Introduction
5S Ribosomal DNA is a highly conserved tandemly repeated multigenic family transcribed by RNA polymerase III. There is substantial literature on 5S rDNA but most reports concern the size of the repeat, its copy number and chromosomal localization obtained from in situ hybridization. The first results on the regulation of 5S rDNA transcription were obtained in Xenopus laevis, where a developmental control of the 5S rRNA genes expression occurs. Two 5S RNA gene families are transcribed in oocytes, firstly the major oocyte type (there are 20,000 copies per haploid genome) and secondly the somatic type (there are 400 copies per haploid genome). In somatic cells, only the somatic 5S RNA genes are transcribed, while the oocyte genes are repressed (Chipev and Wolffe, Reference Chipev and Wolffe1992).
Active and silent genes are distinct from one another with respect to their chromatin configurations. Active genes are located in euchromatin, whereas silent genes are usually located in heterochromatin (Santoro et al., Reference Santoro, Li and Grummt2002). Some recently studies have established that epigenetic mechanisms are involved in marking the transcriptional state of rRNA genes (28S–5.8S–18S) in human and mouse somatic cells, which is transmitted to daughter cells in order to assure a correct chromatin conformation dynamics. The promoter of active rRNA genes is scarce in 5-methyl-cytosine and is associated with acetylated histones, an opposite pattern being predominant among silent genes (Lawrence et al., Reference Lawrence, Early, Pontes, Silva, Chen, Neves, Viegas and Pikaard2004). The epigenetic mechanism of rRNA gene silencing was extensively studied for the nucleolar dominance phenomenon (Reeder, Reference Reeder1985; Santoro & Grummt, Reference Santoro and Grummt2005; McStay, Reference McStay2006), which is common in plant and animal hybrids, whereby rRNA genes inherited by one parent are transcribed and those from the other parent are silent.
In contrast with rRNA genes from somatic cells, epigenetic networks that mediate transcriptional state of rDNA from gametes have been poorly studied. Our previous work investigated amphibian oocyte chromatin remodelling process at the level of 28S–5.8S–18S ribosomal gene promoter by RE-ChIP assay (a variation of the chromatin immunoprecipitation assay). Our findings suggested that there is an involvement of some epigenetic modifications in chromatin architecture dynamics during crested newt oogenesis (Burlibașa et al., Reference Burlibașa, Zarnescu, Cucu and Gavrilă2008).
Only one report in the literature has been linked to the relationship between histone acetylation and transcription of the Xenopus laevis oocyte and somatic 5S ribosomal RNA genes (Howe et al., Reference Howe, Ranalli, Allis and Ausio1998).
The aim of this study was to investigate the dynamics of H4 hyperacetylation at the level of oocyte and somatic 5S rRNA genes on Triturus cristatus.
Materials and methods
Animals
Female newts (Triturus cristatus) were obtained from Bucharest Botanical Garden Pool, University of Bucharest. All the females were sexually active and they were collected from the pool at the end of March when it is the beginning of breeding period.
Animals were sacrificed in accordance with stipulations of European Council law (86/609/CEE/24.11.2004) regarding the protection of animals used for experimental and scientific aims.
Preparation and classification of oocytes
Ovaries were removed from the anesthetized animals and placed in Eagle's minimal essential medium diluted 1:1 with distilled water, and oocytes of different size classes were manually isolated at the binocular microscope. For the purpose of the present study, oocyte development was divided into two basic stages. Small transparent oocytes with a diameter of up to 0.35 mm are referred to as previtellogenic. Oocytes 0.5–1.4 mm in diameter are considered vitellogenic. In the early phase of this stage, yolk is deposited first in the periphery of the oocyte. In later stages (1.2–1.4 mm large oocytes), the yolk platelets fill the entire cytoplasm, except for a small juxtanucleolar zone. We used large vitellogenic oocytes in our experiments.
Chromatin immunoprecipitation assay (ChIP)
Chromatin containing acetylated histone H4 was immunoprecipitated using rabbit polyclonal antibody against the penta-acetylated peptide corresponding to histone H4 (Upstate Biotechnology). The specificity of antibody on newts was previously tested (Burlibașa et al., Reference Burlibașa, Zarnescu, Cucu and Gavrilă2008; Burlibașa & Zarnescu, Reference Burlibașa and Zarnescu2013).
ChIP studies were performed using a Quick ChIP Kit (Imgenex) according to a variant adapted by us from the quick ChIP user manual and published previously (Burlibașa et al., Reference Burlibașa, Zarnescu, Cucu and Gavrilă2008). Vitellogenic oocytes and hepatocytes derived from the liver of the same newt were incubated for 10 min with 1% formaldehyde by gentle agitation. The ChIP assay requires the segmentation of chromatin into 200–800 bp immuno-precipitable fragments. The chromatin isolated from oocytes and hepatocytes was fragmented using 50 UI/ml MNase (micrococcal nuclease) (Biomol). We determined the optimal incubation condition (15 min at 37°C incubation for both type of cells). The reaction was stopped with an equal volume of Buffer X (90 mM HEPES pH 7.9, 220 mM NaCl, 10 mM EDTA, 2% Triton X 100, 0.2% SDS, 0.5 mM PMSF) containing 10 μl 10× PIC after incubation. Next steps of ChIP were described previously (Burlibașa et al., Reference Burlibașa, Zarnescu, Cucu and Gavrilă2008). In the final step of ChIP procedure, the DNA was recovered by phenol/chloroform extraction and etanol precipitation. PCR amplification of a region of 224 bp from oocyte and somatic 5S rRNA genes was performed using primers having sequences chosen from NCBI with accession numbers: M10635, J01010, J01009; V01455 (the first set of primers: forward 5′-ATTAAGGTCCGCCGGACT-3′ and reverse 5′-GCACCTCGACTCGGATAA-3′, and the second set of primers: forward 5′-TAGAGGAGTCCATCGCCT-3′ and reverse 5′-CCTCGTAATCGACGACAG-3′) and Amplitaq polymerase (Biomol), in a Perkin Elmer thermocycler. Two control samples: positive control (input) sample and negative control sample were run through each step of ChIP. PCR products were run in an agarose 1% gel.
Results and Discussion
The majority of amphibians produces two major types of 5S rRNA: the somatic type, which is transcribed in most cells, and the oocyte type, which is produced only during oogenesis and early embryogenesis. Each 5S rRNA type is transcribed from a distinct multigene family, and some research have focused on explaining the differential expression of these genes in somatic cells. Only one report has shown that histone acetylation enhances RNA polymerase III transcription of dinucleosomal 5S rRNA gene templates, suggesting a possible correlation between histone acetylation and transcription of RNA polymerase III transcribed 5S ribosomal RNA genes in Xenopus laevis (Howe et al., Reference Howe, Ranalli, Allis and Ausio1998).
It has been reported that certain Xenopus tissue culture cell lines express low levels of oocyte 5S rRNA (Howe et al., Reference Howe, Ranalli, Allis and Ausio1998), and thus it was necessary to determine whether the oocyte genes were truly repressed in the somatic cell line used in this investigation.
In our study, starting from Howe and coworkers investigations (1998), the dynamics of H4 hyperacetylation at the level of oocyte and somatic 5S rRNA genes of Triturus cristatus has been analyzed.
In order to ensure accurate results in the ChIP assay, chromatin was fragmented by MNase incubation for several time periods, thus obtaining fragments with a range of 300–1000 bp. We tested four different MNase (50 UI/ml) incubation times (5, 10, 15 or 30 min) for both vitellogenic oocytes and hepatocytes from Triturus cristatus. We choose the length of chromatin fragments to be less then 1000 bp (confirmed by electrophoretic analysis) at 15 min MNase incubation time for both types of cells.
PCR results of hyperacetylated H4 immunoprecipitates indicate a positive result for ChIP assay for both type of 5S rRNA genes (somatic and oocytes) in vitellogenic oocytes (Table 1 and Fig. 1). In hepatocytes only somatic 5 S rRNA gene was positive for ChIP assay (Table 1 and Fig. 2).
Table 1 PCR results after ChIP with hyperacetylated H4 antibody

C+, positive control, input sample; C−, negative control without antibody.

Figure 1 PCR analysis of H4 hyperacetylated (H4hac) immunoprecipitates from vitellogenic oocyte. 1, somatic 5S rDNA; 2, oocyte 5S rDNA; C+, positive control; C–, negative control.
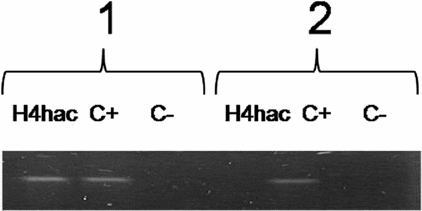
Figure 2 PCR analysis of H4hac immunoprecipitates from hepatocytes. 1, somatic 5S rDNA; 2, oocyte 5S rDNA.
ChIP experiments revealed that histone H4 at the level of 5S rDNA are presented in a highly acetylated state in both type of gene families in oocytes, whereas in hepatocytes only somatic rRNA genes are packed with hyperacetylated H4.
In conclusion, chromatin immunoprecipitation experiments have shown that H4 is hyperacetylated in the promoter regions and along of active genes (Shestakova et al., Reference Shestakova, Bandu, Doly and Bonnefoy2001, Workman, Reference Workman2006). It has been demonstrated that distinct histone modification patterns function as recognition codes for the recruitment of different transcription factors upon transcriptional activation (Agalioti et al., Reference Agalioti, Chen and Thanos2002). Thus, the histone code may function as an epigenetic marker that is directly associated with transcriptional activation.
Our results taken together with those of others demonstrating the presence of acetylated histones on oocyte genes in cells in which oocyte 5S rRNA is synthesized at high levels (Reynolds et al., Reference Reynolds, Smith, Bloomer and Gottesfeld1982), suggest a link between histone hyperacetylation and 5S rRNA transcription.
Acknowledgements
This work was supported by CNCSIS Grant AT-227–2008.





