Introduction
The techniques of radiotherapy, chemotherapy and surgery, are methods to treat different kinds of tumors. Physicians, using treatment planning systems (TPS), try to prescribe doses as high as possible to the tumour, and low doses to the at-risk organs surrounding the tumour.Reference Cox, Stetz and Pajak 1 Intensity-modulated radiation therapy (IMRT), compared with other techniques like two-dimensional conformal and three-dimensional conventional radiotherapy (3D-CRT), allows higher radiation doses to be delivered to the tumor while keeping the surrounding organs at minimum doses.Reference Lee, Pfister and Zhang 2 Unfortunately, the unwanted and common side effect from using radiotherapy is the exact incidence of skin reaction, and the most of skin reactions are unknown.Reference Faithfull and Wells 3 External radiation therapy produces high incidence of skin toxicity from multi-beams, especially for head and neck, rectal or anal malignancies, skin reactions appeared in more than 90% out of 755 patients receiving treatment.Reference Elliott, Wright and Swann 4
Skin toxicity is a well-known complication during radiation therapy for head and neck cancer.Reference Lee, Pfister and Zhang 2 , Reference Ezzell 5 – Reference Radaideh 9 The most common acute effects of radiation are skin erythema and desquamation, followed by late toxicity in long-term damage such as xerostomia.Reference Radaideh and Malatqah 8 , Reference Bhandare 10 Skin is a deterministic factor for radiation, especially when the threshold dose has been exceeded.Reference Koenig, Mettler and Wagner 11 The expression and severity of radiation injury depend on many factors, such as the radiation dose, the interval between irradiations, the size of the skin area irradiated and patient-related factors, for example, individual sensitivity and the presence of coexisting diseases.Reference Koenig, Mettler and Wagner 11 Typically, skin erythema occurs at very high skin doses, exceeding 6–8 Gy, when treated with IMRT.Reference Radaideh and Malatqah 8 , Reference Rosenthal, Williams and Mahesh 12 , Reference Mettler, Koenig, Wagner and Kelsey 13 On the other hand, fractionation of the dose to several fractions can reduce the skin dose and the possibility of skin burn, as the effect of radiation tends to be cumulative.Reference Lee, Pfister and Zhang 2 Consequently, thermoplastic devices for the head and neck are used for fixation of the patient in order to achieve accurate radiation.Reference Bahl, Ghosal, Kapoor, Bhattacharya and Sharma 14 Typically, thermoplastic material such as Orfit masks (Orfit Industries, America, Wijnegem, Belgium) is used to cover the head and neck of the patient during radiation therapy.
Many researchers have described techniques to reduce the total radiation skin dose.Reference Norbash, Busick and Marks 15 Recently, it was reported that head and neck cancer patients treated with IMRT had reduced skin toxicity compared with volumetric modulated arc therapy (VMAT).Reference Bredfeldt, Sapir, Masi, Schipper, Eisbruch and Matuszak 16 This study was designed to compare the severity of skin toxicity induced by two different techniques: IMRT vs. 3D-CRT, using a fabricated anthropomorphic perspex head and neck phantom for dosimetric verification of treatment delivery and fixation using a Klarity mask (Orfit Industries America, Wijnegem, Belgium) for five patient plans treated for nasopharyngeal cancer (NPC). These results were then compared to the doses estimated by the TPS for each of the plans.Reference Radaideh, Matalqah, Tajuddin, Fabian and Bauk 17 , Reference Radaideh, Matalqah, Tajuddin, Fabian Lee, Bauk and Eid Abdel Munem 18
It is important to study and compare the effect of these two techniques (IMRT and 3DCRT) on tumour coverage, minimisation of skin dose and to understand the effects of immobilisation on skin toxicity with different techniques, like IMRT and 3D-CRT, using a Klarity mask.
Material and methods
Patient selection
The five patients were scanned in the computed tomography (CT) simulator. Patients had histologically diagnosed NPC and all had pathology extending to the lymph nodes in the supraclavicular fossa region of the neck. Patients were aged between 40 and 50 years. Informed consent was obtained from each patient to use their data before commencing TPS.
For the CT scans, a 2-mm slice thickness was chosen for the head and neck imaging before the patient’s scan was transferred to the TPS (Oncentra MasterPlan V3.3, Baltimore, MD, USA). Plans were then calculated for each technique: IMRT and 3D-CRT. Ten patient contour plans for nasopharyngeal tumors were transposed on to a Perspex phantom with simulated Thermoluminescent dosimeters (TLDs); five patients’ plans used IMRT techniques and five patient plans used 3D-CRT. These plans were positioned with the help of immobilisation devices (Klarity mask) created for each patient and covering the head, neck, and shoulders, in the supine position. Three tattoo points were fixed using a laser on the Klarity mask during CT simulation.
Treatment planning and delivery
Two treatment plans with different techniques: 3D-CRT and IMRT, were created by TPS. After phantom’s irradiation using the two techniques, the TLDs’ surface doses were measured and compared with calculated doses using TPS. Once the overall target volume and dose has been decided, the TPS planner chooses beams’ energy, shapes, intensity, and the directions, and then calculates the dose distributions.Reference Ezzell 5 Physicians chose the suitable IMRT plan and then calculate the dose and dose distribution using the TPS.Reference Cox, Stetz and Pajak 1 The skin surface is designated as unspecified tissue outside the targets; the dose absorbed by the skin should be less than 5% of the prescribed dose (70 Gy), and no more than 1% of the planning target volume (PTV) area can reach 77 Gy. All guidance advises to keep to these limits.Reference Lee, Pfister and Zhang 2
The mechanical structure of the linear accelerator (Siemens Mevatron MX2 linear accelerator; Siemens Inc., USA) consisted of jaws and 66 pairs of the multi-leaf collimator (MLCs) to collimate the beam. The gantry rotates clockwise in two dimensions, and counterclockwise at 360°; this machine provides a one-monitor unit (MU) that is approximately equal to 1 cGy at Dmax in water.
The plans for IMRT techniques were generated so that the primary PTV should receive at least 70 Gy overall in 33 fractions. During each fraction (2·12 Gy), 95% of it should be targeted to the secondary PTV to receive at least 2 Gy. Less than 1% of the primary and secondary PTVs should receive less than 93% of the prescribed dose. No organs at risk (OARs) such as eyes, salivary gland, brain stem and the spinal cords should receive a dose exceeding 1·5 Gy, and no more than 110% of the prescribed dose should be delivered to normal tissue, with dose constraints according to the Radiation Therapy Oncology Group (RTOG 0615).Reference Lee, Pfister and Zhang 2 For 3D-CRT, the primary PTV plans were to receive 70 Gy in 33 fractions, with 95% of the prescribed dose for each fraction to the primary PTV, and 60 Gy to the anterior neck.Reference Lee, Pfister and Zhang 2 It should be noted that the planning dose and number of fractions for IMRT and 3D-CRT were considered the same for this study.
TLD calibration
TLDs have commonly been used for measuring skin doses in previous studies that have focused on entry and exit doses.Reference Radaideh and AlZoubi 19 The International Commission on Radiological Protection (ICRP), recommended using TLDs for skin dose measurement, as they have a very small thickness (about 0·009 mm), which is comparable to the skin reference depth of 0·007 cm. 20 Other researchers showed that the TPS does not give an accurate estimation of skin dose, overestimating it by 10–18·5%.Reference Chung, Jin and Dempsey 21 – Reference Kinhikar, Murthy, Goel, Tambe, Dhote and Deshpande 23 In addition, TLDs have an accuracy of ±5% compared with Monte Carlo calculations and measurements in water.Reference Stathakis, Li, Paskalev, Yang, Wang and Ma 24
Chip-shaped LiF:Mg,Ti TLDs with the dimensions 0·03 cm × 0·03 cm, length and width, 0·009 cm height, as obtained from the manufacturer (Bicron NE, USA), were used in this study. Annealing treatment was performed prior to each irradiation using a Nabertherm oven (Nabertherm, Germany).Reference Olko 25 A Harshaw TLD reader model 3500 performed the readout of the TLD (Harshaw, Bicron NE, USA).
The calibration factor (Fcal) was calculated for each TLD from the ratio of ionisation chamber doses (Dic) to TLD reading (TLDr) at reference conditions,Reference Radaideh, Matalqah, Tajuddin, Luen, Bauk and Moneim 26 and the fading effect within one day was negligible. For calibration of TLDs, a solid water phantom was used to determine the percentage depth dose (PDD), by which a correction factor was determined.Reference Radaideh and AlZoubi 19 All ion chamber readings were corrected for water temperature and atmospheric pressure. TLDs were selected after a careful initialisation procedure.
Skin dose measurement
A total of 30 TLDs were taped on the skin inside the thermoplastic mask; six TLDs on each lateral side of Buccal, and 18 TLDs placed in fixed regions over the right, left and mid-neck. Five patients were planned, according to the PTV, to receive at least 70 Gy in 33 fractions, for both techniques. Surface doses were measured after each given fraction. The discrepancies between the three readings (right, left and mid neck) and the averages were calculated, and then compared with the calculated dose from the TPS.
Results
The average percent standard deviation of the three surface TLD measurements was less than 5%. Furthermore, the Klarity mask increased the skin doses for IMRT and 3D-CRT approximately 18·6% and 8·6%, respectively. The average measured dose discrepancies between the mean IMRT measured dose and the 3D-CRT dose were 30·9% and 24·9%, with standard deviation 3·2% and 6·0% with and without the immobilization mask, respectively (Tables 1 and 2).
Table 1 The average of three delivered doses at different skin regions using patients’ plan transferred on phantom covered with Klarity mask in comparison with two techniques

Abbreviations: Mav (Gy), average measurement cumulative doses in Gy using TLD; Mav (cGy), average measurement fraction dose in cGy; IMRT, intensity-modulated radiation therapy; 3D-CRT, three-dimensional radiation therapy.
Table 2 The average of three delivered doses at different skin regions using patients’ plan transferred on phantom covered without Klarity mask in comparison with two techniques

Abbreviations: Mav (Gy), average measurement cumulative doses in Gy using TLD; Mav (cGy), average measurement fraction dose in cGy; IMRT, intensity-modulated radiation therapy; 3D-CRT, three-dimensional radiation therapy.
Clinical verification of IMRT and 3D-CRT patient treatment plans was implemented using a phantom, and all delivered doses at all surface regions were measured and compared with both the TPS doses and with a previous study that found skin doses constrained between 6 and 8 Gy.Reference Cox, Stetz and Pajak 1 , Reference Radaideh and Malatqah 8 , Reference Rosenthal, Williams and Mahesh 12
The current study revealed that surface doses for IMRT and 3D-CRT were 95% and 66%, respectively, when using a Klarity mask, and 77% and 57%, respectively, without using a Karity mask.
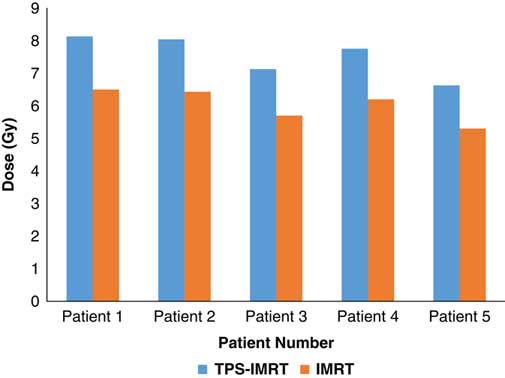
TPS was found to overestimate the skin dose by 20% for IMRT (Figure 1) and 16·6% for 3D-CRT (Figure 2). Using a head and neck phantom, the standard deviation of TLD/TPS was 2·4%.
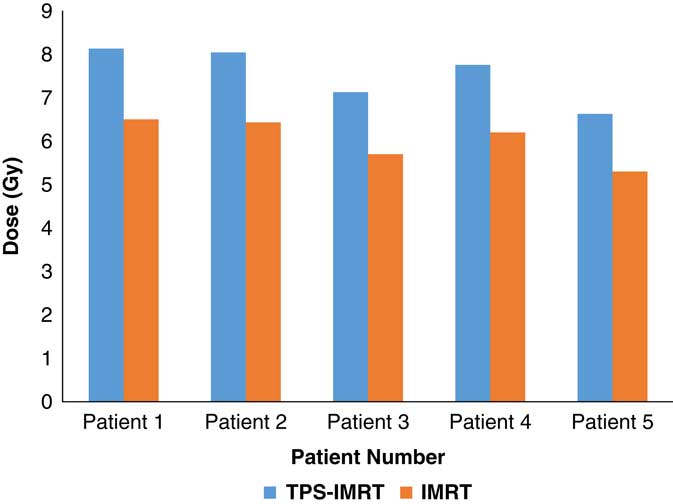
Figure 1 Comparison between measured average skin dose using IMRT and skin dose obtained from TPS.
Abbreviations: IMRT, intensity-modulated radiotherapy; TPS, treatment planning system.
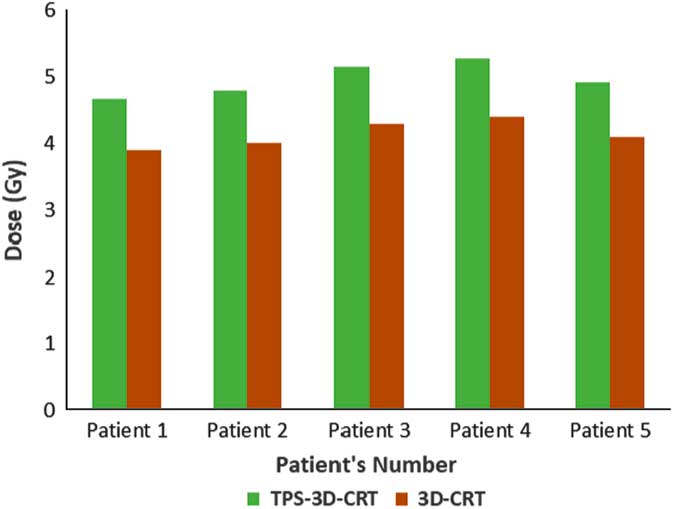
Figure 2 Comparison between measured average skin dose using 3D-CRT and skin dose obtained from TPS. (Abbreviations, TPS-3D-CRT (Gy): Calculated doses using treatment planning system for three-dimensional conformal radiation therapy).
Abbreviations: 3D-CRT, 3-D conventional radiotherapy; TPS, treatment planning system.
Discussion
The reproducibility of the TLDs was examined, and the average of TLD measurements for three inter fraction techniques exhibited standard deviations ranging from 4% to 5%, for IMRT and 3D-CRT, respectively.Reference Radaideh and Malatqah 8 , Reference Radaideh, Matalqah, Tajuddin, Fabian Lee, Bauk and Eid Abdel Munem 18 , Reference Radaideh, Matalqah, Tajuddin, Luen, Bauk and Moneim 26 These findings are in agreement with studies that have found the reproducibility of TLD to be within the range of 3–5%.Reference Lee, Pfister and Zhang 2 , Reference Harris, Elson, Lamba and Foster 27
The correction value of the phantom material was evaluated in previous studies and found to be 1·05.Reference Radaideh and Malatqah 8 , Reference Radaideh, Matalqah, Tajuddin, Luen, Bauk and Moneim 26 This correction replaces all corrections for measurement of TLD doses which resulted from the air gaps. The air gap within the TLDs holes and the slabs, is developed because of the difference in size between the TLD and the hole itself, to avoid scratching the TLD when constructing the slices of the phantom. This air gap could increase exposure to the TLDs, thus leading to such dose discrepancies.Reference Radaideh, Matalqah, Tajuddin, Fabian and Bauk 17
IMRT and 3D-CRT are commonly used for the treatment of NPC.Reference Radaideh and Malatqah 8 In terms of clinical outcomes, both offer comparable survival rates, locoregional control, and metastasis-free survival.Reference Nutting, Morden and Harrington 28 However, IMRT is still the preferred treatment for NPCReference Xia, Fu, Wong, Akazawa and Verhey 29 as it better spares the adjacent OAR, particularly the parotid glands, and reduces the risk of xerostomia, compared with 3D-CRT.Reference Kam, Leung and Zee 30 – Reference Lee, Xia and Quivey 32 However, this study found that the average skin doses, for all patient plans transferred to the phantom, were higher with IMRT when compared to 3D-CRT. This could be explained by the use of multiple beams that tangentially enter the skin.
This study revealed that skin doses using IMRT were increased by 24·9% without a Klarity mask, when compared to 3D-CRT. This finding was in agreement with previous studies which found that IMRT increased the skin doses by about 27%, without using a mask.Reference Lee, Xia and Quivey 32 , Reference Lee, Chuang and Quivey 33 Consequently, reducing the skin dose and applying measurements using IMRT sparing techniques for the head and neck are highly recommended.Reference Radaideh and Malatqah 8
However, previous researchers have reported that IMRT using immobilization masks can lead to skin toxicity and increase the surface dose by 19%,Reference Radaideh and Malatqah 8 , Reference Radaideh 9 , Reference Półtorak, Fujak and Kukołowicz 34 whereas in this study it increased significantly by 30·9%. The increased skin doses while using a Klarity mask may be caused by two main factors; the contaminant of electrons from the collimator air, and the scattering material in the beam path.Reference Nilsson and Brahme 35
The results of this study are consistent with a previous study that showed that skin doses for the first patient were 90% and 92% of the prescribed dose, as measured by metal–oxide–semiconductor field effect transistor MOSFET (TN-502RD, Springfield, VA, USA) and TLDs, respectively, while skin doses for the second patient were 88% and 86% of the prescribed dose.Reference Kinhikar, Murthy, Goel, Tambe, Dhote and Deshpande 23 Our study found that skin doses with the IMRT technique were 95% and 77% of the prescribed dose, with and without a Klarity mask, respectively, and 66% and 57%, with and without Klarity mask, respectively, with the use of 3D-CRT techniques. The average percentage dose on the neck surface when using a Klarity mask was increased, and when using IMRT, the skin dose was higher as compared with 3D-CRT.
Our study found that the average surface doses for IMRT were approximately 6·7 Gy and 5·4 Gy, with and without a Klarity mask, respectively. For 3D-CRT, the average surface doses were approximately 4·6 Gy and 4 Gy, with and without a Klarity mask, respectively. This is comparable with a previous study that estimated skin injuries among 86 patients undergoing intracoronary brachytherapy procedures; beta sources in this study reached 3·5 Gy and 4·6 Gy.Reference Vano, Prieto, Fernandez, Gonzalez, Sabate and Galvan 36 Other researchers have found that cumulative doses on the neck region exceeded 7 Gy, causing burns on the neck area during 3D-CRT and IMRT.Reference Radaideh and Malatqah 8 However, other studies have revealed that erythema occurs at skin doses of 6–8 Gy.Reference Radaideh and Malatqah 8 , Reference Rosenthal, Williams and Mahesh 12 On the other hand, previous researchers have shown that cancer treatment using radiation therapy is well tolerated in older patients as the elderly have smaller mitosis indices, and given that radiation destroys cells mainly in the mitosis phase, this results in elderly people having less skin reactions.Reference Porock 37
Dosimetric measurement is highly recommended for estimation of skin doses as TPS overestimates the skin dose. This is consistent with other researchers who have found that TPS overestimates the dose by 18·5%,Reference Chung, Jin and Dempsey 38 and others found that TPS overestimates the skin dose by 10–12% when compared with measurement using MOSFET and TLD.Reference Kinhikar, Murthy, Goel, Tambe, Dhote and Deshpande 23 Furthermore, using cobalt irradiation of a paediatric phantom, the average magnitude of local difference between the TPS doses and measured skin doses was 22%.Reference Kry, Smith, Weathers and Stovall 39
Limitations
It was difficult to ensure the TLDs remained in the same position for all patient plans. The researchers tried to use the same TLD number at the same position during the experiment, for all patients plans transferred to the phantom; however, there may have been some change in TLD numbers.
Conclusion
Skin dose is an important issue of focus during treatment of malignant diseases using radiation. It is concluded that IMRT increases acute skin toxicity significantly when compared with conventional radiotherapy (CRT). Even though the Klarity mask provides superior target coverage and normal tissue sparing, it increases the skin toxicity risk using both techniques.
It is possible to reduce the skin dose, when considering the skin as a sensitive structure, without affecting tumour coverage. Furthermore, dosimetry measurements for individual planning before radiotherapy treatment are highly recommended as TPS is not accurately estimated the skin doses. The severity of skin burns is related to the total cumulative dose received, and burns can become serious after radiotherapy treatment; therefore, surface dose measurements should be taken be into account.
Acknowledgement
The author gratefully acknowledge Qassim University, represented by the Deanship of Scientific Research, on the material support for this research under the number 1339-Cams1-12-1-2016-S-1240 during the academic year 1438 AH/2016 AD.
Conflicts of Interest
Author has no conflict interest to declare.