As long-term survival and clinical outcomes for patients with CHD have improved, the prevalence of adult-onset arrhythmias, such as atrial fibrillation, has increased. Atrial fibrillation occurs in as many as 25–30% of patients with CHD,Reference Triedman 1 , Reference Kumar, Tedrow and Triedman 2 and treatment with pulmonary vein isolation via transseptal access to the left atrium is common. Other indications for access to the left heart with large sheaths include ablation of atrial tachycardia after Fontan procedure, pulmonary vein stenting, device closure of the left atrial appendage, and transcatheter mitral valve repair.Reference Alkhouli, Sarraf and Holmes 3 Therefore, we expect that the need for transseptal access in adults with CHD will increase. Transseptal access is not straightforward in patients with complex cardiac anatomy and histories of multiple cardiac surgeries.
Static balloon atrial septostomy is a well-established procedure in children with CHD and is typically accomplished by transseptal puncture through the thin portion of the fossa ovalis.Reference Tomita, Hatakeyama, Soda, Watanabe and Takagi 4 – Reference Chan, Mashburn and Boucek 6 In adults with CHD, the interatrial septum is usually thick and fibrotic because of a previous open heart surgery or intervention, and it resists manual advancement of the transseptal sheath. This situation is still uncommon and challenges the electrophysiologists. Forceful advancement of the transseptal sheath may diminish fine control of the sheath, and increase the risk of complications such as precarious tear of the septum and cardiac tamponade. These concerns are heightened when large sheaths are necessary for complex transseptal left heart procedures.Reference Alkhouli, Sarraf and Holmes 3 In these cases, static balloon atrial septostomy may be a useful technique to achieve fine control of the transseptal sheath and facilitate structural intervention in the left atrium.Reference Zadeh, Cannom, Macrum and Ho 7 However, there is a paucity of data about static balloon atrial septostomy in postoperative adult CHD patients.
The risk of iatrogenic atrial septal defect following trasseptal puncture with large sheaths remains a topic of debate. A recent study reported a high incidence of persistent iatrogenic atrial septal defect following transcatheter mitral valve repair, and it was associated with worse clinical outcomes and increased mortality.Reference Schueler, Ozturk and Wedekind 8 However, the clinical impact and evolution of iatrogenic atrial septal defect following structural intervention in adults with CHD remain unclear.
To investigate the usefulness and safety of static balloon atrial septostomy in adults with CHD needing left heart interventions, we analysed six static balloon atrial septostomy procedures in five cases. In addition, we studied the evolution of the iatrogenic atrial septal defect post procedure.
Materials and methods
We reviewed five adults with CHD who underwent a total of six static balloon atrial septostomy procedures undertaken to facilitate planned left heart interventions from January, 2014 to December, 2015 at Showa University Northern Yokohama Hospital. Retrospective review of the medical records for data collection included patient characteristics, purpose of intervention, details of interventions, clinical course, and evaluation of iatrogenic atrial septal defect on echocardiography after the procedure. Transthoracic echocardiography was performed with Acuson Sequoia (Siemens Medical Systems USA Inc., Mountain View, California, United States of America).
Results
Patient population (Table 1)
Median age at the time of procedure was 35 years, ranging from 29 to 45 years (Table 1). Underlying CHD diagnoses were: tetralogy of Fallot after total repair (closure of ventricular septal defect and right ventricular outflow tract reconstruction), tricuspid atresia after one and one-half repair (closure of atrial septal defect, bidirectional Glenn anastomosis and right atrial to right ventricular connection), pulmonary atresia with intact ventricular septum after one and one-half repair, and tricuspid atresia after extra-cardiac total cavo-pulmonary connection. The intended primary interventions were pulmonary vein isolation for atrial fibrillation (n=4), stenting for pulmonary vein obstruction (n=1), and radiofrequency ablation for focal atrial tachycardia (n=1).
Table 1 Summary of patient characteristics, procedures, and outcome.

AF=atrial fibrillation; AT=atrial tachycardia; iASD=iatrogenic atrial septal defect; IVC=inferior caval vein; PA/IVS=pulmonary atresia with intact ventricular septum; PV=pulmonary vein; RF=radiofrequency; RFCA=radiofrequency catheter ablation; SBAS=static balloon atrial septostomy; TA=tricuspid atresia; TCPC=total cavo-pulmonary communication; TOF=tetralogy of Fallot
* Embolisation of ruptured guidewire to right PA, which was successfully retrieved
Procedures (Table 1)
Static balloon atrial septostomy was performed after transseptal puncture with a radiofrequency transseptal needle (Baylis Medical, Montreal, Canada) in five procedures (n=5), and was performed for re-dilation of the fenestration, which was created at the time of Fontan completion in one patient. The transseptal puncture occurred across the atrial septum in two procedures – patients 1 and 2 – and the wall between the inferior caval vein and right atrium through the pericardial space in two procedures – patients 3 and 5, in the first session. Dilation of an existing communication was performed in two procedures: patients 4 and 5, in the second session. After transseptal puncture, static balloon atrial septostomy was performed with several types of balloon catheters, including peripheral cutting balloons (Boston Scientific, Natick, Massachusetts, United States of America) (4–6 mm) (n=5) and peripheral angioplasty balloon catheters mentioned below (n=6). In patients whose septum was initially dilated with a small-size peripheral cutting balloon (4–6 mm), it was exchanged for an angioplasty balloon over the wire. In three procedures, balloon size was increased step by step. Serial balloon dilations were performed in order to enlarge the iatrogenic atrial septal defect, and ultra-high-pressure balloons were used in two patients with particularly stiff septa (Conquest balloon 10–12 mm, Bard Peripheral Vascular, Tempe, Arizona, United States of America). All procedures were performed under fluoroscopic and intracardiac echocardiography monitoring. After static balloon atrial septostomy, three sheaths – that is, two 8 Fr and one 8.5 Fr – were introduced across the septa for pulmonary vein isolation or ablation of focal atrial tachycardia (5 procedures) and one 9 Fr long sheath for pulmonary vein stenting (1 procedure).
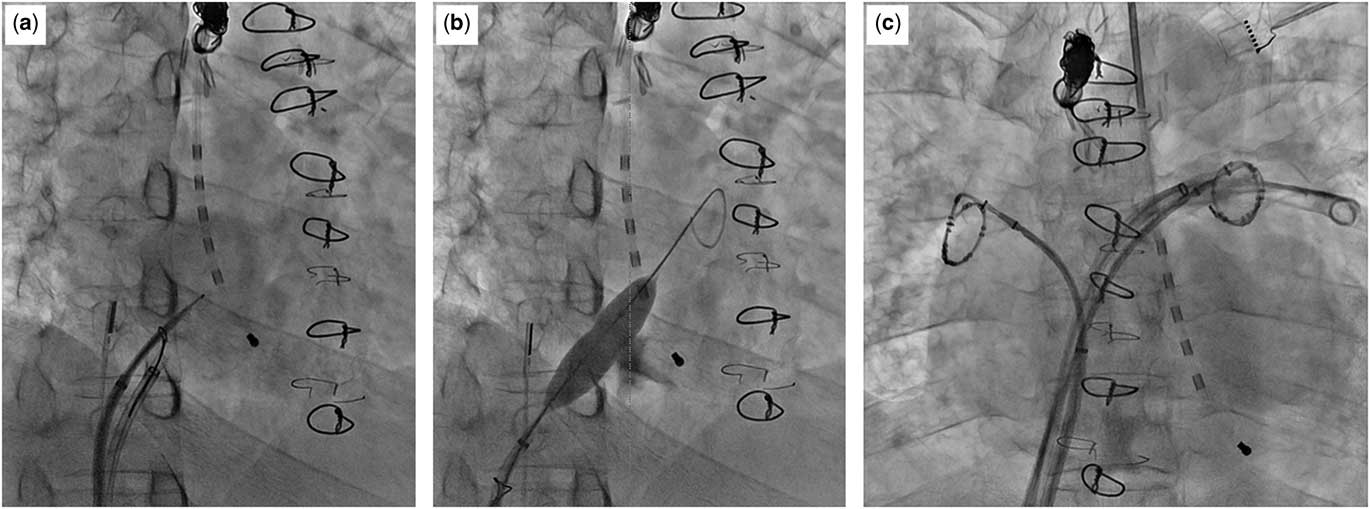
Figure 1 shows the anatomy of patient 5 and the location of the transseptal puncture at the inferior caval vein–right atrium wall and through the pericardial space. Figure 2 shows a three-dimensional reconstruction CT study for patient 5 and axial cuts, including at the level of the transseptal puncture. The puncture was performed under fluoroscopy (Fig 3) and with intracardiac echocardiography monitoring using a radiofrequency needle supported by a goose-neck snare in order to avoid slippage of the needle tip (Fig 3a). Serial balloon dilation was performed with a 4-mm peripheral cutting balloon followed by a 4-mm Jackal balloon (KANEKA Medix, Osaka, Japan), a 6-mm/8-mm Sterling balloon (Boston Scientific), and a 10-mm Conquest balloon (Fig 3b). The fine control of multiple sheaths via the thick septum was achieved during the electrophysiological study and catheter ablation on double lasso technique (Fig 3c).
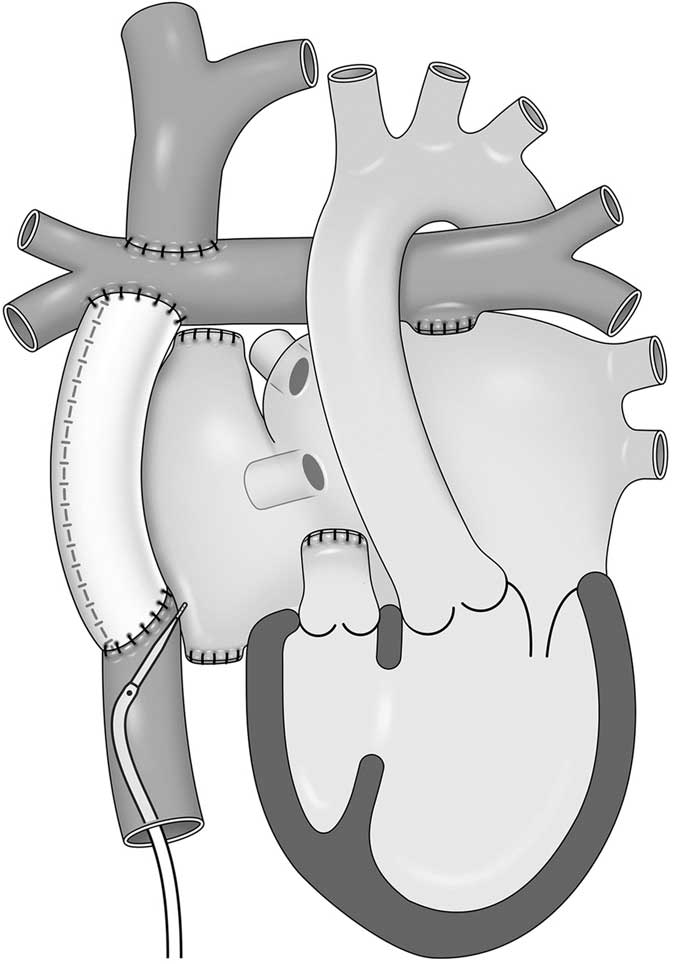
Figure 1 A schema of patient 5. The patient was born with tricuspid atresia and underwent extra-cardiac total cavo-pulmonary connection. The transseptal puncture was performed at the wall between the inferior caval vein and the right atrium through the pericardial space.
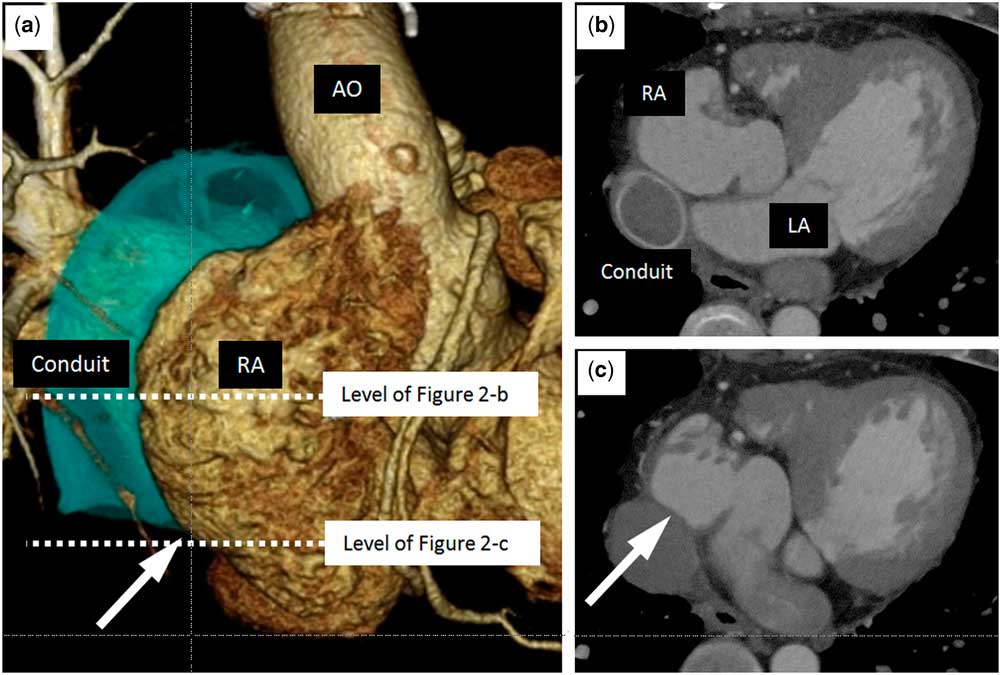
Figure 2 CT image of patient 5. ( a ) A three-dimensional CT image of patient 5. The white arrow shows the location of the puncture. ( b , c ) Horizontal planes of the CT image at the level of the white dotted line in a . The arrow in c shows the location of the puncture. Ao=aorta; LA, left atrium; RA=right atrium.

Figure 3 Fluorography images during the procedure of patient 5 in the first session. ( a ) The puncture was performed under intra cardiac echocardiography monitoring with a radiofrequency needle, which was supported by a goose-neck snare in order to avoid slippage of the tip of the needle. ( b ) Static balloon atrial septostomy at the wall between the inferior caval vein and the right atrium. ( c ) Electrophysiology study and ablation were successfully completed with a double lasso technique.
Outcome (Table 1)
Static balloon atrial septostomy and the intended interventional procedures were successfully completed in all cases. Fine control of multiple sheaths of 8–8.5 Fr was achieved after static balloon atrial septostomy with a final balloon size of 10–12 mm. There were no major complications, including haemopericardium and cardiac tamponade. In session 1 for patient 5, a ruptured guidewire embolised to the right pulmonary artery and was successfully retrieved during the same session.
The most recent transthoracic echocardiograms occurred at a mean follow-up of 9 months, ranging from 5 weeks to 20 months (Fig 4). In three patients, shunt flow across iatrogenic atrial septal defects completely disappeared within 20 months. In the remaining two patients the diameter of the shunt has gradually decreased to <2 mm. Patient 4 originally had a 4-mm fenestration, which was dilated with a 10-mm Sterling balloon, and the fenestration has returned to its original size in 5.5 months. There were no adverse clinical outcomes associated with iatrogenic atrial septal defect.
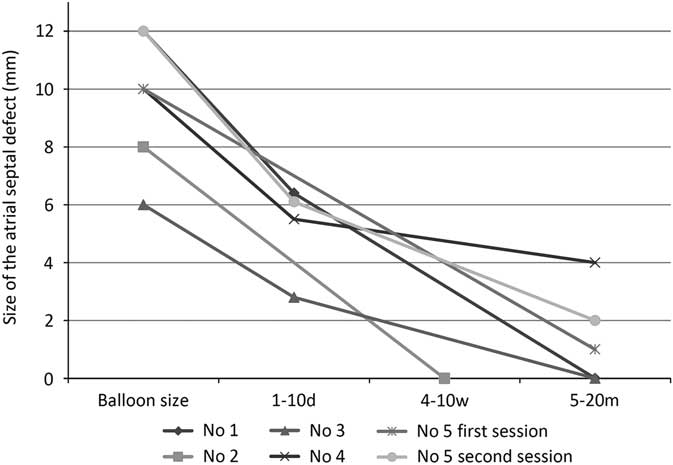
Figure 4 Iatrogenic atrial septal defect (iASD) size on echocardiography after the procedure. The size of iASD regressed over time in all cases. Shunt flow across iASDs completely disappeared in three patients.
Discussion
To perform left heart interventions, we used a radiofrequency transseptal needle that we regularly use for transseptal puncture in all patients. The needle perforates the atrial septum with minimal manual pressure and is easier to advance across the atrial septum than a conventional needle. However, the radiofrequency needle puncture alone was not large enough to accommodate multiple large sheaths through fibrotic and thick septa. To overcome this problem, we performed serial static balloon atrial septostomy, similar to the static balloon atrial septostomy procedures performed in paediatric CHD. We initially used small peripheral cutting balloons in order to minimise the risk of unplanned tears in the septa. After the cutting balloons, we used angioplasty balloons for further expansion of the puncture site.
The most novel use of static balloon atrial septostomy described in this manuscript is in the case of patient 5, who had an extra-cardicac total cavo-pulmonary connection. This technique is challenging transseptal puncture, as the needle traversed the pericardial space. This technique may be more feasible and effective than conventional direct puncture of conduit, in which a higher manual force is required and the needle tends to slip towards the upper side.
The incidence of iatrogenic atrial septal defect and the time course of their resolution are critical issues that may affect the haemodynamics after the interventional procedures. Transseptal interventions with sheaths <14 Fr are associated with a very high rate of spontaneous closure (80–90%), whereas persistent iatrogenic atrial septal defects are not uncommon when larger sheaths are used: 4.4–20% with 14 Fr sheaths and 27–50% with 22 Fr sheaths.Reference Alkhouli, Sarraf and Holmes 3 Schueler et al reported that the persistence of iatrogenic atrial septal defects after transcatheter mitral valve repair using a 21 Fr steerable sheath in MitraClip procedures (Abbott Vascular, Abbott Park, Illinois, United States of America) is 50% at the 6 month follow-up. Spontaneous right-to-left shunt via iatrogenic atrial septal defects was detected in 6% of cases, and persistence of iatrogenic atrial septal defects was associated with a 6-month survival.Reference Schueler, Ozturk and Wedekind 8 These findings suggest that excessive dilation of the interatrial access may be associated with high rates of iatrogenic atrial septal defects. In this study, we performed static balloon atrial septostomy with balloons larger than a 21 Fr sheath, and all of the iatrogenic atrial septal defects disappeared or regressed even in patients in whom we performed static balloon atrial septostomy with a much larger-size balloon than the 21 Fr sheath, except for patient 3 in whom a 6-mm balloon was used.
We speculate that the histologic characteristics of the atrial septum influence the incidence of spontaneous closure or persistence of iatrogenic atrial septal defects. Rich et al suggested that a thicker and more muscular septum allows for compaction and minimisation of the tissue disruption, whereas the thin-walled fossa ovalis does not have enough tissue to establish closure by tissue contact.Reference Rich, Tseng, Lim, Wang and Su 9 Thus, dilation of a thick and fibrotic septum, such as those in adults with CHD, may be associated with a low incidence of significant iatrogenic atrial septal defects, because of compaction and minimisation of the tissue disruption by balloon dilation. These results are clinically important because ophysiologists and interventionists can be reassured that iatrogenic atrial septal defects are likely to resolve in adults with CHD.
Sheath movement via the interatrial access point may also increase the risk of significant iatrogenic atrial septal defects other than excessive dilation with static balloon atrial septostomy. As for transcatheter mitral valve repair with MitraClip, Schueler et al described iatrogenic atrial septal defect persistence caused by requirement of extensive sheath movement and longer procedural time, which was associated with local trauma in the septum.Reference Schueler, Ozturk and Wedekind 8 Moreover, they also described that one of the factors associated with iatrogenic atrial septal defect persistence after pulmonary vein isolation was the use of a single transseptal sheath, demanding more extensive interatrial movement in comparison with double transseptal catheters.Reference Hammerstingl, Lickfett and Jeong 10 From this point of view, we suppose that the use of forcible sheath manipulation via inadequately small septal communication could have the possibility to increase the risk of persistent iatrogenic atrial septal defect. Furthermore, sufficient dilation of the communication with static balloon atrial septostomy can not only facilitate sheath manipulation but also reduce the risk of persistent iatrogenic atrial septal defect.
Caution concerning the potential risks such as heart failure, desaturation, and paradoxical embolism is appropriate, especially in patients with increased ventricular end-diastolic pressure and high venous pressure after Fontan procedure. Static balloon atrial septostomy for native fossa ovalis should be avoided because of the high risk of iatrogenic atrial septal defect.
To our knowledge, this is the first report of static balloon atrial septostomy for the purpose of left heart intervention in adult patients with repaired CHD. These findings suggest that static balloon atrial septostomy is a safe and useful tool for pulmonary vein isolation, ablation of atrial tachycardia after Fontan procedure, and pulmonary vein stenting, which require introduction of sheaths of large size into the left heart. Even in patients after Fontan procedure, puncture from the inferior caval vein to native atrial wall may be possible following careful imaging such as three-dimensional CT. The thickened interatrial septa in adults with CHD may be associated with a reduced rate of persistent iatrogenic atrial septal defects after static balloon atrial septostomy.
Limitation
Measurements of iatrogenic atrial septal defects on transthoracic echocardiograms are less accurate than measurements from transoesophageal and intracardiac echocardiography. We typically perform transesophageal echocardiography only if the clinical management demands more precise information. We consider that any small shunt not detected by transthoracic echocardiogram is not clinically significant. We did not evaluate the size of iatrogenic atrial septal defect immediately after the procedure, so the timing of iatrogenic atrial septal defect regression is still unclear.
Conclusion
We report performing static balloon atrial septostomy to facilitate difficult transseptal puncture in postoperative adults with CHD. Static balloon atrial septostomy is a safe and useful technique in adults with CHD undergoing left heart interventions by means of a transseptal approach. The thick atrial septum found in postoperative adults with CHD may reduce the risk of persistent iatrogenic atrial septal defects.
Acknowledgements
The authors thank Dr Peter M. Olley, Professor Emeritus of Pediatrics, University of Alberta, and Dr Setsuko Olley for language consultation.
Financial Support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of Interest
None.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation (Health and Disability Ethics Committees) and with the Helsinki Declaration of 1975, as revised in 2008, and has been approved by the institutional committees.