INTRODUCTION
Fiddler crabs (Ocypodidae, Uca) are a well-known group of small, intertidal brachyuran crabs (Backwell & Christy, Reference Backwell and Christy2000). They are widely distributed in nearly all tropical and subtropical regions of the world. They play an important role in these ecosystems and are a rich food source for birds. Fiddler crabs burrow in intertidal areas and are important consumers of detritus, bacteria, fungi, and benthic micro algae in coastal marsh, mangrove, sand flat, and mudflat habitats (Backwell et al., Reference Backwell, Jennions, Wada, Murai and Christy2006). Their burrowing activities aerate the soil and enhance the growth of marsh plants. Their presence also increases the amount of meiofauna in salt marshes (Burford et al., Reference Burford, McGregor and Oliviera2001). Fiddler crabs have long been a favourite animal for ethological studies (Christy et al., Reference Christy, Backwell and Goshima2001). They are highly social animals with a rich behavioural repertoire. Their behaviours are easily studied under natural conditions (Crane, Reference Crane1975). They are animals with simple nervous systems that have a rich social behaviour in comparison with other animals (Iribarne & Martinez, Reference Iribarne and Martinez1999). Unlike most intertidal organisms, fiddler crabs are semi-terrestrial and are active at low tide, returning to their burrows at high tide. Males have one greatly enlarged claw, which can be up to 50% of the body weight (Klassen & Ens, Reference Klassen and Ens1993). The claw is used for mating displays and in combat with other males. In mating displays, males wave their claw to attract females. The pattern of waving is species-specific (Backwell et al., Reference Backwell, Jennions, Wada, Murai and Christy2006). Females, with two feeding claws, can feed more efficiently than males. Fiddler crabs are diurnally active (Land & Layne, Reference Land and Layne1995; Macia et al., Reference Macia, Quincardete and Paula2001; Mokhtari et al., Reference Mokhtari, Savari, Rezai, Kochanian and Bitaab2007). The weather, tidal conditions and the physiological condition of a crab all affect its activity. Surface activity terminates when the tide covers the area and the crabs retreat into their burrows and plug the entrances with sediment. Burrow plugging also occurs at night and on hot days when the sediment is dry (Klassen & Ens, Reference Klassen and Ens1993). Some species feed in flocks (droves) at the edge of the water while others feed in the small areas around their burrow entrances. In some species, e.g. U. pugilator, foraging can be affected by the sediment water content and particle size. It has been shown in U. uruguayensis that the greater the distance a crab is from its burrow; the greater is the risk of predation by birds (Morgan & Christy, Reference Morgan and Christy1994). In U. tangeri, small crabs spend more time near their burrows than larger individuals (Morgan & Christy, Reference Morgan and Christy1995).
In many tropical environments more than one species of Uca coexist in similar habitats. They may have different patterns of behaviour (mating, feeding, etc.) and microhabitat preferences, so that their ecological niches are not identical (Backwell et al., Reference Backwell, Jennions, Wada, Murai and Christy2006). In this study, we examined behaviours and habitats of two sympatric species of Uca on the Abi estuary of the Persian Gulf (Figure 1) to see how their behaviours might differ and how these differences relate to their habitat.
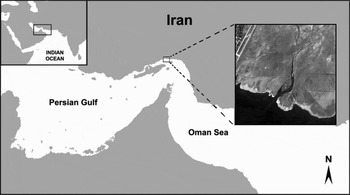
Fig. 1. Abi estuary is located in behind of Bandar Abbas airport of Iran (27°11′N and 56°24′E).
MATERIALS AND METHODS
The study populations were located on a 1000 m2 area of the Abi estuary (Figure 1). There are two species of fiddler crabs in the area: the common Uca annulipes and the less common U. sindensis. We plotted their distributions and documented sediment characteristics by collecting sediment samples from within the populations for analysis in a particle size-distribution analyser. Samples for sediment particle size were taken of each species territory using a coring tube, which was driven into the sediment to a depth of 15 cm. Specifically, according to the Wentworth grade scale gravel-sized particles have a nominal diameter of ≥2.0 mm; sand-sized particles have nominal diameters from <2.0 mm to ≥62.5 mm; silt-sized particles have nominal diameters from <62.5 to ≥4.0 mm; and clay is <4.0 mm. Graphical techniques were used for analyses and grain-size classification sediments (Oliviera et al., Reference Oliviera, McGregor, Burford, Custodio and Latruffe1998). Percentage of sand, silt and clay were measured by Shepard (Reference Shepard1954) who utilized a single ternary diagram (Figure 2).
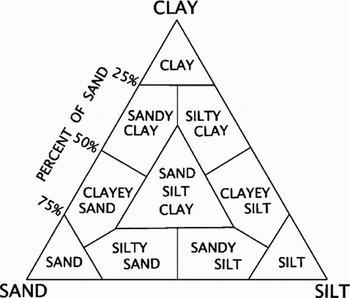
Fig. 2. Diagram of the sediment classification scheme, modified from Shepard (Reference Shepard1954).
We observed the two species with binoculars, noting and describing their behaviours at low tide over a one year period (September 2007 to August 2008). Observations were made throughout the day during a three hour period relative to low tide (one hour before low, one hour at low and one hour after low). Observations were made on two days of each week throughout the year.
We used 1 m2 quadrats to measure the density of burrows and crabs. Individuals were counted within the quadrats 15 minutes after putting down the quadrat markers, giving them time to re-emerge from their burrows and resume natural behaviour (Backwell et al., Reference Backwell, Jennions, Wada, Murai and Christy2006). We measured feeding rates of Uca annulipes by counting the number of scoops of sediment made by the small feeding claws. Male and female fiddler crabs of both species feed by picking up bits of muddy sand from the substrate using the minor cheliped. The number of feeding motions of male and female crabs was recorded by observing individuals feeding on substrate for l minute. A feeding motion consisted of a single cheliped moving from the substrate to the buccal cavity.
We documented the crab behaviour over three 10 minute periods each day (a total of 30 minutes per crab) and scored it according to the following categories: feeding, walking, feeding while walking, standing, aggression and defence (agonistic behaviours), waving, sexual interaction, mating, in burrow and burrowing. ‘Burrowing’ was defined as digging a burrow (Backwell et al., Reference Backwell, Jennions, Wada, Murai and Christy2006), moving mud balls removed from the burrow or mud balls that it later put into the burrow, or constructing a hood and other muddy structures around the burrow. Whenever the crab was below the surface and not visible, it was considered ‘in burrow’ (Backwell et al., Reference Backwell, Jennions, Wada, Murai and Christy2006). The average percentage of time they spent in each activity was calculated and compared.
We further documented crab behaviour by noting the occurrence of burrow cleaning and structure building by each species. During a 30 minute observation period each day of observation, we counted and measured the mud structures built by crabs.
RESULTS
Sediment analysis and species distribution
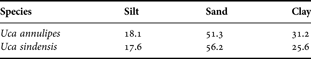
Analysis of the substrate is presented in Table 1. For both species, the percentage of silt, sand and clay was determined. In the U. annulipes areas, the substrate had much higher percentages of silt and clay particles. The U. sindensis areas had more sandy particles. The U. annulipes areas were lower and wetter while the U. sindensis areas were higher and drier. Uca sindensis occurred in small patches throughout the estuary while U. annulipes populations were larger and distributed along the edges of the small estuarine streams.
Table 1. Particle type distribution of substrate for each species (%).
Burrow and crab density
Quadrats placed in the U. annulipes populations (N = 100 quadrats) had an average of 53.19 ± 21.26 burrows/m2. Average number of surface crabs was 12.1 ± 4.37 (N = 1210), while the sex-ratio of crabs on the surface was about 71.42% males and 28.57% females. Approximately equal numbers of left-handed and right-handed (i.e. side of major claw) males were observed (N = 863; R = 442 (51.21%) and L = 421 (48.78%)). The average carapace width in males was 12.15 ± 2.41 mm (mean ± SD) and in females it was 10.3 ± 2.35 mm (mean ± SD).
Quadrats placed in the U. sindensis populations (N = 100 quadrats) had an average of 39.02 ± 20.15 burrows/m2, and contained about 14.2 ± 6.49 surface active crabs per m2 (total of 1049 crabs). There were more males than females with 58.13% of surface active crabs being male. There was a skew in handedness with 33.82% of males being left handed and 66.17% males being right handed (N = 604; R = 399 and L = 205). This difference is significant: binomial test P < 0.001. This species was slightly smaller than U. annulipes. In the U. sindensis average carapace width in males was 10.37 ± 2.67 mm (mean ± SD) and in females it was 9.06 ± 2.52 mm (mean ± SD).
Feeding rates
In U. annulipes, females fed about 111.7% faster than males. The feeding rate of U. annulipes was, on average, 71.23 ± 34.25 scoops per minute for males (N = 84) and 150.87 ± 42.16 scoops per minute for females (N = 84) (t = –25.26, P < 0.001). The equivalent data for U. sindensis females fed about 96.1% faster than males. This was 66.26 ± 22.9 scoops per minute for males (N = 84) and 129.98 ± 47.56 scoops per minute for females (N = 84) (t = −21.80, P < 0.001). Many individuals of both species fed in droves at the edge of the water.
Behavioural observations
Both species spent most of their time feeding during the breeding season. Feeding was less common off the breeding season and, during the winter months; both species were inactive for a period of 2–3 weeks when the temperatures were below 14°C. During the hotter months of the breeding season (mean temperature = 34.17°C), males walked, fed and waved at the same time. In both species, males frequently waved at each other when no females were in the vicinity. Elevated aggression was seen when, after waving at each other, two males dashed forward towards each other. We also observed fights in both species, including manus touching and pushing until one of the males finally retreated. In both species, aggressive interactions were also seen between females. Fights consisted of manus pushing and lasted about 20 seconds to 50 seconds (31.27 ± 8.96 in U. sindensis and was 34.84 ± 9.10 in U. annulipes; (mean ± SD)). Males were not aggressive toward females in either species.
In both species, the wave is a slow extension and flexion of the major chela (Backwell et al., Reference Backwell, Jennions, Wada, Murai and Christy2006). The average number of waves in U. sindensis was 43.5 ± 3.7 waves per minute (N = 10) while in the U. annulipes was 47.1 ± 2.9 waves per minute (N = 10).
Waving was seen frequently and was directed towards females with many males waving at one or more females. In both species we observed burrow mating: after courtship, the female approaches a selected male and enters his burrow. The male follows her down and they stay in the burrow to mate.
We also observed frequent grooming behaviour. After emerging from their burrows, males of both species used the minor chela to wipe the major chela, mostly on the pollex and dactyl but also on the joints. They also wiped their eyestalks with their minor chela. To clean their legs, they flexed and rubbed them together, presumably because they could not reach these surfaces with their minor chela.
Environmental temperature was an important factor in the both species' behaviours. Towards the end of September the temperature decreased substantially and many of the activities of both species ceased. Towards the end of February the temperatures rose and the behaviours were resumed.
Burrow cleaning and structure building
For both species, individuals were observed digging burrows, maintaining them, and covering them. Burrow excavation involved the crab digging with the legs on one side, turning around, and then digging with the legs on the other side. Burrow maintenance involved emerging from the burrow with mud balls and depositing them on the surface. They emerged during the early part of the low tide as their burrows were uncovered by the tide, and retreated back into their burrows late in the low tide, as their area was being covered by water. They excavated their burrows and removed mud balls. Later, they picked up mud balls or newly excavated substrate, entered their burrows and sealed them.
We observed two types of mud balls: feeding pellets that are the result of the crab spitting out the sediment it has processed in its mouth after extracting the organic matter; and burrow balls that are the result of cleaning out the burrow. Burrow balls are much larger than feeding pellets (Figure 3). During the breeding season (February–September), we also observed three types of mud structures constructed by U. annulipes populations. We found an abundance of pillars (1.3 ± 1.62 pillars in a 0.5 m2 area; N = 92) (Figure 4). Pillars are upright piles of mud that sit behind the burrow entrance and are 3.2 ± 0.51 cm tall (N = 30). We also found much rarer hoods that consist of mud piled behind the burrow entrance to half surround it and cover the top of it with an overhanging lip (an average height of 2.1 ± 0.3 cm, N = 30) (Figure 5) and semi-domes that were a collection of mud balls placed behind the burrow entrance in a low semi-dome shape (average height = 2.3 ± 0.39 cm, N = 30) (Figure 6). We found no such structures in the populations of U. sindensis.

Fig. 3. Food pellets (are small) and burrow balls (are big) in Abi estuary area.

Fig. 4. Pillar structure in Abi estuary area.

Fig. 5. Hoods structure in Abi estuary area.

Fig. 6. Semi-domes structure in Abi estuary area.
DISCUSSION
The two species live in very different microhabitats. Uca annulipes lives in large populations on low lying muddy areas along the edges of streams and are covered by even small amplitude high tides. Nutrient-rich filamentous algal, detritus and bacteria are deposited by the receding tide each day, giving this species an ample supply of fresh food. Uca sindensis lives in small patches on higher areas that are not covered by all high tides; their habitat can remain uncovered by tides for several consecutive days in each 14 day semi-lunar tidal cycle. The species therefore has temporally limited access to fresh food which may affect growth rates. The behavioural differences between the two species appear to be strongly related to their different habitats, as found in other fiddler crab species (Backwell et al., Reference Backwell, Jennions, Wada, Murai and Christy2006). For example, burrowing was observed by both species but was less common in the higher, Uca sindensis areas where the sediment was hard. As in other fiddler crab species, these high burrows did not get damaged by incoming tides and remained intact for several days (Klassen & Ens, Reference Klassen and Ens1993; Backwell et al., Reference Backwell, Jennions, Wada, Murai and Christy2006). Furthermore, in the both sexes, the feeding rate of U. annulipes was faster than U. sindensis (t = 3.79, P < 0.001 for female and t = −1.52, P < 0.001 for male). Previous studies have also found a difference in feeding rates: U. pugnax increased its feeding rate when there was a high concentration of food in the sediment (Rosenberg, Reference Rosenberg2001). In contrast, Weis & Weis (Reference Weis and Weis2004) showed that, in four species of fiddler crabs in Indonesia, the highest feeding rate was in U. vocans, which had poor food, and the lowest proportion of time spent feeding was in U. tetragonon, which had the most abundant food source.
There were many other differences detected between the two study species. Uca l. annulipes is slightly smaller than U. sindensis but they live at similar densities. The sex-ratio of U. l. annulipes was male biased, with 2.5 males per female. In U. sindensis, the bias was less pronounced: 1.4 males per female. There was also a difference between the species in handedness. In U. l. annulipes, there were equal numbers of left and right handed males, while in U. sindensis there was a significant skew to right handed males with 66% of the population being right handed. While it is generally thought that female fiddler crabs feed faster than males because they have two feeding claws, it is not always true. Of the four studied species, Weis & Weis (Reference Weis and Weis2004) found this to hold in only two species. We found that, in both U. annulipes and U. sendensis, females fed faster than males.
The microhabitat had a pronounced effect on the behaviour of these two species. A second factor also seemed to play a major role in their behaviour: seasonal cycles in behaviour were pronounced in both species. The cyclicity is probably related directly to temperature. Environmental temperature was an important factor in the behaviour of both of the species with the level of activity strongly correlated to temperature. For both species, the crabs were more active and spent more time feeding during the breeding season (late February–early September). Off the breeding season, the temperatures were low and the crabs were far less active and were even inactive for the coldest period of the year. This is not unexpected since the reproductive demands of animals are important in shaping their foraging behaviour (Schoener, Reference Schoener1971).
Aggression was observed in both species. Waving was associated with aggression toward other males as well as courtship toward females in both species. But waving and courtship was observed only in the breeding season. Seasonal and lunar-related rhythms in waving displays and combat behaviour have been studied in several species of fiddler crabs (Shepard, Reference Shepard1954; Klassen & Ens, Reference Klassen and Ens1993) and lunar or semi-lunar reproductive cycles are common (Weissburg, Reference Weissburg1992; Skov & Hartnoll, Reference Skov and Hartnoll2001; Backwell et al., Reference Backwell, Jennions, Wada, Murai and Christy2006).
There was a very obvious difference in the structure-building behaviour of these two species. Uca annulipes arranged mud balls at some distance from the burrow and built three types of sand structures near their burrow entrances: semi-domes, pillars and hoods. We did not observe U. sindensis build these structures. Many Uca species build structures such as pillars or hoods from moist sand or mud (Shepard, Reference Shepard1954), some of which play roles in attracting females (Shepard, Reference Shepard1954) or defining territory (Zucker, Reference Zucker1981; Weis & Weis, Reference Weis and Weis2004). Mud balls may be merely a by-product of burrow excavation (Klassen & Ens, Reference Klassen and Ens1993), but may also function as a signal (Zeil et al., Reference Zeil, Hemmi and Backwell2006). Oliviera et al. (Reference Oliviera, McGregor, Burford, Custodio and Latruffe1998) and Burford et al. (Reference Burford, McGregor and Oliviera2001) indicate that for U. tangeri males, mud balls mark territory and are attractive to females. While we do not know the significance of our observations of crabs arranging mud balls and placing them at a distance from burrows and have not tested the functions of semi-domes, pillars and hoods, it is clear that these structures play some role in the social lives of the crabs involved. Future work will illuminate their various roles.
CONCLUSION
There were some similarities and some differences in the behaviours of the two species of fiddler crabs studied here. The similarities were largely due to the strong effect of seasonal cycles in the behaviour of the crabs. Both species responded strongly to changes in temperature over the year. The differences seem to be related, in part, to the different microhabitats used by the two species. The muddy, food-rich sediments allowed for, among other things, the construction of mud structures that probably play some role in the social lives of these crabs. The drier, harder sediment of U. sindensis prevented the construction of such structures.
ACKNOWLEDGEMENTS
We thank M. Saffaii for his guidance and helpful comments on crustacean species in the area. We particularly thank M. Keshavarz, H. Dehghani, R. Dabbagh, M. Afkhami, L. Abdoli and O. Parshan for assistance in the field. Thanks also to A. Javadi, A. Nasrolahi and E. Mokhlesi for their guidance.









