Introduction
The tribe Fordini, with the other two tribes of Eriosomatini and Pemphigini, belongs to the aphid subfamily Pemphiginae (Hemiptera: Aphididae) (Blackman & Eastop, Reference Blackman and Eastop1994; Remaudière & Remaudière, Reference Remaudière and Remaudière1997). Antennae of aphids in this tribe are five- or six-segmented with secondary rhinaria round, oval or large sheet. The media of forewing usually does not branch, and the hind wing has two oblique veins. The alate viviparous female has four or more hairs on the first tarsal segment. All morphs lack siphunculi and a wax plate may or may not be present (Zhang et al., Reference Zhang, Qiao, Zhong and Zhang1999).
The life history of aphids in this tribe is complicated, including holocyclic and anholocyclic species. Anholocyclic species are parthenogenetic on grass roots or mosses all the year round. However, most of the species are heteroeciously holocyclic with distinct primary host specificity. The primary hosts for all species are within two genera, Pistacia and Rhus, which belong to Anacardiaceae. They shift from Rhus to mosses, the secondary hosts (Melaphidina), or from Pistacia to the roots of Graminae (Fordina). Sexual reproduction alternates with parthenogenesis (Wool, Reference Wool and Ananthakrishnan1984; Zhang et al., Reference Zhang, Qiao, Zhong and Zhang1999). Aphids are plant-sucking insects and many of them are agricultural and horticultural pests (Powell et al., Reference Powell, Tosh and Hardie2006), but Melaphidina, which are endemic to East Asia, are a group of beneficial aphids, because they can induce galls containing tannin, an important industrial material (Zhang et al., Reference Zhang, Qiao, Zhong and Zhang1999). The division of subtribes in Fordini has been equivocal. One point of view thinks that Fordini encompasses two subtribes, Fordina and Baizongina (Börner, Reference Börner1952; Zwölfer, Reference Zwölfer1957; Davatchi, Reference Davatchi1958); the other suggests it be divided into Fordina and Melaphidina (Blackman & Eastop, Reference Blackman and Eastop1984; Ghosh, Reference Ghosh1984; Zhang et al., Reference Zhang, Qiao, Zhong and Zhang1999). Almost all the species in Fordini are gall-forming and stimulate species-specific galls on their primary hosts. However, galls of different species vary from each other in size, position, morphology and structure (Zhang et al., Reference Zhang, Qiao and Zhang2006). The position of galls can be on the leaf blade, the secondary vein, the midrib, the common petiole of the compound leaf or the whole twig. The shapes of galls vary from being bag-like, spherical, oval, horn-like, maple leaf-like, and others. The structure of galls may be either single-chambered or multi-chambered. Studies showed that it is not the host but the aphid itself which manipulates the morphology of gall formation (Stern, Reference Stern1995; Crespi & Worobey, Reference Crespi and Worobey1998; Nyman et al., Reference Nyman, Roininen and Vuorinen1998; Stone & Cook, Reference Stone and Cook1998). Inbar et al. (Reference Inbar, Wink and Wool2004) discussed the evolution of galls in Fordini, including the evolutionary tendency and driving force behind it, through reconstructing phylogenetic relationships based on mitochondrial genes COI, COII and combining ecological data (Fordini was raised to subfamily Fordinae in this paper). Unfortunately, no Melaphidina taxa were tested and the evolution of galls in Melaphidina was not considered. Whether melaphidine gall evolution followed the same scenario as that in Fordina was still unknown. In this study, we included the Melaphidina samples which are endemic to East Asia. The aim of this paper is to clarify the subtribe division in Fordini and further shed light on the evolution of galls, especially in Melaphidina, by reconstructing the phylogenetic relationship based on nuclear gene, EF-1α, and mitochondrial gene, COI, and combining the morphological and biological characters of aphids.
Materials and methods
Taxa examined
Taxa examined in this study and information about their collection localities and primary hosts are listed in table 1. Thirteen species and subspecies collected in China and Israel from 2004 to 2005 were used as ingroups. Two representatives of tribe Pemphigini, which is the sister group of Fordini based on morphology (Zhang et al., Reference Zhang, Qiao, Zhong and Zhang1999), were used as outgroups. Voucher specimens were preserved in 95% ethanol and deposited in the Zoological Museum of the Institute of Zoology, Chinese Academy of Sciences, Beijing.
Table 1. Information for species and subspecies examined in this study.

DNA extraction, PCR amplification, and sequencing
Total DNA was extracted from single aphids preserved in 95% or 100% ethanol. Tissue homogenates were incubated at 55°C in lysis buffer (30 mm Tris-HCl (pH 8.0), 200 mm EDTA, 50 mm NaCl, 1% SDS, and 100 μg ml−1 proteinase K) for 5–7 h, followed by a standard phenol-chloroform-isoamyl alcohol (PCI) extraction with a little improvement (Sambrook et al., Reference Sambrook, Fritsch and Maniatis1989). DNA was precipitated from the supernatant with two volumes of cold ethanol, centrifuged, washed, dried and dissolved in 15–20 μl TE buffer, then stored at 4°C for use.
The nuclear gene, EF-1α, and mitochondrial gene, COI, were amplified by polymerase chain reaction (PCR) using published primers EF3, EF2 (EF-1α; see Palumbi, Reference Palumbi, Hillis, Moritz and Mable1996) and CIS, CIA (COI; see Favret & Voegtlin, Reference Favret and Voegtlin2004). PCR reactions for EF-1α were performed in a 50 μl volume with 1×PCR buffer, 1.25 U Taq DNA polymerase, 200 μm dNTPs all from Takara Biotech (Dalian, China) and 0.2 μm primers synthesized in Sangon Biotech (Shanghai, China). The system for COI was the same as that of EF-1 α except for Taq DNA polymerase 2.5 U, primers 0.4 μm. The reactions were done on a GeneAmp PCR System 9700 (Applied Biosystem, USA) with the following conditions for EF-1α: 95°C for 5 min; 35 cycles of 94°C for 1 min, 49°C for 1 min, 72°C for 1 min; a final extension step of 10 min at 72°C was added after cycling. COI PCR amplification was performed with the following cycle protocol: 35 cycles, denature at 94°C for 1 min, anneal at 54°C for 1.5 min, and extend at 72°C for 2 min. These two protocols and the two pairs of primers worked well for all species and subspecies that we examined. Sequencing reactions were performed with the corresponding amplifying primers from both directions with BigDye Terminator Cycle Sequencing Kit v.2.0 (Applied Biosystem, USA) and run with ABI 3730 automated sequencer (Applied Biosystem, USA).
Assembling and alignment of sequences
Chromatograms, including sense and antisense, were analysed and assembled with the Seqman in DNAStar* software package (DNASTAR, Inc. 1996) to obtain single consensus sequence. As for EF-1 α, intron splicing junctions were then identified by the GT-AG rule and by comparison with the cDNA sequence of Schizaphis graminum (Rondani) (GenBank Accession No. AF068479). Introns were removed prior to phylogenetic analysis (von Dohlen et al., 2006). We confirmed the correctness of the sequences by checking whether it can be appropriately translated into protein with Editseq (DNASTAR, Inc. 1996). Sequences were deposited in GenBank under Accession Nos. DQ499605–DQ499630.
Phylogenetic analysis
The partition homogeneity test (Farris et al., Reference Farris, Källersjö, Kluge and Bult1995), as implemented in PAUP*, was used to determine the appropriateness of combining both genes into a single analysis. All phylogenetic analyses were performed with PAUP* 4.0b (Swofford, Reference Swofford2002). A maximum-parsimony (MP) analysis was carried out firstly on each gene region separately and the combined data set under the heuristic search strategy, with all sites weighted equally, gaps treated as missing data, and 1000 random-addition sequences and TBR branch swapping. To assess the support for branching events, non-parametric bootstrapping was performed with 1000 pseudoreplicates under the heuristic search strategy and 100 random-addition sequences in each pseudoreplicate (Felsenstein, 1983, Reference Felsenstein1985).
ModelTest 3.06 (Posada & Crandall, Reference Posada and Crandall1998) was used to select the best-fit nucleotide substitution model under the criterion hLRTs for maximum-likelihood (ML) analysis. ML analysis was performed in PAUP* under the selected optimal model on these three data sets, respectively, under the heuristic search strategy with ten random-addition sequences and TBR branch swapping. Bootstrap analysis was performed under the same model, with 100 pseudoreplicates, 10 random-addition sequences per replicate, and TBR branch swapping.
Bayesian analysis was conducted on the combined data set only, using MrBayes3.1.1 (Ronquist & Huelsenbeck, Reference Ronquist and Huelsenbeck2003) with models set for each partition based on the results of ModelTest. Model parameter values were treated as unknown variables with uniform prior probabilities and were estimated during the analysis for each data partition, independently. Four chains (three heated and one cold) were run, starting from a random tree and proceeding for one million Markov chain Monte Carlo generations, sampling the chains every 200 generations. Two independent runs were conducted to verify results. For all runs, 1000 trees were discarded as burn-in samples. Remaining trees were used to generate a majority-rule consensus tree, in which the percentage of trees recovering a clade portrayed the clade's posterior probability (Huelsenbeck et al., Reference Huelsenbeck, Ronquist, Nielsen and Bollback2001), or the probability that the clade is correct given the data and the model parameters.
Mapping morphological, biological characters of aphids and gall traits on the phylogenetic tree
We mapped aphid morphological characters, primary host plants and the galls' features on the phylogenetic tree in order to further discuss the intimate affinities between aphids and their host plants and the possible evolutionary tendency of galls in this tribe.
Results
Data
For all 15 taxa, including outgroups, approximately 1100 bp were sequenced for EF-1α. Removing the introns, the exons were assembled into a 762 bp sequence used for phylogenetic analysis. Of a total of 762 characters, 604 sites were conserved, 158 variable, and 113 parsimony-informative (625 sites were constant, 137 variable and 90 parsimony-informative for the ingroup only) and average base frequencies were equal. As for COI, two outgroup and nine ingroup taxa were sequenced, and sequences of four other species, Aploneura lentisci, Baizongia pistacia, Slavum wertheimae and Forda formicaria, were downloaded directly from GenBank (Accession Nos: AY227083, AY227079, AY227077 and AY227086). After alignment with Seqman, a sequence of 613 bp for phylogenetic analysis was acquired, with 427 sites conserved, 186 variable and 130 parsimony-informative (for ingroup taxa only, 433 sites were constant, 180 variable and 109 parsimony-informative). These sequences were heavily biased toward A and T nucleotides, as expected from previous studies (Simon et al., Reference Simon, Frati, Beckenbach, Crespi, Liu and Flook1994; von Dohlen et al., Reference von Dohlen, Kurosu and Aoki2002). Base-composition averages were 43.7% T, 9.3% C, 34.1% A and 13.0% G.
Phylogenetic analysis
MP and ML analysis were performed on single gene data sets and the combined data set with PAUP*. As for the combined data set, Bayesian analysis was also conducted. Therefore, we obtained the EF-1 α MP and ML trees, COI MP and ML trees, and three combined trees drawn from different algorithms (MP, ML and Bayesian analysis). The selected optimal models for each data set and the corresponding parameters for ML and MP analysis are listed in table 2. Topologies of these trees are very similar (fig. 1; single gene trees not shown). Two outgroup taxa, Thecabious beijinensis and Epipemphigus niisimae, are at the base of the trees. The 13 ingroup taxa cluster together form a monophyly with robust support (100% support value in combined trees, EF-1α MP and ML trees, 96% in COI MP tree and 86% in COI ML tree). Two clades correspond to the traditional taxonomic dichotomy of the Fordini into two subtribes, Fordina and Melaphidina (Heie, Reference Heie1980; Zhang & Zhong, Reference Zhang and Zhong1983; Blackman & Eastop, Reference Blackman and Eastop1994). Slavum wertheimae, Baizongia pistacia, Geoica wertheimae, Aploneura lentisci, Forda marginata and F. formicaria form a clade with strong support corresponding to Fordina (88% bootstrap value in ML combined analysis, 83% in MP combined analysis and 1.00 posterior probability in Bayesian analysis). Schlechtendalia peitan, S. chinensis, Meitanaphis elongallis and four subspecies of Kaburagia rhusicola are in the other clade corresponding to Melaphidina (97% bootstrap value in ML and MP combined analysis and 1.00 posterior probability in Bayesian analysis). In the Fordina clade, two monophyletic groups are further developed. One is S. wertheimae+B. pistacia+G. wertheimae+A. lentisci, with support percentages of 92 in ML analysis, 75 in MP analysis and 1.00 in Bayesian analysis; the other is F. marginata+F. formicaria with 100% support value in all three algorithms. In the Melaphidina clade, M. elongalis is at the basal position, and four subspecies of K. rhusicola are on the top. The position of S. peitan and S. chinensis is uncertain. In the MP combined phylogenetic tree, S. chinensis is the sister taxon of four subspecies of K. rhusicola. Schlechtendalia peitan is between M. elongalis and the clade of S. chinensis+K. r. rhusicola+K. r. ovatirhusicola+K. r. ovogallis+K. r. ensigallis. In the ML and Bayesian combined trees, S. peitan and S. chinensis are sister taxa, clustering with group of four subspecies of K. rhusicola.
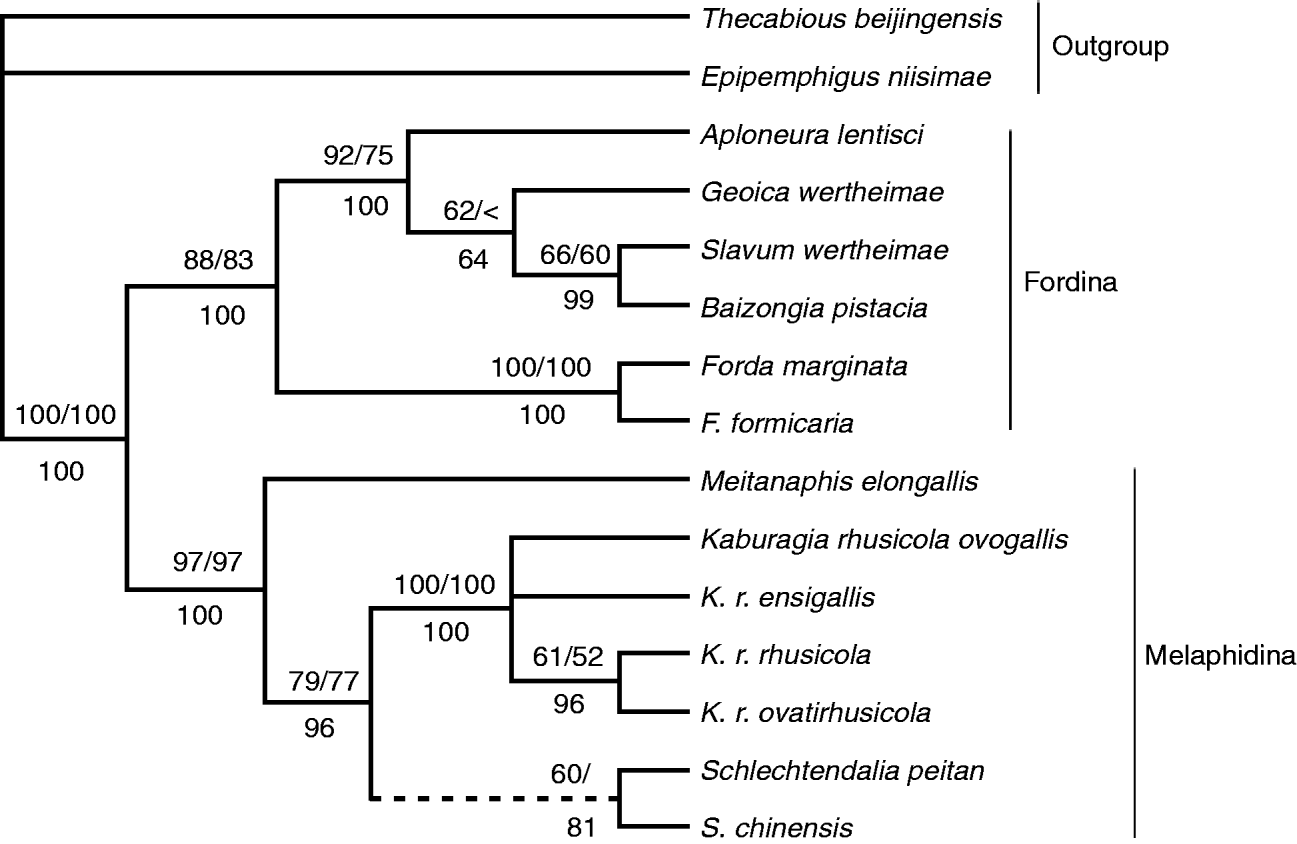
Fig. 1. Maximum likelihood, maximum parsimony and Bayes phylogenetic tree of Fordini reconstructed from combined nuclear EF-1α and mitochondrial COI sequences with dashed showing inconsistent branch. Bootstrap percentages from maximum-likelihood/maximum parsimony (50% and greater) are shown above the branches, and Bayesian posterior probabilities (60% and greater) are shown below the branches.
Table 2. Parameters for ML and MP analysis.

I: Proportion of invariable sites; G: Gamma distribution shape parameter; CI: Consistency Index; RI: Retention Index.
Discussion
Division of subtribes in Fordini
The division of subtribes in Fordini is an equivocal problem that has not been resolved up to now. Börner (Reference Börner1952) considered Fordini as subfamily Fordinae in the light of the arrangement of dorsal hairs, shape of secondary rhinaria, biological association with Anacardiaceae, etc. and divided it into tribe Fordini, having non-ciliated primary rhinaria, and tribe Baizongiini, having ciliated primary rhinaria. The former was represented by Paracletus von Heyden and Forda von Heyden, and the latter was divided into subtribe Baizongina, represented by Baizongia Rondani and Aploneura Passerini, and subtribe Geoicina, represented by Geoica Hartig. Zwölfer (Reference Zwölfer1957), followed Börner's idea, but considered two subtribes of Baizongiini as district tribes. Davatchi (Reference Davatchi1958), while giving a comparative account of biology and polymorphism in gall-forming aphids on Pistacia, pointed out that dividing ‘Fordinae’ on the basis of ciliation of primary rhinaria might lead to confusion, as in some species of ‘Baizongiini’ the ciliation might be absent or indistinct, e.g. alate emigrants of some Geoica species. Similarly, the genus Rectinasus Theobald or the species Forda riccobinii Steff might be difficult to place in ‘Baizongiini’ and ‘Fordini’, respectively. Alternatively, Davatchi (Reference Davatchi1958) separated emigrants of ‘Baizongiini’ and ‘Fordini’ on the basis of pigmentation of mesonotum, sclerotization of ventral head, wax glands on abdominal tergites, wing veins, pigmentation of abdominal dorsum, tarsal chaetotaxy and the ciliation of rhinaria. He placed Smynthurodes Westwood, Paracletus v. Heyden and Forda v. Heyden under Fordini, and Chaetogeoica Remaudière and Tao, Rectinasus Theobald, and Geoica Hartig under ‘Geocina’, and Baizongia Rondani, Aploneura Passesini, Asephonella Theobald and Slaivum Mordvilko under ‘Baizongina’. Ghosh (Reference Ghosh1984) viewed Fordini as a single tribe with two subtribes, Fordina and Melaphidina. The members of Fordina use Pistacia as primary host and roots of Graminae and other plants as secondary host, and members of Melaphidina have Rhus as primary host and mosses and roots of some angiosperms as secondary host.
In our combined molecular phylogenetic tree (fig. 1), the Fordini is monophyletic with 100% bootstrap support and is obviously divided into two groups with bootstrap values higher than 80%. In fig. 2, these diagnostic characters were mapped onto separate branches of the molecular tree. The wax glands are developed in the lower clade, which forms the subtribe Melaphidina, and are arranged into four or six rows on abdominal tergites; and in the upper clade, which forms the subtribe Fordina, most of the species have no wax gland plates. Even though A. lentisci has six rows of wax plates and B. pistacia has four rows, their wax plates and wax gland cells are very small. As for the sensory organ, members of Fordina all have spherical, oval or elongate primary rhinaria. The ciliation of the primary rhinaria has been one of the important diagnostic characters for subdividing Fordina (Börner, Reference Börner1952). Our molecular phylogenetic relationships coincide with Börner's standpoint: primary rhinaria of the A. lentisci+G. wertheimae+S. wertheimae+B. pistacia clade are ciliated, corresponding to his ‘Baizongiini’ and that of the F. marginata+F. formicaria lineage is non-ciliated, corresponding to his ‘Fordini’. Primary rhinaria in Melaphidina are small or absent, but species in this subtribe have large, sheet-like secondary rhinaria on antennal segments III–V, while Fordina only have small, round or oval secondary rhinaria. Another important morphological character is the number of tarsal segments of apterous viviparous females. Aphids of Fordina have a two-segmented tarsus, while those of Melaphidina are all one-segmented. Distribution of this character in our molecular phylogenetic tree also indicates two subtribes, Fordina and Melaphidina are included in tribe Fordini.

Fig. 2. Morphological characters and primary hosts mapped on the phylogenetic tree. Wax gland: absent (); 4 rows (□); 6 rows (





A distinct host-affinity pattern was drawn when the information of primary host plants were mapped onto the molecular phylogenetic tree (fig. 2). The upper clade all use Pistacia plants as their primary hosts, whereas the primary hosts of the lower clade are all Rhus species. Forda marginata and F. formicaria, both having P. palaestina as primary host, cluster together with 100% bootstrap support. Four sub-species of K. rhusicola cluster together, within which K. r. rhusicola and K. r. ovatirhusicola, having the same primary host of R. potanii, are more closely related to each other than to others. This close affinity between aphids and primary hosts indicates that the primary host plant is an important character for subtribe division in Fordini. The division of subtribes here is also consistent with the viewpoint of Blackman & Eastop (Reference Blackman and Eastop1994), who also thought the genera of primary hosts play an important role in subtribal division; and they posited two subtribes, Fordina and Melaphidina. Inbar et al. (Reference Inbar, Wink and Wool2004) reconstructed the Fordini phylogenetic tree with mitochondrial COI/COII sequences (Fordini was raised to subfamily Fordinae in their paper). They thought Fordini only included the group feeding and galling on Pistacia and omitted another group galling on Rhus, endemic to East Asia. So we think that their two main clades (except the third new low-supported lineage – Smynthurodes betae) were in fact two subgroups of the subtribe Fordina. Zhang et al. (Reference Zhang, Qiao, Zhong and Zhang1999) reconstructed the phylogenetic relationship of Fordini based on morphological and ecological characters. Two branches were deeply split, corresponding to two subtribes, Fordina and Melaphidina. Comparing our molecular phylogenetic analysis with previous studies, we posit Fordini consisting of two subtribes, Fordina and Melaphidina. In Fordina, two groups are further evident: one is represented by Forda von Heyden, and the other is represented by Baizongia Rondani.
Evolution of galls in Fordini
Galls are the extended phenotype of aphids (Stone & Schönrogge, Reference Stone and Schönrogge2003). Their position, morphology and structure are important aphid characters. Among the whole Aphididae, only Pemphiginae, Hormaphidinae and some species of Aphidinae produce galls (Blackman & Eastop, Reference Blackman and Eastop1994). In Cerataphidini (Hormaphidinae), ancestral galls are assumed to have a single chamber or cavity, and more recent species induce multi-chambered galls (Fukatsu et al., Reference Fukatsu, Aoki, Kurosu and Ishikawa1994). Galls of Fordini are greatly variable in morphology and position, but interestingly, they are species-specific (Wool, Reference Wool2004). Galls induced by individuals of the same species are remarkably similar, and those induced by different species on the same organ of a given plant are different (Mani, Reference Mani1964).
In Fordina, two monophyletic clades, (A. lentisci (G. wertheimae (S. wertheimae, B. pisticiae))) and (F. marginata, F. formicaria) were reconstructed corresponding to Inbar et al.'s (Reference Inbar, Wink and Wool2004) Baizongiini and Fordini, respectively. In the (A. lentisci (G. wertheimae (S. wertheimae, B. pisticiae))) clade, bag galls of A. lentisci, spherical galls of G. wertheimae and large bud galls of S. wertheimae and B. pistaciae were found (fig. 3). Aploneura lentisci, forming bag galls, is at the basal position of this clade, while S. wertheimae and B. pistaciae, producing big, cauliflower-like bud galls, are more terminal, with G. wertheimae, producing spherical galls, between. Another parallel clade is of two-gall species, belonging to Forda von Heyden (Ghosh, Reference Ghosh1984; Inbar et al., Reference Inbar, Wink and Wool2004). It seems that in Fordina the galls have evolved along two pathways: for some species, such as Forda spp., two types of galls are formed in their life cycles. The fundatrix induces a small pea-shaped gall on the leaflet mid-vein (temporary gall) and the first generation of fundatrigeniae forms a different gall (permanent gall) on the leaflet margin, within which a second generation of apterae fundatrigeniae, followed by a third generation of alate fundatrigeniae, are produced (Bodenheimer & Swirski, Reference Bodenheimer and Swirski1957; Wool & Burstein, Reference Wool and Burstein1991; Burstein & Wool, Reference Burstein and Wool1993). The offspring of the fundatrix can survive more successfully through producing more such final galls. The other scenario of gall evolution is toward enlarging the gall and moving the gall from the leaflet blade to the common petiole of the compound leaf, and eventually to the whole twig. The bag gall and spherical gall are both located on the veins of the leaflets, whereas the bud gall almost covers the whole twig. The latter type can intercept more nutrition and nourish more aphids than the former two types.

Fig. 3. Gall traits (including gall morphology and gall position) mapped on the phylogenetic tree. (▲) On the blade; (▂) On the middle of the midvein; (●) On the base of the midvein (near petiole); (

In Fordina, the gall types show great diversity, especially in size from very small and pea-sized to large and bud-like, while in Melaphidina the gall size has less diversity; almost all of them induce big bursa-like galls (length×width: 50–80 mm×10–40 mm) on the primary hosts, although some are spherical, some oval, and others irregular in shape and with angles. The gall may be on the leaflet blade, the secondary vein, the midrib, the petiole, or the common petiole of the compound leaf (Anacardiaceae leaves are pinnate). In this study, seven species and subspecies belonging to three genera were examined, which represent the main position types in Melaphidina. In the molecular phylogenetic tree, it can be seen that Meitanaphis elongallis, with oval galls on the middle of the midrib of the leaflet, is at the base of the clade. Schlechtendalia chinensis and four subspecies of Kaburagia rhusicola, with galls on the common petiole, are more terminal; and S. peitan, with galls on the base of the midrib (near the leaflet petiole) is between them (fig. 3). Aphids are phloem feeders and divert plant assimilates to the galls. With the gall's location moving from the middle of the midrib to the common petiole, they can intercept more nutrition and raise more aphids. As the galls move to the common petiole of the compound leaf and eventually cover the whole twig, they can enlarge the inner surface by changing the shape from spherical or oval to irregular with angles (e.g. S. chinensis), thereby gaining a much wider area to bear more aphids. Figure 4 shows the probable evolutionary scenario of galls in Fordini.

Fig. 4. Illustrated evolutionary scenario of galls in Fordini, including gall morphology and position. Absolute scaling was not maintained (the bud gall from Ghosh, Reference Ghosh1984; the bag gall and spherical gall from Inbar et al., Reference Inbar, Wink and Wool2004).
Acknowledgements
The authors thank Dr M. Inbar in Department of Biology, University of Haifa-Oranim, Israel, for his kind contribution of some aphid specimens. Thanks are also due to Favret Colin, D.C. Yuan, F.M. Lei and X.L. Huang for revising the manuscript and two anonymous reviewers for their valuable comments on the manuscript. This work was funded by the National Natural Sciences Foundation of China (No.30570214, 30670240) and the National Science Fund for Fostering Talents in Basic Research (NSFC-J0030092).








