Over the past two decades, there has been increasing interest in how social conditions influence health and disease by becoming biologically embedded (Beach et al., Reference Beach, Lei, Brody, Kim, Barton and Philibert2016, Reference Beach, Lei, Simons, Barr, Simons, Ehrlich and Philibert2017; Brody, Lei, Chen, & Miller, Reference Brody, Lei, Chen and Miller2014; Chen & Miller, Reference Chen and Miller2013; Link & Phelan, Reference Link and Phelan1995; Phelan, Link, & Tehranifar Reference Phelan, Link and Tehranifar2010; Rutter, Reference Rutter2016; Simons et al., Reference Simons, Lei, Beach, Barr, Cutrona, Gibbons and Philibert2017, Reference Simons, Lei, Beach, Barr, Simons, Gibbons and Philibert2018). It has become increasingly clear that social circumstances such as lower socioeconomic status and exposure to adversity are associated with a higher prevalence of chronic illnesses in adults, including diabetes, hypertension, cardiovascular disease, and the dysregulation of the immune system (Belsky, Ruttle, Boyce, Armstrong, & Essex, Reference Belsky, Ruttle, Boyce, Armstrong and Essex2015; Evans, Kim, Ting, Tesher, & Shannis, Reference Evans, Kim, Ting, Tesher and Shannis2007; Lei, Beach, & Simons, Reference Lei, Beach and Simons2018; Miller, Chen, & Parker Reference Miller, Chen and Parker2011; Simons et al., Reference Simons, Lei, Beach, Barr, Cutrona, Gibbons and Philibert2017).
Going beyond individual experiences of adversity and individual socioeconomic status, theory and evidence have suggested that the neighborhoods in which people reside are also fundamental contexts for understanding the biological embedding of social circumstances and accounting for effects on physical health and well-being (Aneshensel, Reference Aneshensel, Avison, Aneshensel, Schieman and Wheaton2009; Bosma, Dike van de Mheen, Borsboom, & Mackenbach, Reference Boardman, Onge, Rogers and Denney2001; Causadias, Reference Causadias2013; Diez-Roux, Reference Diez-Roux, Kawachi and Berkman2003; Finegood, Rarick, Blair, & Family Life Project Investigators, Reference Faul, Erdfelder, Lang and Buchner2017; Hill, Ross, & Angel Reference Hill, Ross and Angel2005; Lei et al., Reference Lei, Beach, Simons, Barr, Cutrona and Philibert2016; Lei, Simons, Beach, & Philibert, Reference Lei, Simons, Beach and Philibert2017; Ross & Mirowsky, Reference Ross and Mirowsky2001). This is due to the fact that individuals residing in socioeconomically disadvantaged neighborhoods are exposed to a constellation of social stressors associated with increased presence of social disorder and subculture (Anderson, Reference Anderson1999; Burt, Lei, & Simons, Reference Burt, Lei and Simons2017; Hill et al., Reference Hill, Ross and Angel2005; Latkin & Curry, Reference Latkin and Curry2003; Ross & Mirowsky, Reference Ross and Mirowsky2009). These conditions foster concerns about safety and feelings of powerlessness and social isolation (Ross, Reference Ross2011). Given that youth typically leave home at age 18 (Arnett, Reference Arnett2004), the period of emerging adulthood represents a developmental period in which neighborhood context is of increased importance for trajectories of behavior and health. The present study is concerned with increasing our understanding of how the duration and timing of exposure to neighborhood disadvantage combines with various social and biological processes to forecast the onset of chronic illness and more stringently testing models of biological embedding.
Neighborhood Influences and Health
Sociological and social-epidemiological studies of illness have been informed, in large measure, by the stress process model proposed by Leonard Pearlin (Reference Pearlin1989, Reference Pearlin, Aneshensel and Phelan1999). The model asserts that health inequalities are caused by differential exposure to stressful environments. Prolonged exposure to such conditions is viewed as fostering a physiological stress response that increases the chances of chronic illness by damaging tissue and immune cells. Given that stress exposure often occurs in multilevel contexts, ranging from individual to neighborhood and broader contexts (Cicchetti & Garmezy, Reference Cicchetti and Garmezy1993; Cicchetti & Toth, Reference Cicchetti and Toth2009), in recent years, researchers (e.g., Aneshensel, Harig, & Wight, Reference Aneshensel, Harig, Wight, Gorge and Ferraro2016) have drawn upon the stress process model to develop arguments regarding the role of neighborhood context in explaining health inequalities. Neighborhood context is defined as a distinct geographical area (e.g., residential census tracts) in which people live, interact with each other, and share norms and values (Sampson, Morenoff, & Gannon-Rowley, Reference Sampson, Morenoff and Gannon-Rowley2002). To measure neighborhood context, neighborhood researchers (Ross & Mirowsky, Reference Ross and Mirowsky2001; Sampson, Raudenbush, & Earls, Reference Sampson, Raudenbush and Earls1997) have focused largely on socioeconomic measures of neighborhood context, often called neighborhood disadvantage.
Social disorganization theory (Shaw & McKay, Reference Shaw and McKay1969) posits that neighborhoods characterized by socioeconomic disadvantage are often marked by visible cues indicating a breakdown of social order and control (Browning, Cagney, & Iveniuk, Reference Browning, Cagney and Iveniuk2012; Hill et al., Reference Hill, Ross and Angel2005; Ross & Mirowsky, Reference Ross and Mirowsky2001, Reference Ross and Mirowsky2009). These cues can be physical (e.g., graffiti, scattered trash, vandalism, and rundown and abandoned buildings) or social (e.g., public drinking, people hanging out on the streets, and trouble with neighbors). Given that residents in socioeconomic disadvantaged neighborhoods find such conditions to be threatening and demoralizing, the neighborhood stress process model (Aneshensel et al., Reference Aneshensel, Harig, Wight, Gorge and Ferraro2016) proposes that neighborhoods are a critical context for health. The distress of living under disadvantaged circumstances is seen as promoting psychological distress and physiological stress that can lead to chronic illness. Support for this idea has been provided by studies reporting an association between neighborhood adversity such as poverty or neighborhood socioeconomic disadvantage and various health outcomes (e.g., chronic illness, blood pressure, obesity, and cardiovascular diseases) even controlling for individual socioeconomic measures and health-related variables (e.g., Aneshensel & Sucoff, Reference Aneshensel and Sucoff1996; Blair, Ross, Gariepy, & Schmitz, Reference Blair, Ross, Gariepy and Schmitz2014; Boardman, Onge, Rogers, & Denney, Reference Boardman, Onge, Rogers and Denney2005; Brody et al., Reference Brody, Lei, Chen and Miller2014; Browning et al., Reference Browning, Cagney and Iveniuk2012; Finegood et al., Reference Finegood, Rarick and Blair2017; Hill et al., Reference Hill, Ross and Angel2005; Latkin & Curry, Reference Latkin and Curry2003; Lei, Beach, Simons, & Philibert, Reference Lei, Beach, Simons and Philibert2015; Ross & Mirowsky, Reference Ross and Mirowsky2001).
Although previous studies suggest that neighborhood disadvantage is associated with health outcomes, studies to date have been limited by theoretical and methodological issues that remain to be addressed. First, the timing of neighborhood context effects is rarely examined. Recent studies on obesity suggest this may be problematic (Do & Zheng, Reference Do and Zheng2017; Kravitz-Wirtz, Reference Kravitz-Wirtz2016). Using a longitudinal approach to examine timing-specific exposure to neighborhood disadvantage on body mass index, these studies found support for a temporal model in which durable exposure to neighborhood disadvantage is more important for obesity than exposure during any particular stage of life. These findings highlight the likelihood that neighborhood disadvantage during young adulthood can add to disadvantage experienced in childhood, influencing obesity incrementally. However, it is still unclear how the temporal effects of neighborhood disadvantage become linked with biological age and chronic illness in adulthood. This issue is of considerable interest and importance for developmental psychopathology.
Second, from a methodological perspective, most studies of neighborhood effects have utilized self-report measures of both neighborhood context and general chronic health status. Thus, the associations reported in past research are subject to criticism for potentially reflecting shared method variance (measures from the same source), leading the associations to be inflated (Podsakoff, MacKenzie, Lee, & Podsakoff, Reference Podsakoff, MacKenzie, Lee and Podsakoff2003). We avoid this problem by using Census data to measure neighborhood disadvantage (Lei, Simons, et al., Reference Lei, Simons, Beach and Philibert2017; Sampson et al., Reference Sampson, Raudenbush and Earls1997) and the Framingham cardiovascular risk score to assess accelerated cardiometabolic aging (D'Agostino et al., Reference D'Agostino, Vasan, Pencina, Wolf, Cobain, Massaro and Kannel2008). Accordingly, both the primary predictor and the outcome variable are not based on self-report, and there is little method overlap between them in the current investigation.
In addition, prior research can be criticized as being driven by nonrandom assignment of individuals into neighborhoods, again potentially inflating associations and mispecifying the causal significance of neighborhoods (e.g., Humphrey & Root, Reference Humphrey and Root2017; Sampson et al., Reference Sampson, Morenoff and Gannon-Rowley2002). For example, families with low socioeconomic status are more likely to live in disadvantage neighborhoods. This may produce spurious neighborhood effects that are actually better attributed to socioeconomic status. However, simply incorporating control variables into a regression model has been criticized for overcontrolling potential neighborhood effects (Sampson et al., Reference Sampson, Morenoff and Gannon-Rowley2002) and being inadequate to control for confounding variables that vary over time and are affected by previous neighborhood effects (Robins, Reference Robins, Halloran and Berry2000). In particular, traditional regression models ignore the fact that reciprocal cause-and-effect relationships exist between exposures and covariates. To adjust for nonrandom assignment to neighborhood without overcontrolling potential confounds, we use marginal structural modeling, strengthening our tests of causal hypotheses.
Neighborhood Disadvantage on Chronic Illness Through Biological Aging
Prior research also has provided little guidance regarding the biopsychosocial mechanisms whereby neighborhood factors may become biologically embedded and thereby influence health (Causadias, Telzer, & Lee, Reference Causadias, Telzer and Lee2017; Johnson & Acabchuk, Reference Johnson and Acabchuk2018). It is generally assumed that adverse neighborhood conditions, such as concentrated socioeconomic disadvantage, foster increased physiological stress that, over time, leads to disease (Pearlin, Reference Pearlin1989, Reference Pearlin, Aneshensel and Phelan1999; Thoits, Reference Thoits2010). While this general model is useful as a first approximation, identification of specific potential biopsychosocial mechanisms allows for stronger tests of theory and ultimately the design of stronger preventive interventions.
In the past decade, scholars have proposed different methods to estimate biological age. Recently, the most popular measures of biological aging are the epigenetic measures developed by Horvath (Reference Horvath2013) and Hannum et al. (Reference Hannum, Guinney, Zhao, Zhang, Hughes, Sadda and Deconde2013). Given that methylation at specific CpG sites provides an epigenetic “signature” to track the state of cellular aging, they identified CpG sites scattered throughout the human genome where methylation levels were significantly associated with chronological age. Many of the CpG sites included in Hannum et al.’s and Horvath's measures are within or near genes with known functions in aging-related conditions, including DNA damage, tissue degradation, cardiovascular disease, and chronic diseases. The correlation between a person's chronological age and predicted age based on either of these two indices is roughly .90 (Beach et al., Reference Beach, Dogan, Lei, Cutrona, Gerrard, Gibbons and Philibert2015; Horvath, Reference Horvath2013). Recent studies have indicated that accelerated epigenetic aging is a robust predictor of mortality (Marioni et al., Reference Marioni, Shah, McRae, Chen, Colicino, Harris and Deary2015), and some chronic illnesses (Horvath et al., Reference Horvath, Erhart, Brosch, Ammerpohl, von Schönfels, Ahrens and Deloukas2014; Perna et al., Reference Perna, Zhang, Mons, Holleczek, Saum and Brenner2016; Wallace et al., Reference Wallace, Twomey, Custaud, Turner, Moyna, Cummins and Murphy2017; Zheng et al., Reference Zheng, Joyce, Colicino, Liu, Zhang, Dai and Jafari2016). In addition, there is evidence that accelerated epigenetic aging is affected by social environment conditions (Brody, Miller, Yu, Breach, & Chen, Reference Brody, Miller, Yu, Beach and Chen2016; Simons et al., Reference Simons, Lei, Beach, Philibert, Cutrona, Gibbons and Barr2016; Zannas et al., Reference Zannas, Arloth, Carrillo-Roa, Iurato, Röh, Ressler and Mehta2015).
Although DNA methylomic age provides one window onto accelerated biological aging, use of these assays is limited by relatively high costs. Further, these studies show weak and inconsistent relationships with health risk factors such as elevated blood pressure, cholesterol, glucose, or body mass index, and they do not predict onset of chronic illnesses such as myocardial infarction, stroke, or diabetes (Murabito et al., Reference Murabito, Zhao, Larson, Rong, Lin, Benjamin and Lunetta2017). To overcome these limitations, we use a different approach to assessing the speed of biological aging. Specifically, rather than use methylomic measures of aging, we estimate biological age using the Framingham algorithm. Using data from the Framingham Study, D'Agostino et al. (Reference D'Agostino, Vasan, Pencina, Wolf, Cobain, Massaro and Kannel2008) developed a measure of cardiometabolic age based upon seven metabolic syndrome and cardiovascular risk factors, including systolic blood pressure, diabetes, body mass index, treatment for hypertension, age, gender, and smoking. Studies have established that cardiometabolic aging is a biological process associated with the whole organism that ultimately leads to the onset of chronic illness (Groenewegen et al., Reference Groenewegen, Den Ruijter, Pasterkamp, Polak, Bots and Peters2016; Hirsch, Waits, Li, & Soliman, Reference Hirsch, Waits, Li and Soliman2018; Wallace et al., Reference Wallace, Twomey, Custaud, Turner, Moyna, Cummins and Murphy2017). This measure has been shown to have high validity and reliability (D'Agostino, Pencina, Massaro, & Coady, Reference D'Agostino, Pencina, Massaro and Coady2013) and has been commonly used by physicians to monitor their patients’ health condition (Davies, Gompels, Johnston, Bovill, & May, Reference Davies, Gompels, Johnston, Bovill and May2013; Schaefer et al., Reference Schaefer, Caspi, Belsky, Harrington, Houts, Israel and Moffitt2015).
The gap between cardiometabolic and chronological age can be considered a measure of person's speed of aging. It can be used as a “biological clock” to assess the extent to which an individual is experiencing accelerated or decelerated cardiometabolic aging (Beach et al., Reference Beach, Dogan, Lei, Cutrona, Gerrard, Gibbons and Philibert2015; Simons et al., Reference Simons, Lei, Beach, Philibert, Cutrona, Gibbons and Barr2016). Thus, accelerated cardiometabolic aging or premature aging is defined as people who are biologically older than their chronological age.
As outlined by Arline Geronimus (Reference Geronimus2013), chronic exposure to prolonged stressful environments can result in physiological wear and tear that may lead to onset of chronic illness. She used the term biological weathering to refer to accelerated biological aging that results from the physiological wear and tear that happens in response to stress. Thus, a greater burden of illness among African Americans results from increased biological weathering, a form of accelerated biological aging that is attributable to discrimination and marginalization in a society. This suggests that speed of biological aging is a weathering process fostered by adverse environments and that this biopsychosocial process may explain the relationship between chronic stress and health status. Based on this idea, residing in a disadvantaged neighborhood can exert a weathering effect in the form of accelerated cardiometabolic age. These metabolic and cardiovascular changes, in turn, ultimately lead to chronic illness.
Resilience Mechanisms in Neighborhoods
According to the classical neighborhood assumption, people from the same neighborhood are more similar to each other than to those from different neighborhoods (Foster & Brooks-Gunn, Reference Foster, Brooks-Gunn, Gibson and Krohn2013). However, while individuals living in disadvantaged neighborhoods are more likely to become ill than those residing in more advantaged areas, many, if not most, will remain healthy (Fone et al., Reference Fone, Dunstan, Lloyd, Williams, Watkins and Palmer2007; Lei et al., Reference Lei, Beach, Simons and Philibert2015; Miller et al., Reference Miller, Chen and Parker2011), a phenomenon labeled neighborhood resilience (Brody, Yu, & Beach, Reference Brody, Yu and Beach2016; Masten & Coatsworth, Reference Masten and Coatsworth1998; Norris, Stevens, Pfefferbaum, Wyche, & Pfefferbaum, Reference Norris, Stevens, Pfefferbaum, Wyche and Pfefferbaum2008). This raises the question: why and how do some people living in disadvantaged neighborhoods develop chronic illnesses whereas others do not? Understanding neighborhood characteristics that may moderate the relationship between neighborhood stressors, such as neighborhood disadvantage, and physical health is fundamental to the advancement of neighborhood research.
Sampson et al. (Reference Sampson, Raudenbush and Earls1997) proposed that a set of neighborhood processes, which they termed neighborhood collective efficacy, would promote neighborhood cohesion and protect residents from the adverse effects of disadvantaged neighborhood contexts. The concept of neighborhood collective efficacy is defined as “the linkage of mutual trust and the willingness to intervene for the common good [of the neighborhood]” (p. 917). In neighborhoods with high collective efficacy, residents trust each other, and there is cohesion among neighbors who are willing to intervene to help each other to reach collective goals. The literature on collective efficacy has focused on positive adaptation in response to neighborhood stressors and has demonstrated that collective efficacy can promote resilience (Browning, Gardner, Maimon, & Brooks-Gunn, Reference Browning, Gardner, Maimon and Brooks-Gunn2014; Diez-Roux & Mair, Reference Diez-Roux and Mair2010; Foster & Brooks-Gunn, Reference Foster, Brooks-Gunn, Gibson and Krohn2013; Liu, Mustanski, Dick, Bolland, & Kertes, Reference Liu, Mustanski, Dick, Bolland and Kertes2016; Ross & Jang, Reference Ross and Jang2000; Simons, Simons, Burt, Brody, & Cutrona, Reference Simons, Simons, Burt, Brody and Cutrona2005; Wickes, Hipp, Sargeant, & Homel, Reference Wickes, Hipp, Sargeant and Homel2013). For example, among African Americans from disadvantaged neighborhoods, Liu et al. (Reference Liu, Mustanski, Dick, Bolland and Kertes2016) found that exposure to neighborhood stressors and racial discrimination was associated with comorbid problems. However, the association was less pronounced for those who perceived higher levels of neighborhood collective efficacy.
As this example suggests, perceived collective efficacy has the potential to promote resilience to neighborhood stressors, indicating that perceived neighborhood collective efficacy may help to buffer against other facets of the neighborhood that may be stressful and so enhances an individual's well-being despite ongoing stressors. Although social scientists have long been interested in the extent to which social support affects health (Phelan et al., Reference Phelan, Link and Tehranifar2010; Turner & Marino, Reference Turner and Marino1994) and have proposed the stress-buffering hypothesis, which argues that social support moderates the relationship between stress and health (Cohen, Reference Cohen1988; Lin & Ensel, Reference Lin and Ensel1989), to our knowledge, no prior research has examined perceived neighborhood collective efficacy buffers in modifying the impact of neighborhood-level disadvantage on biological mechanisms linked to health.
Why Focus on African Americans?
People living in disadvantaged environments have greater prevalence, earlier onset, and more complications for several age-related diseases (Aneshensel, Reference Aneshensel, Avison, Aneshensel, Schieman and Wheaton2009; Bosma et al., Reference Bosma, Dike van de Mheen, Borsboom and Mackenbach2001). This greater burden of illness results from increased biological weathering (Geronimus, Reference Geronimus2013), a form of accelerated biological aging that is attributable to chronic stress and marginalization in a race-conscious society. This is particularly true for African Americans, who are more likely to reside in extremely disadvantaged neighborhoods (Peterson & Krivo, Reference Peterson and Krivo2010). In More Than Just Race, William Julius Wilson (Reference Wilson2009, p. 43) states that “one of the effects of living in a racially segregated, poor neighborhood, is the exposure to cultural framing, habits, styles of behavior, and particular skills that emerged from patterns of racial exclusion; these attributes and practices may not be conducive to facilitating social mobility.” Thus, African Americans experience health inequality as a consequence of the cumulative impact of life in a society where they suffer social, economic, and racial exclusion. Supporting this contention, several studies have found that this disproportionate burden is due, in large measure, to disparities in stress-related weathering and wear and tear of African Americans’ bodies, processes that often begin years or decades prior to the onset of disease (Genovese et al., Reference Genovese, Friedman, Ross, Lecordier, Uzureau, Freedman and Bernhardy2010; Konen, Summerson, Bell, & Curtis, Reference Konen, Summerson, Bell and Curtis1999; Simons et al., Reference Simons, Lei, Beach, Barr, Simons, Gibbons and Philibert2018; Thomas, Eberly, Davey Smith, Neaton, & Stamler, Reference Thomas, Eberly, Davey Smith, Neaton and Stamler2005).
Because differences in acculturation status and racial–ethnic backgrounds may condition response to, and interpretation of, neighborhood contexts, assessments of neighborhood disadvantage may not be fully comparable across ethnic groups even when using similar items. In addition, studies that search for biomarkers using multiple distinct ethnic groups are at increased risk for spurious findings due to background variation in biomarkers across ethnic groups (Hamer & Sirota, Reference Hamer and Sirota2000). Consequently, a focus on African Americans seems appropriate for examination of the weathering model that may have implications for health effects and possible points of preventive intervention for African Americans.
The Present Study
Summarizing, Figure 1 illustrates the theoretical model tested in the present study using multilevel data from a sample of approximately 400 African Americans. According to the neighborhood stress process model (Aneshensel et al., Reference Aneshensel, Harig, Wight, Gorge and Ferraro2016) and the weathering hypothesis (Geronimus, Reference Geronimus2013), the first set of hypotheses addresses the relationships among neighborhood disadvantage, speed of cardiometabolic aging, and chronic illness after controlling for covariates. Further, the temporal effects of neighborhood disadvantage have often been ignored in previous research (e.g., for an exception, see White, Zeiders, Knight, Roosa, & Tein, Reference White, Zeiders, Knight, Roosa and Tein2014). To address this issue, we examine timing-specific (ages 18, 21, 24, and 29) exposure to neighborhood disadvantage on accelerated aging and chronic illness. Then, we test durable effects of neighborhood disadvantage on accelerated aging and chronic illness using data from ages 18 to 29. Our hypotheses were as follows:
Hypothesis 1a: At each wave, neighborhood disadvantage will be associated with a small to medium effect on accelerated cardiometabolic aging and chronic illness (Pathways a and b), even after controlling for potential confounders (e.g., gender, income, education, romantic relationship, health insurance, healthy diet, exercise, and sleep quality).
Hypothesis 1b: Durable exposure to neighborhood disadvantage will be more important for accelerated aging and chronic illness than exposure during any particular stage of life.
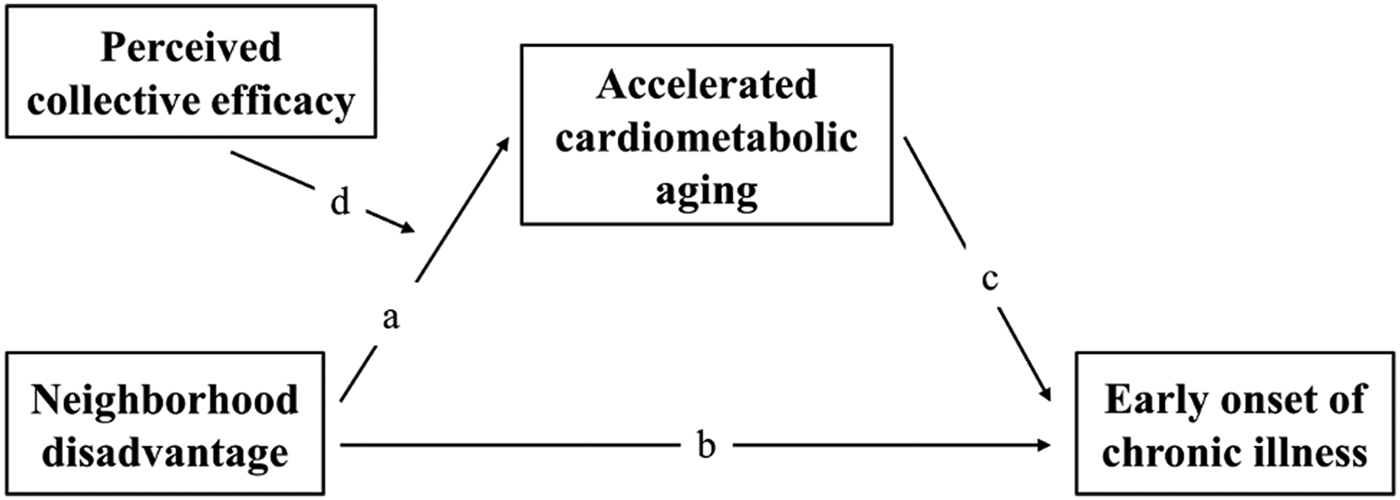
Figure 1. Theoretical model linking neighborhood disadvantage to cardiometabolic aging and ultimately chronic illness response. The model indicates that the theoretical link is mediated and moderated by perceived neighborhood collective efficacy.
As discussed, past research has reported that stressful circumstances (e.g., neighborhood disadvantage, racial discrimination, stressful life events, and harsh parenting) have a small- to medium-sized association with biological weathering and accelerated aging (Brody, Miller, et al., Reference Brody, Miller, Yu, Beach and Chen2016; Geronimus, Reference Geronimus2013; Lei, Beach, Dogan, & Philibert, Reference Lei, Beach, Dogan and Philibert2017; Lei, Simons, et al., Reference Lei, Simons, Beach and Philibert2017; Miller, Yu, Chen, & Brody, Reference Miller, Yu, Chen and Brody2015; Moffitt, Belsky, Danese. Poulton, & Caspi, Reference Moffitt, Belsky, Danese, Poulton and Caspi2017; Simons et al., Reference Simons, Lei, Beach, Philibert, Cutrona, Gibbons and Barr2016). In addition, studies have shown that accelerated aging is a robust predictor of all chronic age-related diseases, such as heart disease, liver disease, type II diabetes, hypertension, and Alzheimer disease (Marioni et al., Reference Marioni, Shah, McRae, Chen, Colicino, Harris and Deary2015; Murabito et al., Reference Murabito, Zhao, Larson, Rong, Lin, Benjamin and Lunetta2017). Building on these findings, the present study tests the hypothesis that it is speed of cardiometabolic aging that links adverse neighborhood conditions to adult illness.
Hypothesis 2: The effect of neighborhood disadvantage on chronic illness will be mediated by accelerated cardiometabolic aging (Pathways a and c) even after potentially confounding variables (e.g., sociodemographic and health-related variables) and neighborhood selection bias are controlled.
Finally, given the modest association that has been shown to exist between neighborhood disadvantage and health outcomes, researchers (Aneshensel, Reference Aneshensel, Avison, Aneshensel, Schieman and Wheaton2009; Brody et al., Reference Brody, Lei, Chen and Miller2014; Fone et al., Reference Fone, Dunstan, Lloyd, Williams, Watkins and Palmer2007) have turned their attention to protective and resilience factors that might buffer adversity and so account for why some people become ill in the face of adversity whereas others do not. The present study investigates the extent to which perceived neighborhood collective efficacy buffers the deleterious effects of neighborhood disadvantage on cardiometabolic aging and chronic illness.
Hypothesis 3: Drawing upon the stress-buffering hypothesis (Phelan et al., Reference Phelan, Link and Tehranifar2010), the associations between neighborhood disadvantage and accelerated cardiometabolic aging will be stronger for individuals who perceived lower collective efficacy from their neighborhood than those who did not (Pathway d).
Hypothesis 4: Based on Hypotheses 2 and 3, we hypothesize that the indirect effect of neighborhood disadvantage on chronic illness through accelerated cardiometabolic aging will vary as a function of the degree to which individuals perceive collective efficacy from their neighborhood.
Research Design
Sample
To examine the effects of neighborhood concentrated disadvantage during emerging and young adulthood, we tested hypotheses using data from the four waves of data (ages 18 to 29) from the Family and Community Health Study, an ongoing research project designed to increase understanding of the contextual risk and protective factors associated with the health and well-being in adulthood for African Americans (see Beach et al., Reference Beach, Lei, Simons, Barr, Simons, Ehrlich and Philibert2017; Simons et al., Reference Simons, Lei, Beach, Barr, Simons, Gibbons and Philibert2018). The sample strategy was intentionally designed to generate families representing a range of socioeconomic status and neighborhood settings. At the first wave (1997–1998), the Family and Community Health Study sample consists of 889 African American fifth-grade children. The mean ages were 10.56 years (SD = 0.631; range 9–13). The sample had an average family per capita income of $6,956. Thirty-six percent of the families were below the poverty line, and 51% of the respondents identified as single parents. The second, third, fourth, fifth, and sixth waves of data were collected in 1999–2000, 2001–2002, 2004–2005, 2007–2008, and 2011–2012 to capture information when the target children were ages 12 to 13, 14 to 15, 17 to 18, 20 to 21, and 23 to 24, respectively.
In 2014–2015, a Wave 7 of data collection was completed that included blood draws. The mean age was 29 years. Given the logistics of scheduling home visits by phlebotomists, only members of the sample residing in Georgia, Iowa, or a contiguous state were identified as eligible. After also excluding persons who were deceased, incarcerated, or otherwise unreachable, we were left with a pool of 545 individuals, 470 (86%) of whom agreed to be interviewed and to provide blood. In the current study, analyses are based on the 408 respondents (150 men and 258 women), nested within 201 Census tracts at Wave 7, who agreed to provide blood samples at age 29, and for whom data was available on all study measures from Wave 7. To further assess attrition bias, we used Heckman's (Reference Heckman1979) two-step procedure to estimate sample selection bias. The results showed that the inverse Mills ratio was not significant, and including this ratio parameter in our models did not change the findings. There were no significant differences between those remaining in the panel and those dropping out with regard to a variety of measures such as neighborhood characteristics, education, income, and health-related variables.
Procedures
The protocol and all study procedures were approved by the University of Georgia Institutional Review Board. To enhance rapport and cultural understanding, African American university students and community members served as field researchers to collect data from the families in their homes. Prior to data collection, the researchers received 1 month of training in the administration of the self-report instruments. The questions were administered in the respondent's home and took on average about 2 hr to complete. Some of the instruments administered in Waves 5–7 included questions regarding illegal or potentially embarrassing sexual activities. Hence, in an effort to further enhance anonymity, we used audio-enhanced, computer-assisted, self-administered interviews. Using this procedure, the respondent sat in front of a computer and responded to questions as they are both presented visually on the screen and auditorily via earphones. In addition to the interview data, participants were also asked to provide a blood sample at Wave 7. After blood was drawn into serum separator tubes by certified phlebotomists, it was frozen and shipped via courier to a laboratory at the University of Iowa to allow assessment of Hemoglobin A1c (HbA1C), a marker of elevated blood sugar, as well as other blood-based indices.
Measures
Chronic illness
Self-reported chronic illness was measured at Wave 7. Respondents were asked “Have you even been diagnosed from any of the following health problems?” The list of health problems consisted of eight illnesses: coronary heart disease, hypertension, diabetes, peptic ulcer, kidney disease, liver disease, thyroid disease, and other chronic disease. For each illness, “no” was coded as 0 and “yes” was coded as 1. Items were summed to form an index of chronic illness that ranged from 0 to 8. The mean score for this variable was 0.267 (SD = 0.564), with roughly 22% of the sample reporting that they had at least one diagnosed chronic disease.
Speed of cardiometabolic aging
Cardiometabolic age at Wave 7 was calculated following the gender-specific Framingham algorithm proposed by D'Agostino et al. (publicly available online tool: https://www.framinghamheartstudy.org/fhs-risk-functions/cardiovascular-disease-10-year-risk/). To estimate cardiometabolic aging, the Framingham algorithm uses systolic blood pressure, body mass index, and blood glucose level (HbA1C), plus it adjusts for an individual's chronological age and gender, and whether they currently smoke (1 = yes, 24.26%) or take antihypertensive medication (1 = yes, 8.09%). Resting systolic blood pressure was monitored with Dinamap Pro 100 (Critikon; Tampa, FL) while the participants sat reading quietly. Three readings were taken every 2 min, and the average of the last two readings was used as the resting index. Mean systolic blood pressure was 122.476 (SD = 16.291). An individual's body mass index score is calculated by weight in kilograms divided by the square of height in meters. Mean body mass index was 31.564 (SD = 8.522). Hemoglobin A1c (HbA1c) level is an indicator of average blood glucose concentrations over the preceding 2 to 3 months. It was determined by the University of Iowa Clinical Pathology Laboratories using a protocol previously described (Philibert et al., Reference Philibert, Beach, Gunter, Todorov, Brody, Vijayendran and Cutrona2011). In the current study, about 4% had HbA1c above 6. Finally, we formulated a measure of accelerated cardiometabolic aging using the unstandardized residual scores from the regression of cardiometabolic age on chronological age (Lei, Simons, et al., Reference Lei, Simons, Beach and Philibert2017). These residuals had a mean of zero and represented both positive and negative deviations from chronological age (in years), with positive scores indicating accelerated aging.
Neighborhood disadvantage
Neighborhood disadvantage was assessed with the US Census Bureau's American Community Survey (ACS) 5-Year Estimates (2008–2012), which was mapped onto study participants’ residential addresses in 2004 (Wave 4), 2007 (Wave 5), 2011 (Wave 6), and 2014 (Wave 7) using a Federal Information Processing Standard census tract code. Following previous studies (Sampson et al., Reference Sampson, Raudenbush and Earls1997), the scale included six items: median household income (reverse coded), percentage unemployed, percentage of residents below the poverty threshold, percentage who are single-mother families, percentage receiving public assistance, and percentage of residents less than age 18. The six items were standardized and summed. A higher score represented a more disadvantaged neighborhood. At age 29, 37.7% never moved, 52.5% moved one or two times, and 9.8% of respondents moved three or more times, and the average number of moves was 1.034 (SD = 1.037) in the past 10 years. The correlations among neighborhood disadvantage across waves were over .40. The mean of the proportion of households in participants’ tract level neighborhoods whose income fell below the federal poverty level was .21 at Wave 4, .20 at Wave 5, .20 at Wave 6, and .19 at Wave7. That is, despite the relatively high rate of relocation, the percentage of participants residing in economically disadvantaged neighborhoods remained relatively constant across waves.
Racial composition
An item from the US Census Bureau's ACS 5-Year Estimates (2008–2012) assesses the percentage of residents in the respondent's census tract who are non-Hispanic Black at waves 4 to 7 (![]() $\bar x$ = 0.332 to 0.346).
$\bar x$ = 0.332 to 0.346).
Perceived collective efficacy
At the time of the blood draw, participants completed a short form (5 items) of the collective efficacy scale (Sampson et al., Reference Sampson, Raudenbush and Earls1997) that asked them to indicate how much they agree (1 = not at all true, 3 = very true) with various statements about their neighborhood (e.g., many of the adults in the neighborhood do get along with each other, adults in the neighborhood would call the police if they saw someone breaking the law, and adults in the neighborhood would tell them to behave if individuals got loud or disorderly). A higher score indicates greater perceived collective efficacy from neighborhoods. Coefficient α for the scale was above 0.70.
Covariates
To account for measures that could provide plausible rival explanations, all analyses statistically controlled for gender and a comprehensive set of time-varying covariates at Waves 4 to 7. Annual income at each wave was assessed by asking participants to report their income in the past year. Education was measured in years of education completed at each wave. At Waves 4 to 7, respondents reported their relationship status with a romantic partner (1 = married or cohabiting; 0 = other). Employment status was measured by a question that asked respondents if they have a part-time or full-time job (1 = yes). Living with parents at each wave was assessed using the following item: “In the past 12 months, did you live with your parents?” The item assessed the victimization of violence in the past 12 months. In addition, we included health-related time-varying covariates at Waves 4 to 7. First, the item asks respondents about their health insurance status (1 = having health insurance) during the previous year. Second, healthy diet across waves was assessed using two items that asked about frequency of fruit and vegetable consumption during the previous 7 days. Responses ranged from 1 (none) to 6 (more than once every day) and were averaged to form the healthy diet variable. Third, exercise at Waves 4 to 7 was measured with two items (e.g., On how many of the past 7 days did you exercise or participate in physical activity for at least 30 min that made you breathe hard such as running or riding a bicycle hard?) The response categories ranged from 1 (0 days) to 5 (all 7 days). Scores on the two items were averaged to form the exercise measure. Finally, sleep quality was measured using the subjective item (1 = very bad, to 4 = very good): “During the past month, how would you rate your sleep quality overall?”
Analytic strategy
All analyses were run using STATA 15 (StataCorp, 2017). Because missing data might influence our results, we used the last observation carried forward approach for imputing missing values at Waves 4 to 6. In order to test the duration effects, we will first run a set of traditional multilevel models to examine the impact of timing-specific exposure to neighborhood disadvantage on accelerated cardiometabolic aging and chronic illness. This method is comparable to previous human development studies.
Given that respondents are not randomly assigned to residential locations, we used marginal structural models with inverse-probability-of-treatment weighting to adjust for a potential neighborhood selection bias. This approach allows us to identify and control individual characteristics that could potentially confound the relationship between neighborhood disadvantage and outcomes. Then, using inverse-probability-of-treatment weighting of the marginal structural model, we adjust for potential neighborhood selection bias (e.g., Humphrey & Root, Reference Humphrey and Root2017; Wodtke et al., Reference Wodtke, Elwert and Harding2016). This method involves transforming neighborhood from a continuous to a categorical variable. This allows for calculation of propensity scores using a multinomial logistic regression with time-varying and invariant covariates.
Recently, Lei, Simons, et al. (Reference Simons, Lei, Beach, Barr, Cutrona, Gibbons and Philibert2017) extended previous studies by using a continuous measure of neighborhood disadvantage to estimate inverse-probability-of-treatment weights through conditional densities (Robins, Reference Robins, Halloran and Berry2000) with clustered standard errors. Let N i denote neighborhood disadvantage and C i be a set of time-varying and invariant covariates. The inverse-probability-of-treatment weights are given by
For individual i, the inverse-probability-of-treatment weighting calculates conditional densities of exposure to disadvantaged neighborhoods at time t, conditional on the set of neighborhood disadvantage and time-varying and invariant covariates at t – 1. However, the weight (w i) would have infinite variance. Hernán, Brumback, and Robins (Reference Hernán, Brumback and Robins2000) recommend using stabilized inverse-probability-of-treatment weighting to reduce bias:
where stabilized weights (sw i) has the same denominator as in the inverse-probability-of-treatment weighting. However, the numerator of the stabilized weights (sw i) comprises the conditional densities of exposure to a disadvantaged neighborhood given neighborhood disadvantage at t – 1. This weighting method creates a distribution reflecting sample characteristics except that there is no association between neighborhood measures and covariates (e.g., individual socioeconomic measures). The new distribution retains the effect of exposure on outcomes. Thus, marginal structural modeling not only provides more accurate estimates of time-varying measures than traditional regression models but also makes causal inference possible with observational data if the assumptions of exchangeability (i.e., no unobserved confounding), positivity (i.e., positive probability of exposure at every level of observed confounders), consistency (i.e., well-defined exposure), and correct model specification are met. Accordingly, the result is a more stringent test of causal hypotheses. Finally, pooled regression models of panel data are weighted by the stabilized inverse-probability-of-treatment weights to adjust for a potential neighborhood selection bias. Thus, individuals who are underrepresented in exposure assignment are given proportionately higher weights, while those who are highly represented in exposure assignment are given proportionally lower weights.
To test the second set of hypotheses, pooled regression models with inverse-probability-of-treatment weighting were used with cardiometabolic aging as the outcome. We began by testing the main effect of neighborhood disadvantage, controlling for sociodemographic and health-related variables. Next, we estimated a model that included a measure of chronic illness to test the mediating hypothesis. Then, pooled Poisson models with inverse-probability-of-treatment weighting were used for chronic illness as the outcome. Poisson models were used at this stage because this measure is a count variable. We first tested the main effect of neighborhood disadvantage. Finally, we included a measure of cardiometabolic aging to test the mediating hypothesis.
Turning to the final set of hypotheses, the studies included two models with inverse-probability-of-treatment weights to test for the direct effect and the buffering effect of perceived collective efficacy. In these two models, the first model included a measure of perceived neighborhood collective efficacy to test the main effect. Model 2 included the interaction term (Neighborhood Disadvantage × Collective Efficacy) necessary to test the moderating hypothesis. When the interaction effects were significant, post hoc analyses of interaction terms were conducted using the average marginal effects. Finally, the moderated-mediation model (Preacher, Rucker, & Hayes, Reference Preacher, Rucker and Hayes2007) was used to examine speed of cardiometabolic aging as a possible mediator of the two-way interaction effect of neighborhood disadvantage and collective efficacy on chronic illness. The logic of the moderated-mediation model is similar to traditional mediated models except that it focuses on testing the mediated effect of the interaction term on the outcome rather than examining the effect of the independent variables.
Results
Initial findings
At the time of the blood draw, about 22% of participants reported that they had at least one or more symptoms using self-reported questionnaires. Turning to each of biomarkers, about 49% of samples had a body mass index greater than 30, and about 4% of those had an HbA1c greater than 6. Approximately 23% of participants (143 individuals) had hypertension (systolic blood pressure ≥130 mm Hg and diastolic blood pressure ≥80 mm Hg; American Heart Association, 2017). The mean chronological age of the respondents was 29.158 years (SD = 0.744), and the mean cardiometabolic age, calculated as the algorithm identified by D'Agostino et al. (Reference D'Agostino, Vasan, Pencina, Wolf, Cobain, Massaro and Kannel2008), was 33.016 (SD = 6.574). Using the r-squared values to indicate the magnitude of significant relationships (Cohen, Reference Cohen1992), as expected, cardiometabolic age had a small to medium association with chronological age (r 2= .028, p < .001). Mean cardiometabolic age derived from the Framingham algorithm was 3.858 years higher than the actual chronological age of the sample. Sixty-eight percent of respondents had a cardiometabolic age greater than their chronological age, indicating a tendency in the sample toward accelerated aging. The correlation matrix is presented in Table 1. It shows a medium significant correlation between chronic illness and accelerated cardiometabolic aging (r 2= .157, p < .001), and both of these variables display significant associations with each of cardiovascular risk biomarkers.
Table 1. Correlations between chronic illness, cardiometabolic aging, and biomarkers
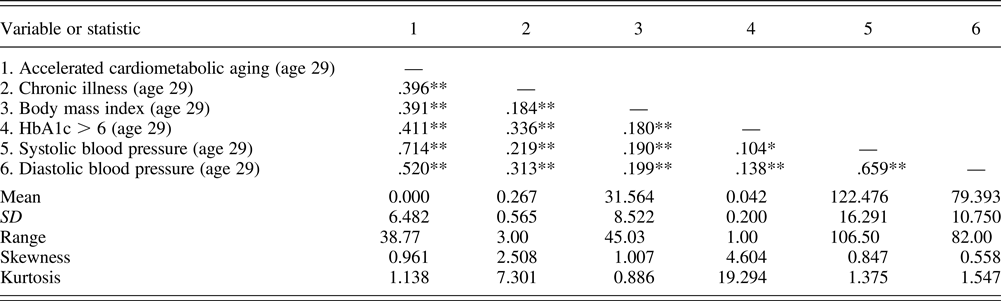
Note: Accelerated cardiometabolic aging was defined as residual from a linear regression model that regressed cardiometabolic age on chronological age. Hemoglobin A1c (HbA1c) was coded as dichotomous (1 = above 6). N = 408.
*p ≤ .05, **p ≤ .01 (two-tailed tests).
Hypothesis 1a: Neighborhood disadvantage will be related to both accelerated cardiometabolic aging and chronic illness even after controlling for potential confounders
Multilevel modeling began with an unconditional model to estimate how much variability in accelerated cardiometabolic aging exists at the neighborhood level. This model has no predictors at the respondent and neighborhood levels. Our results indicate that the neighborhood random effect is significant, τ 00= 1.139, 95% confidence interval (CI) [0.037, 35.475]. About 3% (intraclass correlation coefficient = 0.028) of the total variance in accelerated aging occurs across neighborhoods, suggesting that there is sufficient variation across neighborhoods in biological aging. Table 2 shows the results of using multilevel modeling to examine the impact of time-specific exposure to neighborhood disadvantage on accelerated cardiometabolic aging. Model 1 shows that neighborhood concentrated disadvantage at age 18 (Wave 4) was associated with accelerated cardiometabolic aging (b = 0.707) after taking into account the various controls. It suggests that a 1 SD increase in neighborhood disadvantage is association with 0.707 years increase in cardiometabolic age. We next ran three models, entering neighborhood disadvantage at ages 21, 24, and 29, respectively, as predictors. As shown in Models 2 to 4, there was evidence of a significant effect of neighborhood disadvantage at each wave on accelerated cardiometabolic aging after taking into account all of the various controls.
Table 2. Multilevel regression models examining effects of neighborhood disadvantage on accelerated cardiometabolic aging
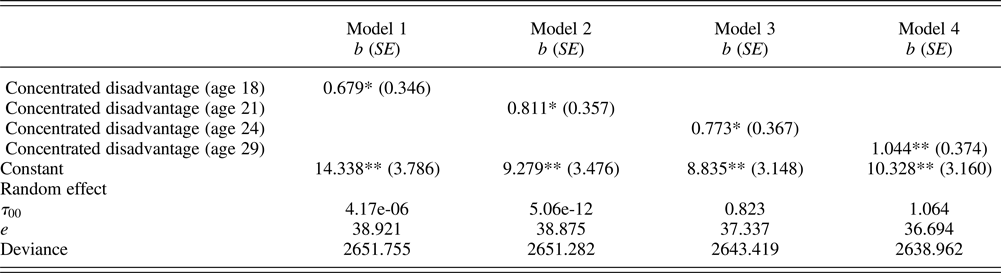
Note: Unstandardized (b) shown with robust standard errors in parentheses; neighborhood concentrated disadvantage are standardized by z-transformation (mean = 0 and SD = 1); racial composition, males, annual income, education, married or cohabiting, employment status, living with parents, victimization, health insurance, healthy diet, exercise, and sleep quality are controlled at the same age as concentrated disadvantage. N (persons) = 408.
*p ≤ .05, **p ≤ .01 (two-tailed tests).
Turning to the models for chronic illness as the dependent variable, Table 3 shows that the results replicate those shown in Table 2. Models 1 to 4 show that neighborhood disadvantage at each wave was associated with chronic illness. Consistent with study hypothesis, neighborhood disadvantage, measured by census ACS data, was significantly associated with both accelerated cardiometabolic aging and chronic illness, and this association held after controlling for a variety of covariates.
Table 3. Multilevel Poisson regression models examining effects of neighborhood disadvantage on chronic illness
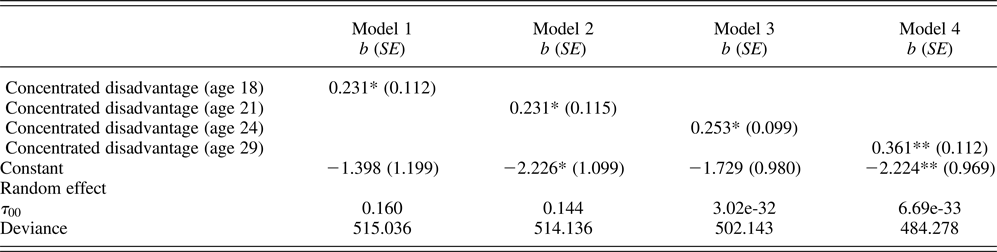
Note: Unstandardized (b) shown with robust standard errors in parentheses; neighborhood concentrated disadvantage are standardized by z-transformation (mean = 0 and SD = 1); racial composition, males, annual income, education, married or cohabiting, employment status, living with parents, victimization, health insurance, healthy diet, exercise, and sleep quality are controlled at the same age as concentrated disadvantage. N (persons) = 408.
*p ≤ .05, **p ≤ .01 (two-tailed tests).
Hypothesis 1b: Durable exposure to neighborhood disadvantage will be more important for accelerated cardiometabolic aging and chronic illness than exposure during any particular stage of life.
Next, we ran a path model to identify whether durable effects of neighborhood disadvantage exist. As shown in Figure 2, the association between neighborhood disadvantage at age 18 and neighborhood disadvantage at age 21 was significant (β = 0.453, p < .001), the relationship between neighborhood disadvantage at age 21 and neighborhood disadvantage at age 24 was also significant (β = 0.373, p < .001), and the association between neighborhood disadvantage at age 24 and neighborhood disadvantage at age 29 was statistically significant (β = 0.158, p = .005). Finally, exposure to neighborhood disadvantage at age 29 was associated with accelerated cardiometabolic aging (β = 0.130, p = .028). Using an indirect effect test, the results revealed that the mediating effect of early neighborhood disadvantage on accelerated cardiometabolic aging through neighborhood disadvantage at ages 21, 24, and 29 was significant: indirect effect = .005, 95% CI [0.001, 0.015], suggesting that it is the durable effect of neighborhood disadvantage that accelerates cardiometabolic aging. Further, Figure 3: indirect effect = .004, 95% CI [0.001, 0.009], indicates a pattern virtually identical to those depicted in Figure 2, indicating durable effects of neighborhood disadvantage on accelerated cardiometabolic aging and chronic illness among African Americans.
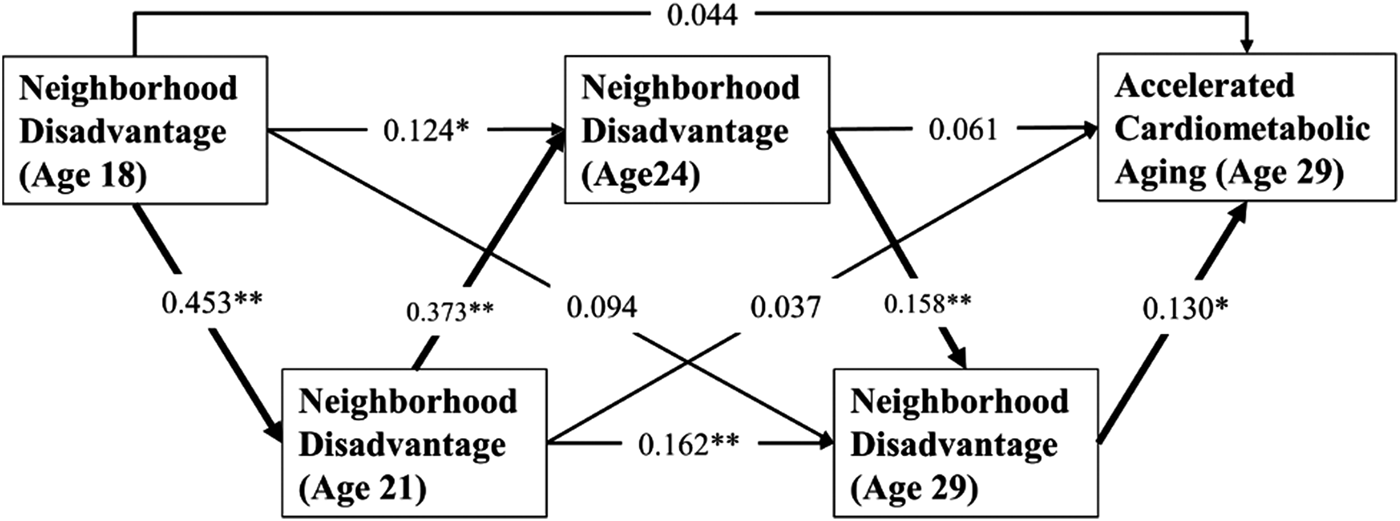
Figure 2. Path model of the relationship between early and later neighborhood disadvantage on accelerated cardiometabolic aging. *p ≤ .05, **p ≤ .01 (two-tailed tests).

Figure 3. Path model of the relationship between early and later neighborhood disadvantage on chronic illness. *p ≤ .05, **p ≤ .01 (two-tailed tests).
Hypothesis 2: The effect of neighborhood disadvantage on chronic illness will be mediated by accelerated cardiometabolic aging even after confounding confounders and neighborhood selection bias are controlled.
Due to nonrandom assignment of individuals into neighborhoods, we used marginal structural modeling to control for selection bias through inverse-probability-of-treatment weighting. The inverse-probability-of-treatment weighting was calculated using a regression model with cluster-adjusted standard errors to estimate each respondent's probability of receiving the treatment of exposure to neighborhood concentrated disadvantage. That is, neighborhood disadvantage was regressed on gender and time-varying sociodemographic covariates from ages 18 to 29. The descriptive statistics for all time-varying covariates in the inverse-probability-of-treatment weighting are shown in Table 4. To avoid an extreme variation of weights, we first checked the variance of inverse-probability-of-treatment weights. Unstabilized inverse-probability-of-treatment weighting (w i) were associated with substantial variability (SD = 11279.82), whereas this variability was eliminated by using stabilized inverse-probability-of-treatment weighting (SD = 0.06). Given that extreme weights can bias the standard error estimates (Hernán et al., Reference Hernán, Brumback and Robins2000), all models were weighted by stabilized inverse-probability-of-treatment weighting to reduce neighborhood selection bias.
Table 4. Descriptive statistics for time-varying and time-invariant covariates in the inverse probability treatment weighting
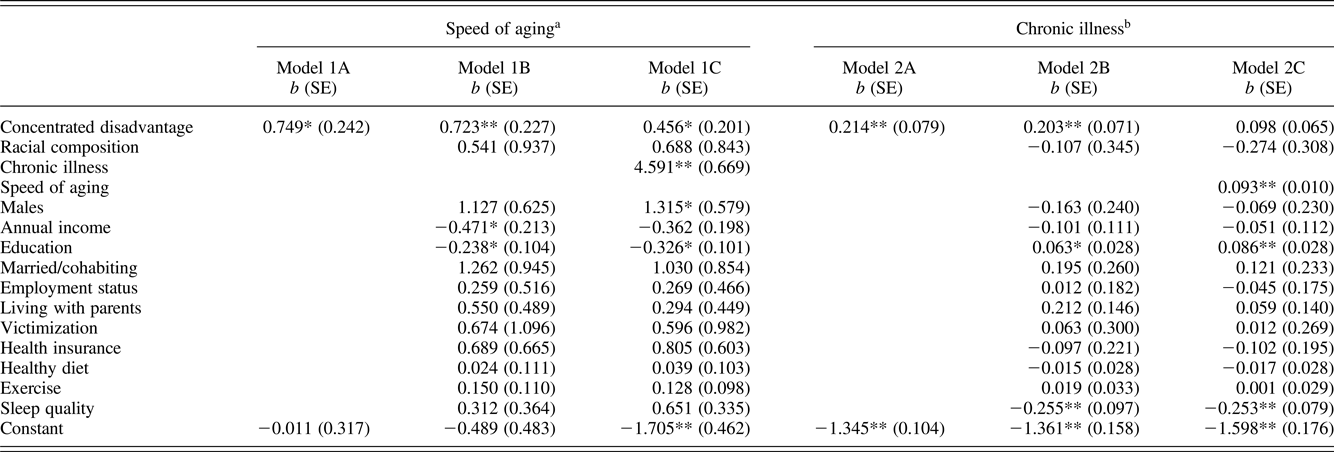
Table 5 presents pooled regression models with stabilized inverse-probability-of-treatment weighting. This model allows us to include time-varying sociodemographic and health-related control variables and control for potential neighborhood selection effects. Model 1A reveals that a measure of time-varying neighborhood disadvantage had a significant effect on accelerated cardiometabolic aging (b = 0.749, p = .002). The result indicates that a 1 SD increase in neighborhood disadvantage is associated with a 0.749 years increase in cardiometabolic age. To further interpret this finding, we graphed the effect in Figure 4 for levels of neighborhood disadvantage that range from –2 to +2 SD from the mean. As can be seen, the regression line crosses the line of deviation of cardiometabolic age from chronological age at zero, suggesting that individuals living in disadvantaged neighborhoods show significantly accelerated aging whereas those living in advantaged neighborhoods demonstrate significantly decelerated aging. Further, Model 1B shows that this association was maintained (b = 0.723, p = .002) after controlling for time-varying socioeconomic status and various health-related behaviors. Next, Model 1C presents the result of entering chronic illness as a mediator. The impact of neighborhood disadvantage on accelerated cardiometabolic aging remained significant, indicating chronic illness is not a mediator of this relationship.

Figure 4. Scatter plot representing the association between neighborhood disadvantage and accelerated cardiometabolic aging using Framingham algorithm. The solid line displays the predicted regression line, and the dashed lines are the 95% confidence interval for the fitted line. Predicted scores represent residual biological age after controlling for chronological age (N = 408).
Table 5. Pooled regression models using four waves (ages 18 to 29) of data examining effects of neighborhood disadvantage on accelerated cardiometabolic aging and chronic illness
Note: Unstandardized (b) shown with robust standard errors in parentheses; models adjust for selection effects through application of inverse-probability-of-treatment weighting; income and neighborhood disadvantage are standardized by z-transformation (mean = 0 and SD = 1); all time-varying covariates are lagged to the previous wave (T – 1). N (persons) = 408.
a Pooled regression model for a continuous outcome.
b Pooled Poisson regression model for a count outcome.
*p ≤ .05, **p ≤ .01 (two-tailed tests).
Given that the measure of chronic illness is a count variable, pooled Poisson models with stabilized inverse-probability-of-treatment weights provide a good fit of the data. Model 2A of Table 5 shows that the main effect of neighborhood disadvantage was significant (b = 0.214, incidence rate ratio [IRR] = 1.239, p = .007), indicating that a 1 SD increase in neighborhood disadvantage increases the expected number of symptoms by 23.9%. Model 2B added the potentially time-varying confounding variables. As hypothesized, long-term exposure to neighborhood disadvantage was related to chronic illness (b = 0.203, IRR = 1.225, p = .004). The results are consistent with numerous studies (Aneshensel, Reference Aneshensel, Avison, Aneshensel, Schieman and Wheaton2009; Hill et al. Reference Hill, Ross and Angel2005; Lei et al., Reference Lei, Beach, Simons and Philibert2015; Ross & Mirowsky, Reference Ross and Mirowsky2001, Reference Ross and Mirowsky2009) reporting that residing in a disadvantaged neighborhood has a deleterious effect on physical health even after controlling for selection bias, socioeconomic status, and health-related behaviors.
Model 2C introduced the measure of accelerated cardiometabolic aging using the residual scores from the regression of cardiometabolic age on chronological age. The difference in deviance between Model 2B and Model 2C was significant (Δχ2 = 229.368, df = 1, p < .001), suggesting that cardiometabolic aging improves the model fit. As predicted, accelerated cardiometabolic aging was positively and significantly related to chronic illness (b = 0.093, IRR = 1.098, p < .001), indicating that each 1-year increase in cardiometabolic age was associated with an estimated 9.8% increase in the incidence of chronic illness. Moreover, consistent with the mediation argument, the relationship between neighborhood disadvantage and chronic illness was no longer significant when the measure of accelerated aging is included in the model. This pattern of results suggests that accelerated cardiometabolic aging is a mediator of the effect of neighborhood concentrated disadvantage on chronic illness.
Hypothesis 3: The associations between neighborhood disadvantage and accelerated cardiometabolic aging will be stronger for individuals who perceived lower neighborhood collective efficacy than those who did not.
Model 1 of Table 6 introduces the variable of perceived neighborhood collective efficacy. Although the effect of neighborhood disadvantage was significant, there was no significant association between perceived collective efficacy and accelerated cardiometabolic aging. The last model in Table 6 incorporates the interaction of neighborhood disadvantage with neighborhood collective efficacy to test stress-buffering effects. As predicted, the interaction effect of neighborhood disadvantage and their collective efficacy was significant (b = –0.611, p = .011); we graphed the effect in Figure 5. In the figure, high perceived collective efficacy is defined as 1 SD above the sample mean, and low perceived collective is defined as 1 SD below the sample mean. Consistent with the stress-buffering hypothesis, the figure demonstrates that individuals living in advantaged neighborhoods showed significantly decelerated cardiometabolic aging. In addition, perceived neighborhood collective efficacy had no effect on accelerated cardiometabolic aging of those living in advantaged neighborhoods. However, individuals living in disadvantaged neighborhoods with low perceived collective efficacy in their neighborhood showed significantly accelerated biological aging. Conversely, individuals with high collective efficacy were protected, supporting the buffering hypothesis.
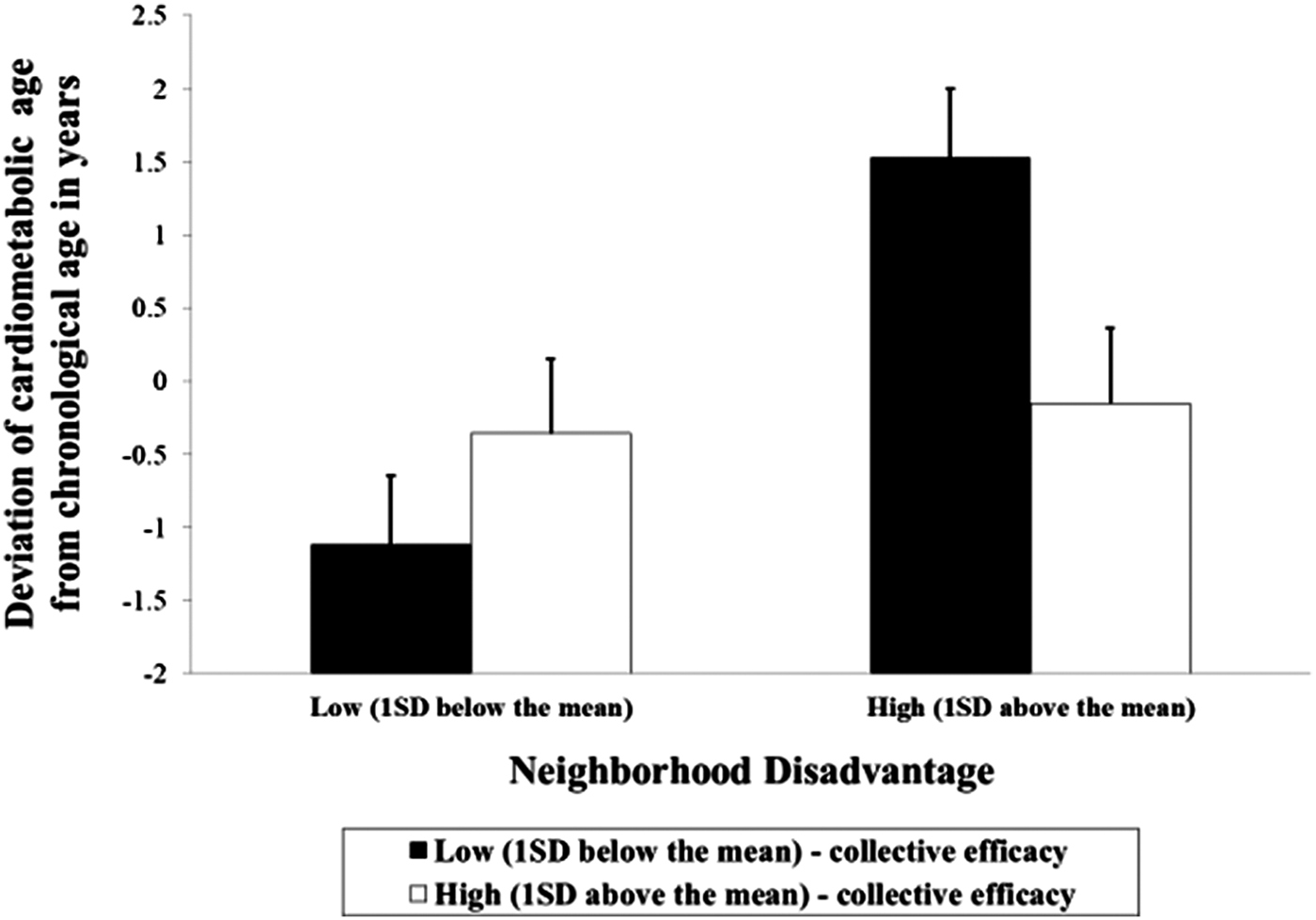
Figure 5. The effect of neighborhood disadvantage on accelerated cardiometabolic aging by level of perceived collective efficacy. Vertical bars indicate the 95% confidence interval (N = 408).
Table 6. Pooled regression models using four waves (ages 18 to 29) of data examining the relationships among neighborhood disadvantage, perceived collective efficacy, and accelerated cardiometabolic aging

Note: Unstandardized (b) shown with robust standard errors in parentheses; models adjust for selection effects through application of inverse-probability-of-treatment weighting; income and neighborhood disadvantage are standardized by z-transformation (mean = 0 and SD = 1); all time-varying covariates are lagged to the previous wave (T – 1). N (persons) = 408.
*p ≤ .05, **p ≤ .01 (two-tailed tests).
Hypothesis 4: The indirect effect of neighborhood disadvantage on chronic illness through accelerated cardiometabolic aging will vary as a function of the degree to which individuals perceive neighborhood collective efficacy
Given that perceived neighborhood collective efficacy buffers the effect of neighborhood disadvantage on accelerated cardiometabolic aging, a conditional indirect effect model (Preacher et al., Reference Preacher, Rucker and Hayes2007) was used to test the extent to which the interaction of neighborhood disadvantage and collective efficacy on chronic illness was mediated by accelerated cardiometabolic aging. As expected, the indirect effect of this interaction term on report of diagnosed chronic illness through accelerated cardiometabolic aging was significant: indirect effect = –0.356, 95% CI [–0.595, –0.117], and accounted for about 88% of the total variance, suggesting it is important in explaining neighborhood-level effects on chronic illness. This pattern of findings supports the hypothesis that the indirect effect of neighborhood disadvantage through speed of aging on chronic illness is conditional on levels of perceived neighborhood collective efficacy.
Power analysis
To ensure adequate power to detect a significant interaction effect given the current sample size, power analyses were conducted based on the results showed in Tables 2 and 3. According to the G*Power program (Faul, Erdfelder, Land, & Buchner, Reference Faul, Erdfelder, Lang and Buchner2007) for a regression model, for an effect of this size, the current sample (n = 408) provided more than 90% power. This suggests that our sample was an adequate size to test our theoretical models.
Discussion
Social stress is related to illness, with much of the supporting research guided by the stress process model (Pearlin, Reference Pearlin1989, Reference Pearlin, Aneshensel and Phelan1999). This model posits that prolonged exposure to stressful conditions chronically activates a physiological stress response that increases the chances of tissue damage, impaired immune functioning, and illness. Studies examining the link between stress and illness have typically focused on personal stressors. Recently, however, researchers have begun to investigate the extent to which adverse neighborhood conditions might also increase the risk for disease (Aneshensel et al., Reference Aneshensel, Harig, Wight, Gorge and Ferraro2016). A popular hypothesis is that it is the ambient threat posed by socioeconomically disadvantaged neighborhoods that is highly stressful and leads to illness beyond the contribution of individual socioeconomic status and individual experiences and health behaviors. Consonant with this idea, several studies have reported an association between neighborhood context and various health outcomes (Brody et al., Reference Brody, Lei, Chen and Miller2014; Browning et al., Reference Browning, Cagney and Iveniuk2012; Chen, Miller, Brody, & Lei, Reference Chen, Miller, Brody and Lei2015; Hill et al., Reference Hill, Ross and Angel2005; Lei, Simons, et al., Reference Lei, Simons, Beach and Philibert2017; Ross & Mirwosky, Reference Ross and Mirowsky2009).
Using marginal structural modeling to account for neighborhood selection effects, the present study was an attempt to overcome methodological and theoretical limitations that have hampered previous studies of the link between neighborhood and health. Our goal was to utilize more objective measures of both neighborhood characteristics and illness, and to expand the basic neighborhood stress process model by incorporating a plausible biological mediator, cardiometabolic aging. Specifically, we extended prior research in several ways.
First, most studies employ self-reported measures from the same source. As a consequence, associations reported between neighborhood characteristics and illness may be inflated due to shared methods variance (Podsakoff et al., Reference Podsakoff, MacKenzie, Lee and Podsakoff2003). We avoided this problem in the present study by using multiple methods of assessment. We used the ACS, collected by the US Census Bureau, to measure objective characteristics present within neighborhoods, self-report to assess perceived neighborhood collective efficacy, blood-derived biomarkers and biometics to assess cardiometabolic age, and report of diagnosed chronic illness to assess morbidity and health.
In addition to the problem of shared methods variance, many neighborhood studies limit their analysis to a single time point of neighborhood characteristics (e.g., for an exception, see White et al., Reference White, Zeiders, Knight, Roosa and Tein2014). As a result, we cannot examine possible developmental mechanisms of health and determine the age at which neighborhood effects began or exert their strongest influence. For instance, is current exposure to disadvantaged neighborhoods more important for accelerated aging and health? Is durable exposure to disadvantaged neighborhoods more consequential for accelerated aging and health than exposure during any specified period of time? Using four waves of data across the span of young adulthood (ages 18–29), the current results indicated that across measurement methods, neighborhood disadvantage at each wave was associated with accelerated cardiometabolic aging and chronic illness. Moreover, long-term exposure to neighborhood disadvantage has durable effects on accelerated biological aging and chronic illness. Thus, neighborhood disadvantage effects on individual outcomes tend to be relatively stable over time among African Americans. Just as Sampson, Sharkey, and Raudenbush (Reference Sampson, Sharkey and Raudenbush2008, p. 852) note, “When we consider moves into and out of concentrated disadvantage among a representative sample of black children, not just the poor, durable inequality matters.” This durable-effects model of neighborhood disadvantage that takes account of a temporal process is particularly important for sociological models of health.
Second, the stress process model posits that heightened physiological stress serves as the mechanism that links adverse conditions to illness. Nevertheless, potential mediating physiological factors are rarely assessed. This is particularly true of neighborhood studies. According to the weathering hypothesis (Geronimus, Reference Geronimus2013), the present study tested the hypothesis that accelerated cardiometabolic aging is an important physiological mechanism through wear and tear on the body that mediates much of the impact of neighborhood disadvantage on chronic illness, and may be a particularly important mechanism for young adult African Americans. Consistent with previous studies (Lei, Simons, et al., Reference Lei, Simons, Beach and Philibert2017; Simons et al., Reference Simons, Lei, Beach, Philibert, Cutrona, Gibbons and Barr2016), we found a robust association between neighborhood disadvantage and accelerated cardiometabolic aging that was robust to controls for neighborhood selection bias, sociodemographics, and health-related variables. Further, we found that speed of cardiometabolic aging mediated much of the association between neighborhood disadvantage and illness. Such association was no longer significant once accelerated aging was entered into the model.
Finally, although we found significant relationships between neighborhood disadvantage and both accelerated aging and chronic illness, the magnitude of these associations, as expected, was modest. Amplifying the importance of neighborhood characteristics, we also found results supporting the stress-buffering hypothesis where high levels of collective efficacy moderated the effect of neighborhood disadvantage on health. Theory and research have emphasized the extent to which neighborhood collective efficacy can operate as a resilience and protective mechanism (e.g., Liu et al., Reference Liu, Mustanski, Dick, Bolland and Kertes2016; Norris et al., Reference Norris, Stevens, Pfefferbaum, Wyche and Pfefferbaum2008; Simons et al., Reference Simons, Simons, Burt, Brody and Cutrona2005) that improve people's ability to cope with neighborhood disadvantages and stressors. Consonant with this idea, our results indicated that the association between neighborhood disadvantage and speed of aging was ameliorated when individuals perceived high levels of collective efficacy from their neighborhood. In addition, findings from the moderated-mediation analysis revealed that a substantial portion of the explained variance in chronic illness was accounted for by the interaction of neighborhood disadvantage and perceived neighborhood collective efficacy. These results provide important evidence that the health consequences of neighborhood conditions may not be the same for all people. Perceived neighborhood collective efficacy may be an important protective factor for those residing in disadvantaged neighborhoods. In the presence of lower collective efficacy, individuals will tend to be less resilient to neighborhood stress effects, and so develop more problematic health outcomes. If so, interventions designed to enhance level of perceived neighborhood collective efficacy and cohesion may counter the stressors, which over time lead to biological embedding and onset of chronic illness.
Unlike in some previous studies, however, our analyses did not reveal a main effect for neighborhood collective efficacy on accelerated aging and chronic illness. The explanations for this finding may be methodological or theoretical. The focus of the current study is on the lived experiences of African Americans. Yet, African Americans are unevenly distributed geographically, being concentrated in disadvantaged neighborhoods (Peterson & Krivo, Reference Peterson and Krivo2010). Given that disadvantaged neighborhoods typically have lower collective efficacy (Sampson et al., Reference Sampson, Raudenbush and Earls1997), the problem of restricted distributions may also have limited our ability to test the main effect hypothesis.
In contrast, the absence of a main effect may be a consequence of the inherent nature of collective efficacy. Studies finding a main effect, such as Sampson et al. (Reference Sampson, Raudenbush and Earls1997), were focused on violence or criminal behaviors as outcomes. Research focused on health (e.g., Burdette, Wadden, & Whitaker, Reference Burdette, Wadden and Whitaker2006; Franzini, Caughy, Spears, & Esquer, Reference Franzini, Caughy, Spears and Esquer2005) often has not found a main effect of collective efficacy on health outcomes. This may be because collective efficacy, at least as it is defined and measured by Sampson et al., emphasizes trust and the belief that one's neighbors will intervene for the good of the community (e.g., call the police, pick up trash, and reprimand children engaging in antisocial behavior). Thus collective efficacy is a cultural resource, a set of norms and commitments shared with other members of the neighborhood, which can be relied upon when the order and safety of the neighborhood are threatened. Collective efficacy is a type of social capital, but it is a property of the neighborhood rather than the individual, and its value is most evident when social disorder poses a threat.
This is in contrast to social capital in the form of social support. Social support is an individual-level resource that involves access to emotional and instrumental assistance from the members of one's social network. Social support implies social connections, social integration, and close ties to others. As such, it would be expected to directly affect an individual's mental and physical health. Although social support may help an individual cope with various personal stressors encountered in life, it may be that support from a friend can do little to help a person deal with the anxiety and helplessness of living in a neighborhood characterized by crime and social disorder. Collective efficacy is a community resource that directly addresses the potential dangers and threats of life in a disadvantaged neighborhood. All of this suggests that collective efficacy is unlikely to have a main effect on health; rather, its importance is as a moderator of the effect of community disadvantage on health. Conversely, social support might be expected to have a main effect on health, but fail to moderate the effect of community disadvantage. There is a need for future research that focuses on the different ways in which these two types of social capital influence health.
Together, the current study presents an integrated model that combines environments and biology to understand the influence of neighborhood disadvantage on health during the transition to adulthood. Beyond this contribution, findings from the link between neighborhood and health also advance the literature on the field of culture and biology. Given that culture is embedded in multiple social contexts (Causadias, Reference Causadias2013), research has emphasized that exposure to neighborhood disadvantage is often associated with cultural framing, habits, norms, and values (Anderson, Reference Anderson1999; Wilson, Reference Wilson2009). For example, in Code of the Street, Elijah Anderson (Reference Anderson1999) indicated that residents of disadvantaged neighborhoods tend to adopt the norms of the street code subculture to handle their everyday routines. Studies of African Americans have also highlighted cultural promotive and protective factors (e.g., cultural pride, spiritually, and cultural socialization) among African American neighborhoods and cultures and its protective role in the face of adversities and stressors (Burt et al., Reference Burt, Lei and Simons2017; Causadias, Reference Causadias2013; Constantine, Donnelly, & Myers, Reference Constantine, Donnelly and Myers2002; Gaylord-Harden, Burrow, & Cunningham, Reference Gaylord-Harden, Burrow and Cunningham2012).
In addition to individual behaviors, recent research has established ways to integrate cultural experiences and biological processes in development (Causadias, Reference Causadias2013). It may be that culture, as both social and individual sets of processes, has an effect on our biological systems. Their findings combined with those for the present study, suggest that durable exposure to disadvantaged neighborhoods gives rise to cultural (e.g., social norms and values) and social schemas (e.g., street code and cultural pride) that increase or decrease the probability of accelerated biological aging and chronic illness during adulthood. Accordingly, an important future direction will be to integrate culture into the biopsychosocial models and to show that cultural effects mediate the association between neighborhood disadvantage and aging/chronic illness.
Limitations
Although our study was able to extend past research in several respects, it also suffered from various limitations. First, and chief among them, is the fact that our sample was limited to African Americans, suggesting the need to see whether similar processes can be identified in other vulnerable groups who may experience elevated levels of neighborhood disadvantage. In some respects, however, this shortcoming might be seen as a strength. Myriad studies have established that African Americans are at higher risk than other ethnic groups for chronic illness (Adler & Rehkopf, Reference Adler and Rehkopf2008), biological weathering due to social context (Geronimus, Reference Geronimus2013; Simons et al., Reference Simons, Lei, Beach, Philibert, Cutrona, Gibbons and Barr2016), and exposure to disadvantaged neighborhoods (Peterson & Krivo, Reference Peterson and Krivo2010). All of this argues for the relevance of research investigating the effect of neighborhood conditions on the health of African Americans. That said, clearly there is a need to replicate our findings with samples that are more ethnically diverse.
Second, several studies (Clark, Anderson, Clark, & Williams, Reference Clark, Anderson, Clark and Williams1999; Pascoe & Richman, Reference Pascoe and Richman2009; Simons et al., Reference Simons, Lei, Beach, Barr, Simons, Gibbons and Philibert2018) have indicated a relation between discrimination and health outcomes among African Americans through biopsychosocial mechanisms. Given that racial discrimination is a common experience for those living in disadvantaged neighborhoods (Massey, Reference Massey2004), this may be the case for African Americans who live in these neighborhoods. However, this was not tested in the present work. Future research should incorporate discrimination into the link between neighborhood context and health outcomes.
A third limitation that deserves to be mentioned is our use of marginal structural modeling to adjust neighborhood selection bias. Although we included a series of potential confounders that have been previously reported to be associated with neighborhood disadvantage (Sampson et al., Reference Sampson, Sharkey and Raudenbush2008) and biological aging (Lei, Simons, et al., Reference Lei, Simons, Beach and Philibert2017), our results may have been influenced by unobserved confounders that violated the assumption of marginal structural modeling. Accordingly, findings should be viewed as tentative pending replication with other samples and sets of control variables.
Another caveat is related to our specification of theoretical pathways. In line with the weathering hypothesis (Geronimus, Reference Geronimus2013; Simons et al., Reference Simons, Lei, Beach, Philibert, Cutrona, Gibbons and Barr2016), we hypothesized that accelerated cardiometabolic aging as an index of wear and tear on the body would mediate the association between neighborhood disadvantage and chronic illness. It is also possible, however, that chronic illness is a mediator of neighborhood disadvantage on speed of aging during early adulthood. To investigate this idea, we tested a model using chronic illness as a mediator. Our results did not support this reversal of the mediating hypothesis. However, because measures of cardiometabolic age and chronic illness in our data set were measured in age 29, our ability to test a causal mediator may be limited (MacKinnon, Fairchild, & Fritz, Reference MacKinnon, Fairchild and Fritz2007). Accordingly, the results obtained in the current study need to be replicated in a data set with multiple assessments of biological aging and chronic illness.
Finally, although it seems likely that shared method variance would result in inflated associations (Podsakoff et al., Reference Podsakoff, MacKenzie, Lee and Podsakoff2003), and so the effect of self-reported neighborhood characteristics on health would be inflated if both were self-reported, we did not directly test this hypothesis. Future studies should replicate and extend the current study by including self-reported measure of neighborhood characteristics to compare with census tract measures such as those used in the current study. In addition, our assessment of biological aging relied on the Framingham algorithm. While this aging score is commonly used and comprises biomarkers that can be obtained easily and inexpensively, future studies should replicate the current study with other measures of age acceleration including measures of accelerated epigenetic aging.
Conclusion
In conclusion, while our study suffered from certain constraints, it also extended prior research in a number of respects. In addition to proffering more convincing evidence of an association between neighborhood socioeconomic disadvantage and illness, we found durable effects of neighborhood disadvantage on accelerated aging and chronic illness. This is particularly important in the study of health and aging. We also identified speed of cardiometabolic aging as an important mediating biopsychosocial mechanism that links stressful neighborhood conditions to chronic illness. Finally, our analyses indicated that collective efficacy from neighborhoods serves to buffer the association between neighborhood and health. Overall, our findings suggest neighborhoods as fundamental causes of health and illness. In addition, they stress the importance of constructing integrated models that bring together social and biological variables. By specifying biopsychosocial mechanisms that link social conditions to illness, models of biological embedding of social circumstances become more comprehensive and precise. They also become more useful and compelling for medical and public health professionals concerned with designing social policies and preventive interventions to enhance health.













