Introduction
Low temperature (LT) presents a severe environmental challenge to plant growth and distribution in temperate and subtropical regions, and is a crucial factor affecting global food production. Rice (Oryza sativa L.) is one of the three major food crops in the world and is the staple food for more than half of the world's population (Mohanty, Reference Mohanty2013). Being a temperature-sensitive field crop, yield loss due to LT is a worldwide problem in rice production (Peyman and Hashem, Reference Peyman and Hashem2010). About 15 million hectares of rice fields in 24 different countries are under threat from cold weather (Zeng et al., Reference Zeng, Yang, Cui, Yang, Xu, Du, Pu, Li, Cheng and Huang2009). In India, LT affects rice production in parts of Jammu and Kashmir and Uttaranchal and in the northeastern hill states. Rice plants growing in these areas are regularly exposed to LT during different phases of growth. The vegetative growth phase comprises germination, seedling and tillering stages. Occurrence of LT stress at the germination or seedling stage affects seedling establishment and can lead to non-uniform crop maturation (Lou et al., Reference Lou, Chen, Sun, Xing, Li, Xu, Mei and Luo2007) and reduced crop production. Therefore, LT germinability (LTG) of rice is one of the major determinants for stable stand establishment and rice production in temperate and high-elevation areas.
Studies at the molecular level to decipher the mechanisms underlying cold tolerance in rice have led to the identification of major quantitative trait loci (QTLs) at different developmental stages using different phenotypic traits for scoring LT tolerance. However, most of the QTLs identified to date have a small effect (few explaining more than 20% of the phenotypic variation), suggesting the complexity of the genetic mechanism of tolerance to LT stress in rice. Extensive studies have been carried out on qLTG3-1, a major QTL controlling tolerance to LT at the seed germination stage in rice. Using a population of 122 backcross inbred lines derived from a cross between temperate japonica varieties, Italica Livorno from Italy and Hayamasari from Japan, the LT germination QTL viz-qLTG-3-1 explaining 30% of the total phenotypic variation, was identified on chromosome 3 (Fujino et al., Reference Fujino, Sekiguchi, Sato, Kinuchi, Nonone, Takenuchi, Ando, Lin and Yano2004). Map-based cloning for qLTG-3-1 led to the identification of a 555 bp plant-specific gene, encoding a novel protein of unknown function (Fujino et al., Reference Fujino, Sekiguchi, Matsuda, Sugimoto, Ono and Yano2008). Two distinct alleles for LTG were discovered when a 1735 bp qLTG3-1 gene was sequenced (Fujino and Sekiguchi, Reference Fujino and Sekiguchi2011). The two functional nucleotide polymorphisms, a 71 bp insertion found in Italica Livorno (Fujino et al., Reference Fujino, Sekiguchi, Matsuda, Sugimoto, Ono and Yano2008) and an amino acid substitution found in Nipponbare (Fujino et al., Reference Fujino, Sekiguchi, Matsuda, Sugimoto, Ono and Yano2008; Fujino and Sekiguchi, Reference Fujino and Sekiguchi2011), have been identified for qLTG3-1. Furthermore, haplotype analysis across a population of 64 rice accessions from Hokkaido, Japan has revealed that the selection of the tolerant allele qLTG3-1 may be a result of adaptation to the local environment (Fujino and Sekiguchi, Reference Fujino and Sekiguchi2011). In this study, we carried out a survey of the 71 bp tolerant allele in a set of 65 Indian rice accessions that were selected on the basis of diverse geographic distribution. These rice accessions were also genotyped for 33 randomly distributed simple sequence repeat (SSR) markers to confirm genetic diversity background.
Materials and methods
Plant material
Selection of the rice accessions was based on the available literature on mapping parents used for LT stress, geographical regions affected by LT and landraces/varieties adapted to different types of rice-growing regions. Accordingly, 65 diverse rice accessions including 43 landraces, 14 varieties and 7 accessions obtained from the International Rice Research Institute including the tolerant parent Italica Livorno were used in the current study (Table S1, available online). The seeds of these rice accessions used in the current study were grown under the same environment to produce seeds for testing.
SSR marker polymorphism
Genomic DNA was extracted using the CTAB-Cetyl Trimethyl Ammonium Bromide method (Murray and Thompson, Reference Murray and Thompson1980). Approximately 15–20 ng of genomic DNA were used as a template for performing the polymerase chain reaction (PCR) analysis with previously reported SSR markers (RM; HvSSR) (Garris et al., Reference Garris, Tai, Coburn, Kresovich and McCouch2005; Singh et al., Reference Singh, Deshmukh, Singh, Singh, Gaikwad, Sharma, Mohapatra and Singh2010). A set of 33 SSR markers distributed across different chromosomes were selected. Briefly, a standard PCR was carried out with the following profile: 5 min at 94°C, 30 cycles, 30 s at 94°C, 45 s at 55–64°C, 60 s at 72°C, followed by 10 min at 72°C for a final extension. Genomic DNA (15–20 ng) was used as a template in a total volume of 20 μl [5 pmol of each primer, 2 μl PCR buffer (100 mM Tris–HCl, 500 mM KC1, 15 mM MgCl2, pH 9.0, and 0.01% gelatin), 1 μl of 10 mM dNTPs and 0.5 unit Taq polymerase (D1806; Sigma Aldrich, St. Loius, MO, USA)]. Scoring was carried out by using 3–3.5% agarose gel (UltraPure Agarose 1000; Sigma). For loading in the gel, 3 μl of the PCR product were mixed with 3 μl of (1 × ) gel loading dye; a 100 bp DNA ladder (GeneRuler; Fermentas) was used as a standard size. The gels were run for around 3 h and stained with ethidium bromide for 15 min (3 μl) and then destained using distilled water. The gel was observed under UV using a Bio-Rad gel documentation system. Bands were scored based on the size of the amplified products. The number of alleles per locus, major alleles per locus, genetic diversity and the polymorphism information content were determined using PowerMarker ver 3.25 (statgen.ncsu.edu/powermarker/index.html). The C.S. Chord distance (Cavalli-Sforza and Edwards, Reference Cavalli-Sforza and Edwards1967) was used for the construction of the tree using the UPGMA method with PowerMarker software (version 3.25).
Screening for LTG
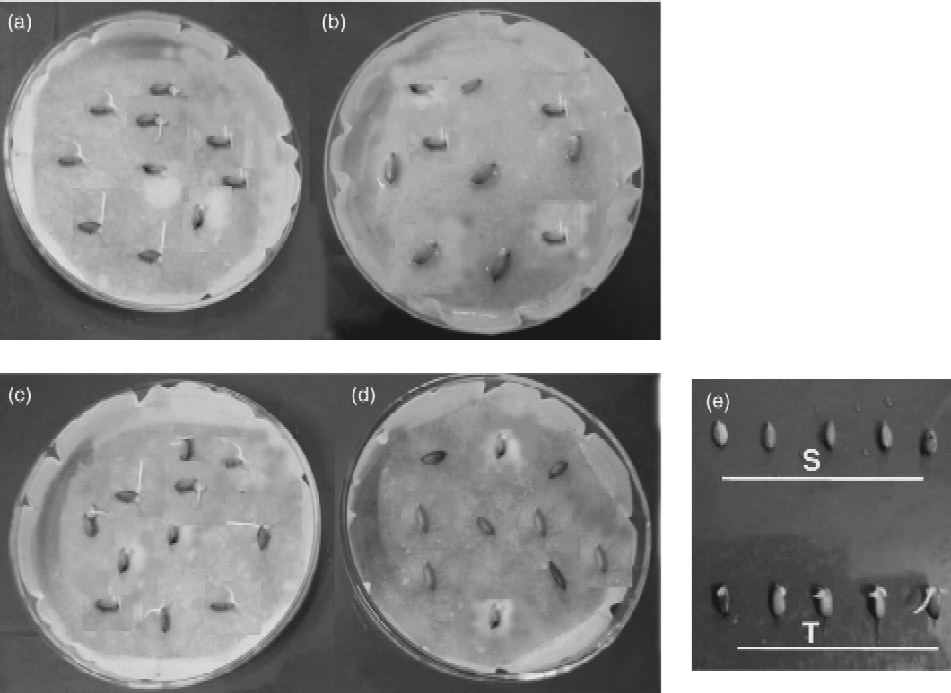
For LTG, the accessions were tested as described previously by Fujino et al. (Reference Fujino, Sekiguchi, Sato, Kinuchi, Nonone, Takenuchi, Ando, Lin and Yano2004). Briefly, ten seeds of each rice accession were placed in a 9-cm Petri dish on a filter paper soaked in 10 ml of distilled water at 15°C in the dark in a controlled growth chamber (Fig. 1(b) and (d)). The number of germinated seeds (as indicated by coleoptile growth) was counted daily until 10 d after sowing. To determine the viability of the seeds used, the same set of seeds was placed at 25°C for a few days (Fig. 1(a) and (c)). The experiment was replicated twice. Evaluation of cold tolerance of the accessions was carried out by calculating germination percentage. The accessions that showed more than 60% germination of the viable seed under cold conditions were designated as tolerant (Fig. 1(e)).

Fig. 1 Germination screening of the rice genotypes using moist paper. Differential germination of genotypes LR 55 and LR 64 at (a, c) controlled and (b, d) low temperature. (e) Difference in germination at LT (15°C) for susceptible (S) and tolerant (T) genotypes.
DNA and PCR analysis
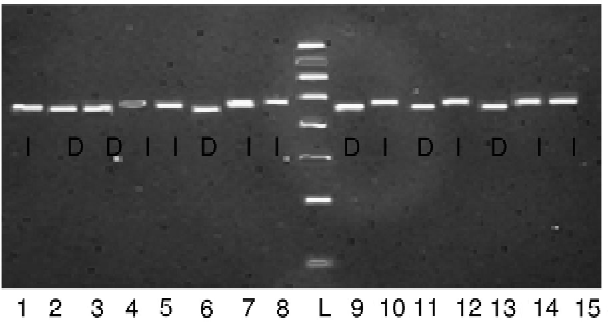
Total DNA was isolated from the young leaves of the crop using the CTAB method. To analyse the allelic variation of qLTG3-1 among the diverse rice accessions, PCR amplification using the primers LTG_F (5′-CCTTGGTCCATAGATGTATC-3′) and LTG_R (5′-CGTATATTAGTTCGTGCACT-3′) was carried out for the 65 accessions (Fujino and Sekiguchi, Reference Fujino and Sekiguchi2011). PCR conditions were 94°C for 30 s, 55°C for 30 s and 72°C for 60 s for 35 cycles and a final extension at 72°C for 3 min. PCR products were run on a 0.8% gel and scored as ‘del’ or ‘D’ and ‘ins’ or ‘I’ based on the absence (Hayamasari) or presence (Italica Livorno) of the 71 bp tolerant allele, respectively (Table S1, available online; Fig. 2). Some of these PCR products were randomly selected for sequencing to confirm that the correct fragment was being amplified and scored for analysis.

Fig. 2 Representative agarose gel showing polymorphism in the rice genotypes using LTG3 primer pairs. Symbols ‘I’ and ‘D’ indicate insertion and deletion, respectively.
Association between the tolerant allele and cold tolerance at the germination stage
To determine the association between the distributions of the 71 bp tolerant allele and cold tolerance at the germination stage, frequencies of the two alleles for qLTG3-1 were analysed in the 65 diverse rice accessions and associated with the cold-tolerant and -susceptible designations. The two allelic frequencies were also studied in four other subgroups, namely upland accessions, lowland accessions, landraces and improved lines/varieties, to study whether there is any association of these alleles with the subgroups. Significance of deviation in allele frequencies within the subgroups when compared with the population mean was tested using the χ2 test.
Results
Genetic classification of the rice accessions
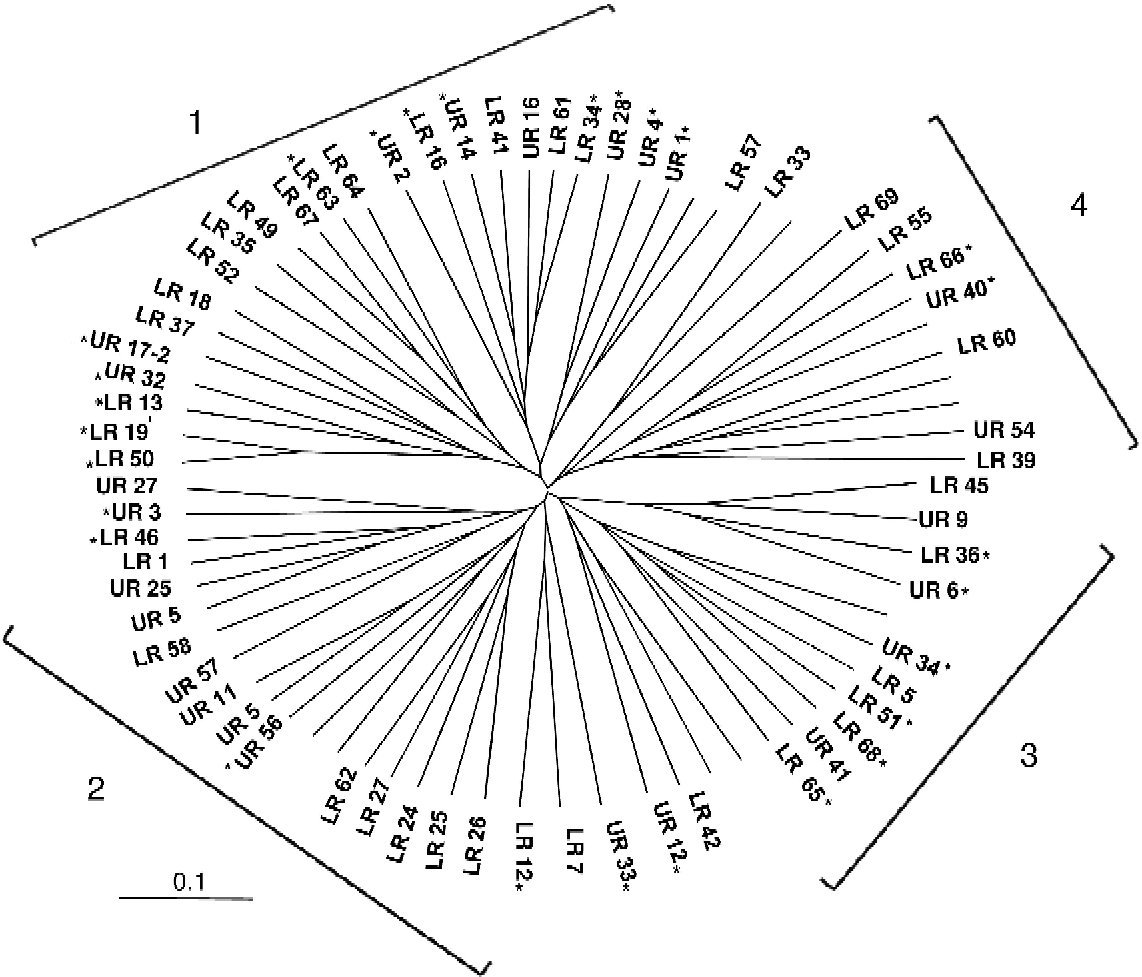
Use of the 33 genome-wide SSR markers (Table S2, available online) led to clustering of the rice accessions into four groups (Fig. 3). One of the subgroups contained 48 of the 65 accessions. This clustering was based on a cut-off value of 70% shared ancestry (Table S2, available online).

Fig. 3 Unrooted neighbour-joining tree showing the genetic relationship among the investigated rice genotypes, based on the C.S. Chord distance (Cavalli-Sforza and Edwards, Reference Cavalli-Sforza and Edwards1967) and distance based on the 33 nuclear microsatellite markers. Rice genotypes carrying the functional allele are indicated by asterisks. The scale bar indicates the genetic distance.
Screening for LTG
Analysis of LTG screening data revealed that 22 accessions (33%; 14 landraces and 8 varieties) showed 0–60% germination, while 43 accessions (66%; 30 landraces and 13 varieties) showed 60–100% germination (Table S1, available online). Interestingly, a few landraces such as LR 52 and LR 63 have evolved in the states of Chhattisgarh and West Bengal (WB), respectively, showing tolerance to LT at the germination stage. Rice in these two states grows under subtropical rain-fed conditions and rarely encounters cold stress.
Polymorphism study for the 71 bp indel for qLTG3-1 in the rice accessions
A panel of 65 rice landraces and varieties from different states of India with a majority from the northeastern hill region along with few international lines was used to determine whether polymorphism exists across qLTG3-1. PCR amplification and scoring was carried out using the 71 bp deletion-specific primer pair (as described in the Materials and methods section). Surprisingly, out of the accessions screened for qLTG3-1, 43 carried the tolerant allele Italica Livorno (71 bp insertion). The 43 accessions came from diverse geographic origin, cultivation type (upland or lowland) and genetic background (landrace or variety). The 71 bp insertion correctly identified 26 of the 43 tolerant accessions, while the deletion correctly identified 17 of the 22 susceptible accessions. It has been reported that selection pressures for traits of interest narrow the genetic diversity of a population (Fujino and Iwata, Reference Fujino and Iwata2011). However, our results suggested that sufficient diversity exists in the screened rice germplasm and the beneficial allele for qLTG3-1 has been selected by farmers and breeders over a period of time.
Genotype–phenotype association for qLTG3-1
The frequencies of two qLTG3-1 alleles were analysed in each of the six population subgroups of accessions, namely cold tolerant, cold susceptible, upland, lowland, landraces and improved lines/varieties against the observed overall distribution of 48% for insertion and 52% for deletion in the whole population (Table 1). There was a significant association of the insertion allele with cold tolerance (χ2= 2.812, P= 0.094) and the deletion allele with cold susceptibility (χ2= 5.496, P= 0.019) (Table 1). No significant association of the two alleles was observed for the remaining subgroups. The distribution of the functional allele was not confined to any particular subgroup derived based on the SSR data (Fig. 3). The genetic structure of the rice accessions used in the current study was analysed using software based on Gaussian distributions (C.S. Chord method). The C.S. Chord distance method employs the likelihood-based criterion that models the distance between accessions as a random variable that follows a Gaussian distribution. Based on the distance analysis, the distribution of the insertion allele seems to be unbiased. This suggests that the functional allele has been retained in diverse rice accessions irrespective of the genetic background.
Table 1 χ2 Analysis of the insertion and deletion alleles of qLTG3-1 in the different population subgroups as against their overall distribution in the population

Discussion
A survey of the tolerant allele qLTG3-1 among landraces and improved rice varieties revealed that this beneficial allele existed in the germplasm surveyed. Diversity estimates based on SSR data suggest that rice germplasm used in the current study is genetically distinct and divergent. Distribution of the tolerant allele is not restricted to any particular genetic group (Fig. 3). However, the tolerant allele was found to be associated with the subgroup of tolerant accessions, implying that the tolerant allele plays an important role and has been favourably selected in tolerant accessions across different genetic backgrounds. The phosphorus uptake locus (Pup1) has been reported to be associated with upland rice accessions (Chin et al., Reference Chin, Lu, Haefele, Gamuyao, Ismail, Wissuwa and Heuer2010), whereas alleles for white pericarp and non-shattering grains have been retained in domesticated rice due to human selection (Zhao et al., Reference Zhao, Wright, Kimball, Eizenga, McClung, Kovach, Tyagi, Ali, Tung, Reynolds, Bustamante and McCouch2010). No such association of qLTG3-1 alleles was observed in this study for upland, lowland, landrace and improved line/variety subgroups, indicating that land type and artificial selection did not play a role in the selection of the tolerant allele.
A previous study has demonstrated that qLTG3-1 has many effects (Iwata and Fujino, Reference Iwata and Fujino2010) as assessed by its effects on different genetic backgrounds of three japonica varieties. However, cultivated rice comprises at least five distinct genetic groups (Garris et al., Reference Garris, Tai, Coburn, Kresovich and McCouch2005) carrying different combinations of alleles and, for quantitative traits such as abiotic stress tolerance, it is rare to find a direct association of a single locus with tolerance in a natural population. Our results suggest a relatively direct role of qLTG3-1 in imparting cold tolerance during germination. Landraces/improved varieties such as Dagaddeshi and Kamlesh, not previously exposed to LT, carry the tolerant allele qLTG3-1 at the same locus as done by cold-tolerant landraces/improved varieties.
Previously, it has also been shown that qLTG3-1 increases tolerance not only to LT but also to high salinity, high osmotic conditions and high temperature at the seed germination stage (Fujino et al., Reference Fujino, Sekiguchi, Sato, Kinuchi, Nonone, Takenuchi, Ando, Lin and Yano2004, Reference Fujino, Sekiguchi, Matsuda, Sugimoto, Ono and Yano2008). However, this had not been tested in diverse genetic backgrounds. Our results show that some landraces belonging to the states of Chhattisgarh and WB (where rice encounters other stresses such as drought and salinity, but not cold) showed tolerance to LT at the germination stage. Such landraces may prove to be a source of novel genes and alleles that may be imparting multiple stress tolerance. Expression profiling of genes targeted by qLTG3-1 revealed the upregulation of a set of 2415 genes including defence response genes (Fujino and Matsuda, Reference Fujino and Matsuda2010). Whether the germplasm carrying the tolerant allele surveyed in the current study expresses similar/same defence response genes is yet to be determined.
It has been suggested that no natural variation across qLTG3-1 exists and all the 62 landraces of rice tested belonging to different countries including India carry the 71 bp gain-of-function allele (Fujino and Iwata, Reference Fujino and Iwata2011). However, our findings suggest that the distribution of both the alleles (Hayamasari and Italica Livorno) is not biased towards any genetic group and that both the alleles can be easily found in Indian rice germplasm. Furthermore, our findings indicate that function of qLTG3-1 is critical for seed germination under diverse environmental conditions. It has been reported that the short arm of chromosome 3 carries genes for two major traits; eating quality and heading date (Fujino and Iwata, Reference Fujino and Iwata2011). An association of these traits with qLTG3-1 could be an area for future study. Also, the tolerant amino acid substitution reported from Nipponbare needs to be investigated across diverse germplasm to find its utility for breeding purposes. In summary, this study provides better understanding of the prevalence of the 71 bp tolerant allele in Indian rice germplasm, and this understanding will lead to the identification of donors for potential marker-assisted breeding programmes using the 71 bp marker.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S1479262113000142
Acknowledgements
Funding from the National Agriculture Innovative Project (NAIP; grant no. C30033/415101-036), Indian Council of Agriculture Research (ICAR), New Delhi, India to W.T. is gratefully acknowledged.