The current strategy for management of univentricular type of congenital heart disease culminates in performance of the Fontan operation. If nature has provided a congenital heart defect in which only one viable ventricle is present, then that ventricle must be assigned the task of systemic perfusion. Initial newborn palliation is specific to the type of univentricular heart present with the goal of providing balanced flow between the systemic and pulmonary circulations. Ultimately, the two circulations are separated at the Fontan operation. Pulmonary blood flow can be adequately achieved absent a pulmonary ventricle so long as the existing conditions will allow for passive blood flow, first through a superior cavopulmonary connection at a few months of age and then inferior vena caval flow through the Fontan operation at a few years of age.
This strategy is now standard of care and has created survival for thousands of children with a univentricular heart. However, as our experience grows and a greater number of survivors make their way into the second and third decades of life, enthusiasm for this strategy is tempered by the sobering appreciation of its inadequacy as a permanent long-term solution for all. Although mortality early after Fontan operation is quite low,Reference Rogers, Glatz and Ravishankar1 end-organ consequences of the unique physiology generated by the Fontan operation are of significance. Francois Fontan's prescient declarationReference Fontan and Baudet2 in 1972 that “this procedure is not an anatomical correction, which would require the creation of a right ventricle, but a procedure of physiological pulmonary blood flow restoration, with suppression of right and left blood flow mixing” is quite evident today in the many survivors of the surgery that carries his name.
In this report, we review the circulatory deficiencies of the Fontan operation and discuss its impact on the development of hepatic fibrosis, protein-losing enteropathy and plastic bronchitis.
Limitations of the Fontan circulation
Upon completion of the Fontan operation, systemic venous return makes its way “passively” from the peripheral veins through the vena cavae into the central branch pulmonary arteries. Once oxygenated, blood returns through the pulmonary veins to a receiving atrium – left or right – then across an atrioventricular valve to the systemic ventricle, before ejection to the systemic arterial circulation.Reference de Leval and Deanfield3
Without any active force propelling systemic venous return forward, congestion and central venous hypertension follows. A variety of factors influence flow in this system.Reference Gewillig, Brown and Eyskens4 The type of Fontan connection and the geometry of the connection between the vena cavae and pulmonary arteries influence the kinetics and dynamics of flow as it enters into the lungs.Reference de Leval, Dubini and Migliavacca5 During systemic ventricular diastole, blood normally rushes forward across the atrioventricular valve, emptying the pulmonary venous atrium, which then draws blood forward through the pulmonary veins and capillaries. In a Fontan circuit, this impacts flow upstream in the pulmonary arteries and systemic veins. Abnormalities of ventricular or atrial compliance can negatively influence diastolic filling, ultimately diminishing the impetus for forward flow.Reference Penny and Redington6 Hypertrophy, myocardial fibrosis and dis-coordinate relaxation of the systemic ventricle, common findings in the univentricular heart, can lead to impaired filling.Reference Ho, Lai, Wong and Cheung7 Abnormalities of the pulmonary vasculature, which may occur naturally as a consequences of altered pulmonary flow patterns in utero, or secondarily because of abnormal development as a consequence of initial newborn palliative surgery or lack of pulsatile flow,Reference Henaine, Vergnat and Bacha8 may contribute to impedance to passive forward flow.
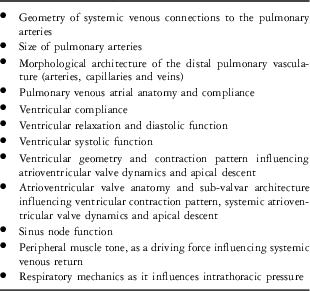
Whereas diastole plays an important role, systole also influences systemic venous flow after Fontan operation. We studied Doppler flow patterns in the pulmonary veins and superior vena cava of patients after superior cavopulmonary connection and found that the predominance of flow during the cardiac cycle appears to occur in systole in both the pulmonary veins and the superior vena cava.Reference Rychik, Fogel and Donofrio9 As there is no pulmonary ventricle, this cannot be due to ventricular thrust. Thus, what is the impetus for systolic forward flow in the systemic veins after Fontan operation? Atrioventricular valve descent towards the apex during systole is the likely explanation. Systemic ventricular contraction typically involves a downward atrioventricular valve motion, which creates expansion of the pulmonary venous atrium. This is an attempt to fulfil the physiological law of conservation of volume within the heart, in which total atrial plus ventricular volumes remain constant throughout the cardiac cycle. Negative intra-atrial pressure is generated by this valve motion, which draws blood out of the lungs and pulls systemic venous blood forward.Reference Steding-Ehrenborg, Carlsson, Stephensen and Arheden10 Ventricular geometry and ventricular contraction patterns, highly variable in the univentricular heart, likely influence the phenomenon of systolic atrioventricular valve motion and the impetus for systemic venous forward flow. Additional factors that influence forward flow after Fontan operation are listed in Table 1.
Table 1 Examples of factors that influence systemic venous forward flow after Fontan operation.
Despite systemic venous hypertension, patients after Fontan operation exist in a ventricular preload deficient state.Reference Gewillig, Brown and Heying11 Systemic venous return must obligatorily traverse the pulmonary circuit before filling the ventricle, resulting in low end-diastolic volume and decreased stroke volume. Furthermore, the capacity to increase flow through this system at periods of increased demand is limited, which explains why exercise capacity after Fontan operation is impaired. The chronic conditions of relatively low cardiac output and systemic venous congestion form the basis for an indolent, morbid state that forms the basis for deleterious end-organ consequences.Reference Rychik12
Impact upon the liver
The liver is positioned in close proximity to the heart and is directly affected by the venous hypertension and congestion generated by the Fontan circulation. The first report of liver fibrosis after Fontan operation occurred in 1983.Reference Lemmer, Coran, Behrendt, Heidelberger and Stern13 In some instances, hepatic pathology may pre-exist before Fontan operation as a consequence of haemodynamic instability at initial presentation or at early surgical palliation.Reference Schwartz, Sullivan and Cohen14
Serious concerns were raised when in 2005 cases of hepatocellular neoplasm were identified after Fontan operation in a 9- and 18-year-old.Reference Ghaferi and Hutchins15 Since then, a number of reports describe the presence of hepatic fibrosis of varying degrees in almost all patients following Fontan operation.Reference Schwartz, Sullivan and Glatz16–Reference Kiesewetter, Sheron and Vettukattill18
The ostensible cause of liver fibrosis is chronic hepatic venous congestion, although hypoperfusion may play a role as well. Arterialised nodules in the periphery of the liver have been noted on computerised tomography scan, arising in regions of potential watershed hypoperfusion.Reference Bryant, Ahmad and Millward-Sadler19 The liver after Fontan operation is uniquely situated in a vulnerable position. Afferent flow, into the liver, is derived from two sources – the hepatic arterial system and venous return from the mesenteric circulation. Both of these afferent systems are potentially compromised as a consequence of sub-optimal cardiac output to the mesenteric circulation and altered portal venous flow. Efferent flow – exiting the liver – is into the congested systemic venous system directly draining into the Fontan pathway. Tissue perfusion, as reflected by the pressure difference between the afferent and efferent systems of the liver, is limited in comparison to normal.
Although both inflow and outflow into the liver may be affected, the primary pathophysiology is likely related to hepatic venous congestion. Hepatic venous hypertension stimulates liver stellate ganglion cells to transform into fibroblasts, through mechanisms not yet fully delineated. The result is collagen deposition and fibrosis, first in a peri-venular manner, then progressively extending further into the hepatic parenchyma, ultimately leading to bridging fibrosis, broad scar formation and cirrhosis (Fig 1).

Figure 1 (a) Core liver biopsy, 10× magnification, trichrome stain from an 18-year-old patient with heterotaxy syndrome, after Fontan operation. There is marked collagen deposition and broad bands of significant fibrosis (blue colour). (b) Increased magnification (40×) shows evidence for cirrhosis.
Diagnosis and characterisation of hepatic fibrosis after Fontan operation is a challenge.Reference Friedrich-Rust, Koch and Rentzsch20, Reference Baek, Bae and Ko21 Routine blood laboratory testing typically reveals trivial elevation of liver enzyme values in all patients after Fontan operation, findings that do not correlate with the severity of fibrosis. A diminished platelet count may reflect hypersplenism with platelet sequestration, and may be seen in advanced stages of hepatic fibrosis, of any cause. Detection of early stages of hepatic fibrosis is difficult even in the more common settings of alcohol or Hepatitis C-induced liver injury and is often overlooked in Fontan physiology because of the absence of overt signs of liver injury. Non-invasive assays surveying for biomarkers in these conditions have not been extensively studied and correlated with liver pathology seen after Fontan operation. Imaging modalities such as ultrasound or magnetic resonance imaging provide information concerning gross structure, but do not provide adequate information concerning histology, thereby not providing an accurate means for grading disease severity. Elastography, the analysis of shear wave propagation through biological tissue, can provide information concerning tissue density and holds promise as a means for assessing liver fibrosis in this population.Reference Ferraioli, Tinelli and Dal Bello22 Studies distinguishing liver tissue density characteristics due to venous congestion from that of hepatic fibrosis will need to be performed through correlation of haemodynamic data and liver biopsy material. Unfortunately, at this point in time, liver biopsy is the “gold standard” optimal means available for evaluating hepatic status after Fontan operation.
There is growing evidence that hepatic fibrosis is a ubiquitous complication after Fontan operation. Realising the importance of characterising the health status of survivors after Fontan operation, in order to optimise long-term outcome, and with published data suggesting that no patient is spared this complication, our group at The Children's Hospital of Philadelphia recently decided to offer systematic, comprehensive evaluation to our patients who are 10 years or more past their Fontan operation.Reference Rychik, Veldtman and Rand23 We currently recommend performance of a liver biopsy, with concurrent evaluation of the cardiovascular system through cardiac catheterisation and cardiac magnetic resonance imaging. Although controversial, the decision to undertake an aggressive evaluation of liver status with concurrent assessment of haemodynamics at this 10-year mark provides the best opportunity to identify variables that might be modifiable for the individual patient, leading to a tailored, patient-specific plan for continued care into the adult years.
A number of goals remain concerning management of the liver after Fontan operation. Identifying modifiable variables along the course of univentricular management that may be causative is important. We also need to identify strategies that minimise the conditions that permit ongoing fibrosis after Fontan operation. Understanding the biological basis for fibrosis under conditions of elevated central venous pressure, relative hypoxaemia and diminished arterial perfusion will allow us to develop better strategies of care, and perhaps offer opportunities for treatment aimed at reversal of hepatic fibrosis.
Protein-losing enteropathy
Protein-losing enteropathy is defined as the loss of serum proteins into the gut lumen due to variable loss of integrity of the enteric mucosa. It can occur because of conditions such as inflammatory bowel disease or primary gut lymphatic abnormalities. The incidence of protein-losing enteropathy after Fontan operation is not fully known. The prevalence is believed to be anywhere from 1 to 5%.Reference Mertens, Hagler, Sauer, Somerville and Gewillig24, Reference Meadows and Jenkins25 Although a sub-clinical entity is highly suspected,Reference Thorne, Hooper, Kemp and Somerville26 this possibility has not yet been adequately characterised. However, if a sub-clinical entity is present as we suspect, then the frequency of this condition is substantially increased. The clinical manifestations of protein-losing enteropathy are many (Table 2).
Table 2 Manifestations of protein-losing enteropathy after Fontan operation.

Protein-losing enteropathy can occur at any point after Fontan operation. Early onset within weeks to months of surgery is typically related to an acute problem, such as anatomical obstruction of the systemic or pulmonary venous pathways, or ventricular dysfunction. More often, it occurs years after Fontan operation, and without any known trigger or specific cause. Cardiovascular assessment with cardiac catheterisation may indicate a specific problem, but more often than not it typically reveals haemodynamic information that is satisfactory and indistinguishable from other patients with Fontan circulation who do not have protein-losing enteropathy.
The precise pathophysiology of protein-losing enteropathy after Fontan operation and mechanism of onset is poorly understood. Nevertheless, a framework of understanding has allowed for the development of a useful algorithm for management.Reference Rychik27 As best as we currently understand it, the mechanistic hallmarks of protein-losing enteropathy include the elements of an altered mesenteric circulation and inflammation.
Mesenteric haemodynamics is very much affected by the Fontan circulation. One theory holds that chronic low cardiac output evident after Fontan operation is similar to that of a chronic state of circulatory “shock”, with increase in mesenteric vascular tone as compensation. Supporting this notion are data derived from Doppler interrogation of the superior mesenteric artery demonstrating increased mesenteric vascular resistance in children after Fontan operation in comparison to normal, with further increase in resistance in those with protein-losing enteropathy.Reference Rychik and Gui-Yang28 Further supporting the idea that haemodynamics play a key role is the resolution of protein-losing enteropathy is what occurs once actions to improve the abdominal circulation are undertaken. Oral sildenafil has been demonstrated to reduce mesenteric vascular resistance and concurrently resolve protein-losing enteropathy.Reference Uzun, Wong, Bhole and Stumper29 Pacing to create atrioventricular synchrony,Reference Cohen, Rhodes, Wernovsky, Gaynor, Spray and Rychik30 fenestration creationReference Jacobs, Rychik, Byrum and Norwood31, Reference Rychik, Rome and Jacobs32 and heart transplantationReference Bernstein, Naftel and Chin33 have also all led to resolution of protein-losing enteropathy in various reports.
Inflammation is the second mechanistic pillar of protein-losing enteropathy after Fontan operation. A number of clues support this notion. Repeatedly, parents will recount the new onset of protein-losing enteropathy, or worsening of symptoms, within a 2–4-week period of a preceding viral illness. Patients after Fontan operation exist in a relatively increased state of inflammation, with increased levels of circulating tumour necrosis factor α, perhaps related to chronic low cardiac output.Reference Ostrow, Freeze and Rychik34 Direct inspection of the gut through endoscopy in patients with protein-losing enteropathy after Fontan operation reveals both gross and histological evidence for inflammation (Figs 2 and 3). Furthermore, numerous reports have documented quiescence of protein-losing enteropathy and its symptoms following administration of high-dose systemic corticosteroids.Reference Rychik, Piccoli and Barber35, Reference Zellers and Brown36

Figure 2 (a) Endoscopic image of a normal duodenum. (b) Duodenum of a patient with protein-losing enteropathy after Fontan operation demonstrating hyperaemia and oedema.

Figure 3 (a) Endoscopic image of a normal colon. (b) Colon of a patient with protein-losing enteropathy after Fontan operation demonstrating hyperaemia and oedema.
How does altered mesenteric circulation and inflammation lead to enteric protein loss? A rare condition of congenital protein-losing enteropathy has been described, in which there is deficiency of enterocyte sulfated glycosaminoglycan, an essential molecule for maintaining integrity of the intestinal mucosa. To test the role of sulfated glycosaminoglycans in protein-losing enteropathy after Fontan operation, an in vitro model was created.Reference Bode, Eklund, Murch and Freeze37, Reference Bode, Murch and Freeze38 Using a monolayer of intestinal cells, albumin flux across was measured. Loss of heparin sulfate, an important intestinal sulfated glycosaminoglycan through addition of heparinase, increased albumin flux across the intestinal monolayer 1.6-fold above resting state. Treatment of the preparation with tumour necrosis factor α to mimic inflammation, in combination with heparanase, further increased albumin flux to sevenfold above resting state. Addition of a small pressure gradient to mimic venous hypertension and complete the pathological picture characteristic of a Fontan circulatory state dramatically increases albumin flux across the mucosa.
Of interest, intestinal sulfated glycosaminoglycan deficiency has been reported in malnourished children with kwashiorkor, a protein-loss state, but not in those with malnutrition and marasmus.Reference Amadi, Fagbemi and Kelly39 This phenomenon suggests a predisposition to altered sulfated glycosaminoglycan intestinal make-up in a select group, those with Kwashiorkor, but not in all following the stressor of severe malnutrition. In order to assess whether patients with protein-losing enteropathy after Fontan operation may have a similar finding of sulfated glycosaminoglycan deficiency, we have analysed biopsy material from the terminal ileum of 10 patients (unpublished data). Preliminary findings indicate deficiency of sulfated glycosaminoglycan make-up in our patients with protein-losing enteropathy after Fontan operation, but to a variable degree (Fig 4). This suggests a possible common molecular pathway for protein leak that may exist between various stressor conditions to the gut.

Figure 4 (a) Silver stain image of a normal intestinal villous with dark band of stain (dark arrows) indicating presence of glycosaminoglycan in the epithelium. (b) Patient with protein-losing enteropathy after Fontan operation in which there is a paucity of silver stain uptake, suggesting a diminution in intestinal epithelial glycosaminoglycan.
Despite absence of a complete understanding of the pathophysiology, how can we currently manage patients with protein-losing enteropathy after Fontan operation? First, a rigorous investigation for faultiness with the Fontan construct is mandatory through testing including cardiac catheterisation. Pathway obstruction, or other anatomical explanations, which limit cardiovascular functionality should be addressed if possible (Fig 5). Often, no direct causative explanation can be found, in which case we recommend a two-pronged approach to improve circulation and inhibit intestinal inflammation. Aggressive pulmonary vasodilation through the use of phosphodiesterase inhibition (sildenafil) and/or endothelin-1 blockade (bosentan) can be offered, in order to optimise forward flow through the Fontan circuit. Pulmonary vasodilation should be pursued regardless of the pulmonary artery pressures, as even a small reduction in pressure may significantly influence flow in a venous, low-flow system. In addition, phosphodiesterase inhibition may have a direct salutary effect on intestinal haemodynamics independent of its effect on the pulmonary circulation.Reference Uzun, Wong, Bhole and Stumper29 In conjunction, oral controlled-release budesonide (entocort) can be used.Reference Thacker, Patel, Dodds, Goldberg, Semeao and Rychik40 This agent offers potent anti-inflammatory treatment and is released at the target site of the small bowel. Normally, oral controlled-release budesonide is 90% metabolised at first pass through the liver, therefore minimising systemic side effects. However, as most patients after Fontan operation have some liver changes, which may affect metabolism, side effects are common. Since our centre's initial report on the efficacy of oral controlled-release budesonide, multiple other reports have emerged.Reference Thacker, Patel, Dodds, Goldberg, Semeao and Rychik40–Reference Schumacher, Cools and Goldstein44 Experience now shows it to be effective treatment when started in the child or adolescent with new-onset protein-losing enteropathy, but less effective when initiated in the adult patient with chronic, long-standing disease. A trial period of 3–6 months of combination pulmonary vasodilation and intestinal anti-inflammatory treatment is suggested. Positive results are often not seen until a minimum of 3–4 months into therapy, suggesting that perhaps a turnover of intestinal mucosal cells is required under the new treatment conditions in order to prevent protein leak. Protein-losing enteropathy itself leads to calcium loss and bone demineralisation, as can chronic steroid treatment. Hence, once a positive response is noted, slow weaning to a low maintenance dose of oral controlled-release budesonide is indicated.

Figure 5 Algorithm for management protocol of protein-losing enteropathy after Fontan operation.
Failing a response to medical management, other options are available, but each has limitations. Creation of a fenestration may be helpful for some patients.Reference Jacobs, Rychik, Byrum and Norwood31, Reference Rychik, Rome and Jacobs32, Reference Mertens, Dumoulin and Gewillig45 Mechanism of action is thought to be related to improved cardiac output and possible increase in oxygen delivery to the gut. Although relatively well tolerated in childhood, adolescents do poorly with oxygen saturations in the mid 80% range and are often dyspnoeic, resulting in exchange of one set of problems related to protein-losing enteropathy for another. Heart transplantation is effective when it achieves the goals of reducing central venous hypertension and improving cardiac output.Reference Bernstein, Naftel and Chin33, Reference Kanter, Mahle, Vincent, Berg, Kogon and Kirshbom46
Improved understanding of protein-losing enteropathy after Fontan operation has provided the ability to develop a working algorithm for treatment. A condition previously reported to have 50% mortality at 5 years from onset of diagnosisReference Mertens, Hagler, Sauer, Somerville and Gewillig24 is now manageable, leading to a chronic but serious condition in most patients affected. A successful long-term treatment with sustained resolution still evades us.
Plastic bronchitis
Plastic bronchitis is a condition seen after Fontan operation in which proteinaceous material is exuded into the airways leading to cast formation (Fig 6).Reference Larue, Gossett, Stewart, Backer, Mavroudis and Jacobs47 These rubbery casts are either expectorated or swallowed after mobilisation out of the bronchial tree. Bronchial casts may also remain in situ, leading to lobar atelectasis with subsequent hypoxaemia, or if large enough they can obstruct the airway leading to asphyxiation and death. The condition is believed to be similar to protein-losing enteropathy after Fontan operation in a number of ways.Reference Stiller, Riedel, Paul and van Landeghem48 The fundamental problem is a break in the integrity of the bronchial mucosa leading to proteinaceous leak into the airway lumen. Unlike the gut, in which there is rapid transit and hence the potential for abundant loss of serum protein into the stool, in plastic bronchitis the transit time is slow and the space limited; hence, protein accretion and cast formation takes place. Fibrin has been identified as the major protein type making up these bronchial casts.Reference Racz, Mane and Ford49 Some investigators believe that there may also be a wide spectrum to the condition, with a sub-clinical entity resulting in the common expectoration of small fibrin plugs and with a prevalence of 4–14% among all survivors of Fontan operation.Reference Caruthers, Kempa and Loo50

Figure 6 Bronchial cast from a child with plastic bronchitis after Fontan operation.
Similar to protein-losing enteropathy, the pathophysiogy and mechanism of onset of plastic bronchitis is not completely understood. Amelioration of circulatory haemodynamics either through improvement of the Fontan stateReference Wilson, Russell, Williams and Benson51–Reference Barber, Burch, Tripple and Balaji53 or through heart transplantationReference Larue, Gossett, Stewart, Backer, Mavroudis and Jacobs47, Reference Laubisch, Green, Mogayzel and Reid Thompson54, Reference ElMallah, Prabhakaran and Chesrown55 has been demonstrated to be effective. Pulmonary vasodilation with phosphodiesterase inhibition (sildenafil)Reference Haseyama, Satomi, Yasukochi, Matsui, Harada and Uchita56 or through the use of endothelin-1 blockade (bosentan) has been demonstrated to be effective in some patients.Reference Apostolopoulou, Papagiannis and Rammos57 We and other investigators have found great benefit in the administration of aerosolised tissue plasminogen activator as a means for dissolving casts or in limiting development of casts.Reference Costello, Steinhorn, McColley, Gerber and Kumar58–Reference Lubcke, Nussbaum and Schroth61 Daily administration of aerosolised issue-plasminogen activator can be life saving as adjuvant therapy when used in combination with a strategy of aggressive pulmonary vasodilation.
Recently, another approach has been proposed to the possible management of plastic bronchitis after Fontan operation. New imaging techniques utilising magnetic resonance imaging, T2 weighted towards high-water content, can offer a means for imaging the lymphatic system. Preliminary findings of magnetic resonance imaging lymphatic imaging suggest the presence of abnormally dilated and tortuous lymphatic vessels in the abdomen of patients with protein-losing enteropathy and the chest of patients with plastic bronchitis after Fontan operation. Interestingly, reduction in lymph production through dietary meansReference Parikh, Witte and Samson62 or thoracic duct ligation has been reported as a means for treating plastic bronchitis.Reference Shah, Drinkwater and Christian63 One plausible hypothesis is that plastic bronchitis is caused by the underlying pathophysiology of the Fontan circulation leading to abnormal lymphatic drainage surrounding the airway.Reference Ezmigna, Morgan, Witte and Brown64 Spillage of lymph-rich, proteinaceous fluid into the airway through mechanisms of over-production or distal lymphatic channel obstruction results in bronchial cast formation. The risk of leakage of proteinaceous material and cast formation may therefore be present in all survivors of the Fontan operation.
Derangement of lymphatic drainage may therefore be the third component to add to the pathophysiology of plastic bronchitis in addition to an underlying circulatory abnormality and inflammation. Lymphatic leakage alone is unlikely the cause as cast analysis has demonstrated abundant inflammatory cells, and the cytokine profile suggests a pro-inflammatory state.Reference Racz, Mane and Ford49 Nevertheless, with this understanding, a strategy for management can be developed. Rigorous investigation for a possible faultiness with the Fontan construct through cardiac catheterisation is required. Pulmonary vasodilation may lower the impetus for airway protein leakage. Use of aerosolised tissue-plasminogen activator will maintain airway patency by reducing cast volume burden. As work in the field of lymphatic imaging after Fontan operation proceeds forward, one may be able to identify specific abnormal lymphatic vessels surrounding the airway. These abnormal lymphatic structures may then become targets for catheter-based interventional techniques to occlude areas of leakage or to divert lymphatic flow away from luminal structures, thereby potentially reducing lymph spillage into the bronchial airway.
Summary
The Fontan operation, although part of a life-saving surgical strategy, manifests a variety of end-organ complications and unique morbidities that are still poorly understood. Liver fibrosis, protein-losing enteropathy and plastic bronchitis are consequences of a complex physiology involving circulatory insufficiency, inflammation and lymphatic derangement, all manifest in an indolent, chronic state. Management strategies are emerging, which shed some light on the origins of these conditions. Continued focused investigation will lead to a better understanding of these complications and allow us to achieve the goal of creating a fully normal duration and quality of life for those born with a univentricular heart malformation.
Acknowledgements
The authors would like to acknowledge Simon Murch MD, for his collaborative efforts in evaluation of intestinal biopsy specimens. Funding for this work is supported by the Robert and Dolores Harrington Endowment in Pediatric Cardiology, at The Children's Hospital of Philadelphia.





