanos & Tannock, Reference Castellanos and Tannock2002; Bertolino et al. Reference Bertolino, Blasi, Latorre, Rubino, Rampino, Sinibaldi, Caforio, Petruzzella, Pizzuti, Scarabino, Nardini, Weinberger and Dallapiccola2006; Ettinger et al. Reference Ettinger, Joober, De Guzman and O'Driscoll2006; Meyer-Lindenberg & Weinberger, Reference Meyer-Lindenberg and Weinberger2006; Lopez-Leon et al. Reference Lopez-Leon, Janssens, Gonzalez-Zuloeta Ladd, Del-Favero, Claes, Oostra and van Duijn2007). The aim of the present study was to investigate the effects of functional polymorphisms of DAT1 and COMT on WM in healthy volunteers.
An untranslated variable number of tandem repeat (VNTR) polymorphism exists at the 15th exon of the DAT1 gene, where the most common forms present are the alleles with 9 or 10 40-bp repeats (Vandenbergh et al. Reference Vandenbergh, Persico, Hawkins, Griffin, Li, Jabs and Uhl1992 a). Although DAT is marginally present in the prefrontal cortex (PFC), it is abundant in the striatum and midbrain (Sesack et al. Reference Sesack, Hawrylak, Matus, Guido and Levey1998; Lewis et al. Reference Lewis, Melchitzky, Sesack, Whitehead, Auh and Sampson2001). WM is thought to result from the reciprocal interaction of regions within these areas, namely the striato-thalamo-cortical system (Chudasama & Robbins, Reference Chudasama and Robbins2006). Tonic dopamine in the striatum, along with D1 transmission in the PFC, is thought to be responsible for both the stability of this neural network and the maintenance of task-relevant information in WM, whereas phasic dopamine in the striatum regulates the resetting and updating of WM when novel information is presented (Bilder et al. Reference Bilder, Volavka, Lachman and Grace2004; Hazy et al. Reference Hazy, Frank and O'Reilly2006). Individuals who are homozygous for the 10-repeat alleles have been shown to have the most focused engagement of WM networks during an episodic memory task (Schott et al. Reference Schott, Seidenbecher, Fenker, Lauer, Bunzeck, Bernstein, Tischmeyer, Gundelfinger, Heinze and Duzel2006) and a WM n-back task, but similar overall behavioral performances (Bertolino et al. Reference Bertolino, Blasi, Latorre, Rubino, Rampino, Sinibaldi, Caforio, Petruzzella, Pizzuti, Scarabino, Nardini, Weinberger and Dallapiccola2006; Caldu et al. Reference Caldu, Vendrell, Bartres-Faz, Clemente, Bargallo, Jurado, Serra-Grabulosa and Junque2007). Furthermore, 10-repeat allele homozygotes show enhanced evoked gamma band activity (Demiralp et al. Reference Demiralp, Herrmann, Erdal, Ergenoglu, Keskin, Ergen and Beydagi2007) and these 30–70 Hz waves affect cognitive processes including WM (Gray et al. Reference Gray, Konig, Engel and Singer1989; Lutzenberger et al. Reference Lutzenberger, Ripper, Busse, Birbaumer and Kaiser2002; Howard et al. Reference Howard, Rizzuto, Caplan, Madsen, Lisman, Aschenbrenner-Scheibe, Schulze-Bonhage and Kahana2003). Thus, these data implicate the DAT1 gene in aspects of cognition including WM, although the effect of this polymorphism at a cellular level remains disputed (Lynch et al. Reference Lynch, Mozley, Sokol, Maas, Balcer and Siderowf2003; van Dyck et al. Reference van Dyck, Malison, Jacobsen, Seibyl, Staley, Laruelle, Baldwin, Innis and Gelernter2005; VanNess et al. Reference VanNess, Owens and Kilts2005).
A missense mutation for the COMT gene leads to the substitution of methionine for valine (Lachman et al. Reference Lachman, Papolos, Saito, Yu, Szumlanski and Weinshilboum1996), with the Val allele coding for a more thermolabile protein with increased enzymatic activity (Chen et al. Reference Chen, Lipska, Halim, Ma, Matsumoto, Melhem, Kolachana, Hyde, Herman and Apud2004). The COMT alleles are co-dominant with three genotypes possible: the most active Val/Val, followed by Val/Met, and the least active Met/Met. The effects of COMT on cognition have been studied extensively. Healthy individuals with the low-activity Met/Met allele were found to perform significantly better on the Wisconsin Card Sorting Test (WCST; Egan et al. Reference Egan, Goldberg, Bhaskar, Callicott, Mazzanti, Straub, Goldman and Weinberger2001; Barnett et al. Reference Barnett, Jones, Robbins and Müller2007). However, association studies between COMT and WM using n-back tasks remain inconsistent (Egan et al. Reference Egan, Goldberg, Bhaskar, Callicott, Mazzanti, Straub, Goldman and Weinberger2001; Goldberg et al. Reference Goldberg, Egan, Gscheidle, Coppola, Weickert, Kolachana, Goldman and Weinberger2003; Stefanis et al. Reference Stefanis, Van Os, Avramopoulos, Smyrnis, Evdokimidis and Hantoumi2004; Bruder et al. Reference Bruder, Keilp, Xu, Shikhman, Schori, Gorman and Gilliam2005), with a recent meta-analysis showing no significant association (Barnett et al. Reference Barnett, Scoriels and Munafo2008). Functional magnetic resonance imaging (fMRI) studies have demonstrated that Met/Met individuals manifest more focused cortical response in the dorsolateral PFC, with a similar performance on the n-back task compared to the other genotypes (Bertolino et al. Reference Bertolino, Blasi, Latorre, Rubino, Rampino, Sinibaldi, Caforio, Petruzzella, Pizzuti, Scarabino, Nardini, Weinberger and Dallapiccola2006; Caldu et al. Reference Caldu, Vendrell, Bartres-Faz, Clemente, Bargallo, Jurado, Serra-Grabulosa and Junque2007). This suggests a higher signal-to-noise ratio in the WM networks and thus more efficient processing, or less ‘work’ for the same outcome. A gene–gene interaction has also been reported whereby individuals with the Met/Met COMT genotype in combination with the 10/10 DAT1 genotype manifested the most focused responses (Bertolino et al. Reference Bertolino, Blasi, Latorre, Rubino, Rampino, Sinibaldi, Caforio, Petruzzella, Pizzuti, Scarabino, Nardini, Weinberger and Dallapiccola2006; Caldu et al. Reference Caldu, Vendrell, Bartres-Faz, Clemente, Bargallo, Jurado, Serra-Grabulosa and Junque2007).
The aim of this study was to further investigate the effect of the most common DAT1 and COMT polymorphisms on WM performance in healthy volunteers. The WM paradigm deployed is a variant of the n-back task designed to elicit a pure WM manipulation not confounded by response conflicts or other higher executive processes. Most studies investigating these polymorphisms with n-back tasks have had sample sizes of less than 100 participants (Bertolino et al. Reference Bertolino, Blasi, Latorre, Rubino, Rampino, Sinibaldi, Caforio, Petruzzella, Pizzuti, Scarabino, Nardini, Weinberger and Dallapiccola2006; Caldu et al. Reference Caldu, Vendrell, Bartres-Faz, Clemente, Bargallo, Jurado, Serra-Grabulosa and Junque2007), and only two published studies have had sample sizes of more than 200 (Goldberg et al. Reference Goldberg, Egan, Gscheidle, Coppola, Weickert, Kolachana, Goldman and Weinberger2003; Stefanis et al. Reference Stefanis, Van Os, Avramopoulos, Smyrnis, Evdokimidis and Hantoumi2004). In our sample of 291 participants, we predicted a DAT1×NBACK difficulty interaction and a COMT×NBACK difficulty interaction; it was expected that all genetic subgroups would perform similarly on the control trials but differently on the more difficult trials. DAT1 and COMT are analyzed independently of each other, with 10/10 individuals and Met/Met individuals expected to perform the best on the more difficult n-back conditions compared to the other genotypes in their respective groups. Finally, on account of two recent fMRI studies reporting an additive effect of both genotypes on WM (Bertolino et al. Reference Bertolino, Blasi, Latorre, Rubino, Rampino, Sinibaldi, Caforio, Petruzzella, Pizzuti, Scarabino, Nardini, Weinberger and Dallapiccola2006; Caldu et al. Reference Caldu, Vendrell, Bartres-Faz, Clemente, Bargallo, Jurado, Serra-Grabulosa and Junque2007), we also predicted a COMT×DAT1×NBACK difficulty interaction with the 10/10-Met/Met group outperforming the other groups on the more difficult trials of the n-back task.
Method
Participants
Individuals aged 18–45 years were recruited from the general population using media advertisements (Cambridge, UK, and surrounding areas). All participants gave written informed consent. Genotyping and baseline testing was part of a protocol approved by the Cambridge Research Ethics Committee (no. 03/266). Exclusion criteria with regard to lifestyle were: smoking >10 cigarettes per day, use of recreational drugs in the previous 5 years, and average weekly alcohol consumption >25 units per week. With respect to medical history, exclusion criteria were: history of heart failure, hypertension, diabetes, mental illness, stroke, or head trauma. Participants were asked to refrain from drinking caffeinated stimulant beverages on the test day. The National Adult Reading Test (NART; Nelson & Willison, Reference Nelson and Willison1991) and the Barratt Impulsivity Scale (BIS; Patton et al. Reference Patton, Stanford and Barratt1995) were administered to assess intelligence and impulsivity respectively.
WM paradigm
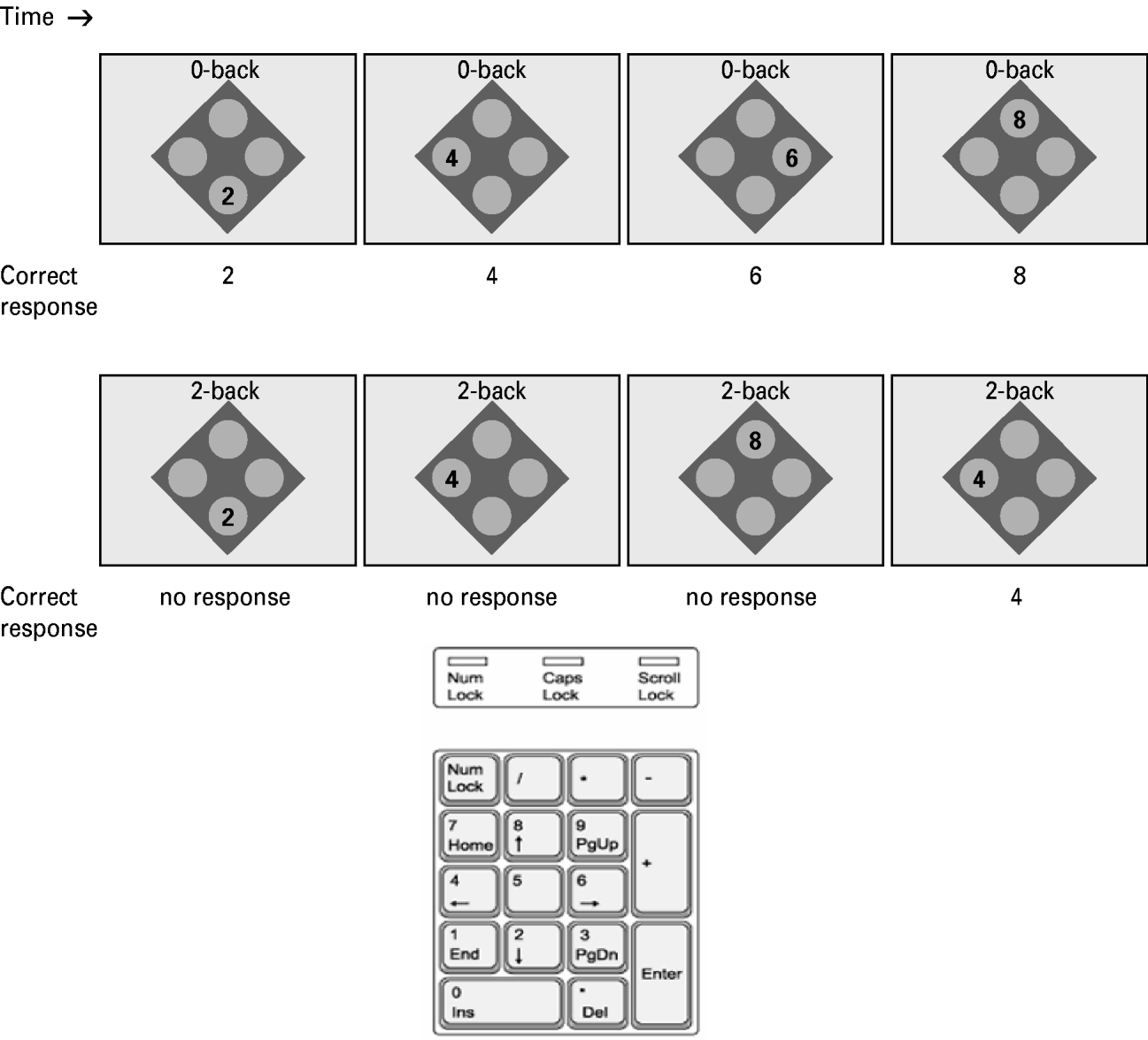
The spatial WM task used in this study was a behavioral variant of the computerized n-back task, which has been widely used in neuroimaging and behavioral research. Stimuli consisted of four numbers on a screen, each appearing within a circle (see Fig. 1). The numbers on the screen corresponded geometrically with the numbers on the right-hand side of a standard keyboard that was used for responding, and stimuli appeared on-screen for 400 ms with an interstimulus interval of 1400 ms. We used non-memory trials (0-back) and three working memory conditions (1-back, 2-back, 3-back) (see Fig. 1 for explanation). Each condition included six blocks of 14 stimuli. Hit rate and reaction times were calculated to assess behavioral performance. Participants who performed very poorly on the task [i.e. below 2 standard deviations (s.d.) away from the mean on at least two measures from the raw data, or 3 s.d. away from the mean in any one measure] were excluded.

Fig. 1. Computerized n-back task. In the 0-back control condition participants were asked to respond to every stimulus presented. During the 2-back condition participants were instructed to respond to a stimulus that was shown two stimuli back. The 2-back condition was an expansion of the 1-back, where participants were asked to respond if the number appearing on the screen was the same as the number that appeared two presentations before the current number.
Genotyping
Blood samples were collected after cognitive testing on-site, and were analyzed at the Molecular Genetics Laboratory, Addenbrooke's Hospital, Cambridge, UK. Genotypes for COMT and DAT1 were analyzed following polymerase chain reaction (PCR) amplification, which was performed using methods and primers described previously (Vandenbergh et al. Reference Vandenbergh, Persico, Hawkins, Griffin, Li, Jabs and Uhl1992 a; Lachman et al. Reference Lachman, Papolos, Saito, Yu, Szumlanski and Weinshilboum1996). Full details of the methodology are available from the corresponding author on request.
Statistical analysis
Demographic characteristics were analyzed using one-way analysis of variance (ANOVA) or χ2 tests as appropriate. To assess gene effects on WM, factorial mixed model ANOVAs were conducted (between-subject factor: genotype; within-subject factor: difficulty level, 0-back, 1-back, 2-back, and 3-back).
To assess a possible gene–gene interaction, a factorial 3 (9/9, 9/10, 10/10)×3 (Val/Val, Val/Met, Met/Met) mixed model ANOVA was performed. The dependent variables were hit rate (%) and reaction times (ms), and a two-tailed level of significance of p<0.05 was applied to all analyses. Contrasts between homozygote groups (Val/Val v. Met/Met and 9/9 vs. 10/10) for the 3-back condition were also conducted. Hit-rate percentages were converted using an arcsine transform to make the data normally distributed. SPSS version 12 (SPSS Inc., USA) for Windows was used for all analyses.
Results
Demographic and control data
The sample demographics and control data are summarized in Table 1. COMT genotype distribution was in Hardy–Weinberg equilibrium but the DAT1 distribution was skewed positively, as expected (Vandenbergh et al. Reference Vandenbergh, Persico and Uhl1992 b; Heinz et al. Reference Heinz, Goldman, Jones, Palmour, Hommer, Gorey, Lee, Linnoila and Weinberger2000). The COMT group was larger than the DAT1 group because DAT1 genotyping was initiated at a later time point and not all participants of the original COMT screening project gave prospective consent for further genotyping. Other than a disparity of age in the COMT group, no significant difference was apparent between genotyping groups for any of the demographic or control variables. NART IQ scores were positively correlated to the 2-back [r(308)=0.133, p<0.05] and 3-back [r(308)=0.129, p<0.05] but not the 0- or 1-back hit rates, indicating a significant effect of intelligence on WM performance. Age was inversely proportional for the 3-back condition [r(303)=−0.133, p<0.05]. Impulsivity scores did not correlate significantly with any measure.
Table 1. Demographic and control data

COMT, Catechol-O-methyltransferase; DAT1, dopamine transporter gene; NART, National Adult Reading Test; BIS, Barratt Impulsivity Scale; Val, valine; Met, methionine; s.d., standard deviation; w, White (mostly European Caucasian); a, Asian; na, North African.
*Indicates significant.
Genotype effect on n-back performance
The statistical analyses are summarized in Table 2. There was no significant association of hit rate or reaction time with either DAT1 (Fig. 2) or COMT (Fig. 3), and no interaction between the two. The results were similar when age was entered as a covariate for COMT. The contrast analysis between the COMT homozygotes in the 3-back condition was initially significant, but after entering age as a covariate the effect disappeared. n-back data are presented in Table 3. The results remained non-significant in a supplementary analysis that included subjects who had difficulty understanding the task.

Fig. 2. Effect of dopamine transporter gene (DAT1) polymorphism on n-back hit-rate performance.

Fig. 3. Effect of catechol-O-methyltransferase (COMT) genotype on n-back hit-rate performance.
Table 2. Statistical analyses of genotype effect on n-back performance

COMT, Catechol-O-methyltransferase; DAT1, dopamine transporter gene.
Table 3. Mean and standard deviation of n-back hit rates stratified by genotype

COMT, Catechol-O-methyltransferase; DAT1, dopamine transporter gene.
Power analysis
For COMT, with 82 participants in the Val/Val group and 61 in the Met/Met group, we had 80% power to detect an effect size of d=0.48 between the genetic subgroups at p=0.05. Previously, Goldberg et al. (Reference Goldberg, Egan, Gscheidle, Coppola, Weickert, Kolachana, Goldman and Weinberger2003) reported an effect size of 0.44 for this contrast in the 2-back condition, which we had 73% power to detect at p=0.05. Our actual detected effect sizes for differences between the two homozygous COMT subgroups (Met/Met >Val/Val) were d=0.13 for the 2-back condition and d=0.35 for the 3-back condition (calculated using transformed data). Note that these calculations do not take into account the small but significant age difference between the genetic subgroups. For DAT1, because of the relative rarity of the 9 allele, the two homozygous groups were very unbalanced, resulting in reduced power to detect effects. With 146 participants in the 10/10 group and 17 participants in the 9/9 group, we had 80% power to detect an effect size of d=0.72 between the genetic subgroups at p=0.05. However, previous studies have not found significant effects of this polymorphism on n-back performance (Bertolino et al. Reference Bertolino, Blasi, Latorre, Rubino, Rampino, Sinibaldi, Caforio, Petruzzella, Pizzuti, Scarabino, Nardini, Weinberger and Dallapiccola2006; Caldu et al. Reference Caldu, Vendrell, Bartres-Faz, Clemente, Bargallo, Jurado, Serra-Grabulosa and Junque2007). Consistent with these reports, our actual detected effect sizes for differences between the two homozygous DAT subgroups (10/10>9/9) were d=0.1 for the 2-back condition and d=−0.005 for the 3-back condition (calculated using transformed data).
Discussion
We report no significant effects of DAT1, COMT or DAT1×COMT on n-back task performance. This is the largest sample so far investigating DAT1 and n-back performance and one of the largest for COMT. An impairment in WM, and visuospatial WM in particular, is an attractive potential endophenotype for psychiatric diseases as it meets several criteria outlined previously, such as being continuously quantifiable, having good psychometric properties, and being more closely related to the genetic underpinnings of the illnesses than the disease itself or the core symptoms (Waldman, Reference Waldman2005). Although we did not find an association in our sample, we offer two potential explanations for our negative results.
Even though this study had a relatively large sample size, it may still have been underpowered to detect subtle overt behavioral effects of genotype. Imaging studies have reported effects for both of these polymorphisms in WM, without differences in behavioral n-back task performance (Bertolino et al. Reference Bertolino, Blasi, Latorre, Rubino, Rampino, Sinibaldi, Caforio, Petruzzella, Pizzuti, Scarabino, Nardini, Weinberger and Dallapiccola2006; Caldu et al. Reference Caldu, Vendrell, Bartres-Faz, Clemente, Bargallo, Jurado, Serra-Grabulosa and Junque2007), indicating that neuroimaging parameters are closer to the neural correlates of gene effects and thus may be more powerful for detecting gene effects (Meyer-Lindenberg & Weinberger, Reference Meyer-Lindenberg and Weinberger2006). Flint & Munafo (Reference Flint and Munafo2007) estimated that a sample of 1700 individuals would be required to obtain 80% power to detect an effect of COMT on n-back performance. Initial reports and publication bias may have overestimated the influence that single genes have on cognitive processes (Barnett et al. Reference Barnett, Scoriels and Munafo2008). Many studies investigating these polymorphisms suffer as a result of low power, leading to increases in the ratio of false positive to true positive findings among studies that achieve nominal statistical significance.
Our non-significant results are in line with the notion that COMT, and possibly other dopamine-modulating genes such as DAT1, may regulate higher-order cognitive functions that involve not only maintenance and updating of information but also mental manipulation (Bruder et al. Reference Bruder, Keilp, Xu, Shikhman, Schori, Gorman and Gilliam2005). From previous studies that investigated the effect of COMT effect on n-back performance (Egan et al. Reference Egan, Goldberg, Bhaskar, Callicott, Mazzanti, Straub, Goldman and Weinberger2001; Stefanis et al. Reference Stefanis, Van Os, Avramopoulos, Smyrnis, Evdokimidis and Hantoumi2004; Bruder et al. Reference Bruder, Keilp, Xu, Shikhman, Schori, Gorman and Gilliam2005; Bertolino et al. Reference Bertolino, Blasi, Latorre, Rubino, Rampino, Sinibaldi, Caforio, Petruzzella, Pizzuti, Scarabino, Nardini, Weinberger and Dallapiccola2006; Caldu et al. Reference Caldu, Vendrell, Bartres-Faz, Clemente, Bargallo, Jurado, Serra-Grabulosa and Junque2007; de Frias et al. Reference de Frias, Marklund, Eriksson, Larsson, Öman, Annerbrink, Bäckman, Nilsson and Nyberg2010), only one found a significant effect, with Met/Met homozygotes performing better than the rest (Goldberg et al. Reference Goldberg, Egan, Gscheidle, Coppola, Weickert, Kolachana, Goldman and Weinberger2003). However, the n-back task used in their study required individuals to respond to each stimulus whereas our study did not (Goldberg et al. Reference Goldberg, Egan, Gscheidle, Coppola, Weickert, Kolachana, Goldman and Weinberger2003). Our version of the n-back task, with a pure WM instruction, makes no heavy demand on higher executive functions (e.g. inhibition of response conflicts) and may therefore be less susceptible to the effects of dopamine-modulating genes. Taken together, these studies and our results suggest that cognition may only be sensitive to COMT after a certain threshold of cognitive load has been passed.
A potential limitation of this study was the small number of participants in the 9/9 genotype group. The 9-repeat allele is rare compared to the 10-repeat allele and the allelic distribution in this present study was similar to previous studies (Vandenbergh et al. Reference Vandenbergh, Persico and Uhl1992 b; Heinz et al. Reference Heinz, Goldman, Jones, Palmour, Hommer, Gorey, Lee, Linnoila and Weinberger2000). Conversely, a strong feature of this study was the use of the NART and the BIS as control variables. Impulsivity is known to affect WM performance and alter dopamine-dependent changes during WM (Cools et al. Reference Cools, Sheridan, Jacobs and D'Esposito2007); however, to our knowledge, no other study investigating COMT or DAT1 in WM has controlled for trait impulsivity.
In summary, we attribute our non-significant results to a lack of statistical power or to the possibility that these two dopamine-modulating genes may affect higher-order cognitive processes that were not present in our n-back task. Thus, more original studies with very large sample sizes are needed. Although meta-analyses are important in this field, the heterogeneity of the n-back tasks used between studies is problematic. We suggest that more multi-centre studies be conducted to attain such large sample sizes and investigate the potential influence that genes may have on cognitive endophenotypes.
Acknowledgments
This study was performed at the Cambridge Behavioural and Clinical Neuroscience Institute, jointly funded by the UK Medical Research Council (MRC) and the Wellcome Trust. We thank Dr S. Bishop for help with recruitment, C.-C. Bo, P. Cameron, F. Gabriel, L. Germine, C. Housden and N. Randhawa for help with cognitive testing, Dr H. Martin and Dr J. Whittaker for DNA extraction, Professor D. C. Rubinsztein and O. Sadiq for high quality genotyping and Dr M. Munafò for critical comments. S.R.C. was supported by an MRC priority studentship and U.M. by an MRC Pathfinder Award. M.M.B. is now at the Faculty of Medicine, Dalhousie University, Halifax, Nova Scotia, Canada. J.R. is now at the Institute of Cognitive Neuroscience, University College London, London, UK.
Declaration of Interest
None.