Introduction
Schizophrenia is associated with significant cognitive deficits (Heinrichs and Zakzanis, Reference Heinrichs and Zakzanis1998; Bora et al., Reference Bora, Yücel and Pantelis2010, Reference Bora, Binnur Akdede and Alptekin2017a; Schaefer et al., Reference Schaefer, Giangrande, Weinberger and Dickinson2013). Cognitive impairment is a stable feature of schizophrenia and significantly contributes to functional deficits in schizophrenia (Fett et al., Reference Fett, Viechtbauer, Dominguez, Penn, van Os and Krabbendam2011; Bora and Murray, Reference Bora and Murray2014; Green, Reference Green2016). Abnormalities in cognitive development which predates the onset of psychosis are major determinants of neuropsychological difficulties in schizophrenia (Weinberger, Reference Weinberger, Nasrallah and Weinberger1986; Bora, Reference Bora2015; Parellada et al., Reference Parellada, Gomez-Vallejo, Burdeus and Arango2017). The fact that cognitive impairment is evident at first-episode schizophrenia and in individuals who have a genetic or clinical high-risk for developing schizophrenia further supports this notion (Mesholam-Gately et al., Reference Mesholam-Gately, Giuliano, Goff, Faraone and Seidman2009; Agnew-Blais and Seidman, Reference Agnew-Blais and Seidman2013; De Herdt et al., Reference De Herdt, Wampers, Vancampfort, De Hert, Vanhees, Demunter, Van Bouwel, Brunner and Probst2013; Bora et al., Reference Bora, Lin, Wood, Yung, McGorry and Pantelis2014; Bora and Pantelis, Reference Bora and Pantelis2015). However, other factors such as metabolic syndrome, alcohol use, chronic stress and antipsychotics can also contribute to cognitive impairment in schizophrenia (Potvin et al., Reference Potvin, Joyal, Pelletier and Stip2008; Thoma and Daum, Reference Thoma and Daum2013; Sahu et al., Reference Sahu, Malavade and Jacob2016; Bora et al., Reference Bora, Akdede and Alptekin2017b; Omachi and Sumiyoshi, Reference Omachi and Sumiyoshi2018).
It is important to investigate potential neurochemical underpinnings of factors associated with cognitive impairment in schizophrenia. Abnormalities in neurotrophic molecules are among important candidates for explaining the relationship between cognitive impairment and factors such as stress, metabolic syndrome and neurodevelopment in schizophrenia. Schizophrenia might be associated with reduced serum levels of Brain-derived neurotrophic factor (BDNF) which might be related to early traumatic experiences and depressive episodes (Fernandes et al., Reference Fernandes, Steiner, Berk, Molendijk, Gonzalez-Pinto, Turck, Nardin and Gonçalves2015; Aas et al., Reference Aas, Dieset, Morch, Steen, Hope, Reponen, Laskemoen, Ueland, Aukrust, Melle, Agartz and Andreassen2019). One may argue that reduced BDNF levels might be one of the factors leading to cognitive impairment in schizophrenia. However, the available studies investigating the relationship between BDNF levels and cognitive deficits in schizophrenia reported inconsistent results (Zhang et al., Reference Zhang, Liang, Chen, Xiu, Yang, Kosten and Kosten2012; Man et al., Reference Man, Lv, Du, Yin, Zhu, Zhang, Soares, Yang, Chen and Zhang2018). A previous meta-analysis which investigated the relationship between BDNF polymorphism and cognition also included a preliminary analysis of only five studies investigating the association between BDNF levels and cognition (Ahmed et al., Reference Ahmed, Mantini, Fridberg and Buckley2015). This study did not provide strong evidence for a significant relationship between BDNF levels and cognition except for a very broad measure of executive function.
Other authors have proposed a potential role for inflammation in schizophrenia (Kirckpatrick and Miller, Reference Kirkpatrick and Miller2013; Khandaker et al., Reference Khandaker, Cousins, Deakin, Lennox, Yolken and Jones2015; Najjar and Pearlman, Reference Najjar and Pearlman2015; Müller, Reference Müller2018). Schizophrenia might be associated with elevated levels of C-reactive protein (CRP) and cytokines (Fernandes et al., Reference Fernandes, Steiner, Bernstein, Dodd, Pasco, Dean, Nardin, Gonçalves and Berk2016; Goldsmith et al., Reference Goldsmith, Rapaport and Miller2016; Fraguas et al., Reference Fraguas, Díaz-Caneja, Ayora, Hernández-Álvarez, Rodríguez-Quiroga, Recio, Leza and Arango2018). There is a paucity of studies investigating the relationship between inflammatory biomarkers and cognitive impairment in schizophrenia (Misiak et al., Reference Misiak, Stańczykiewicz, Kotowicz, Rybakowski, Samochowiec and Frydecka2018; Fourier et al., Reference Fourrier, Singhal and Baune2019). CRP is the most commonly assessed peripheral marker for investigating the relationship between inflammation and cognition in schizophrenia. While some studies suggested elevated CRP is associated with cognitive deficits in schizophrenia (Dickerson et al., Reference Dickerson, Stallings, Origoni, Vaughan, Khushalani and Yolken2012; Bulzacka et al., Reference Bulzacka, Boyer, Schürhoff, Godin, Berna, Brunel, Andrianarisoa, Aouizerate, Capdevielle, Chéreau-Boudet, Chesnoy-Servanin, Danion, Dubertret, Dubreucq, Faget, Gabayet, Le Gloahec, Llorca, Mallet, Misdrahi, Rey, Richieri, Passerieux, Roux, Yazbek, Leboyer and Fond2016), others found no relationship (Joseph et al., Reference Joseph, Depp, Martin, Daly, Glorioso, Palmer and Jeste2015; Boozalis et al., Reference Boozalis, Teixeira, Cho and Okusaga2018). No previous meta-analysis attempted to summarize available research findings to investigate the level of overall relationship between CRP and cognition.
Current meta-analysis was conducted for providing a reliable estimate of the strengths of the potential relationships between cognitive impairment, BDNF and CRP in schizophrenia.
Methods
Data source and study selection
PRISMA guidelines were used in conducting this meta-analysis (Moher et al., Reference Moher, Liberati, Tetzlaff and Altman2009). A literature search was conducted using the databases PubMed and Scopus to identify the relevant studies (1 January 1980 to 31 January 2019). Bipolar disorder and psychotic depression studies were not included in this systematic review as the number of available studies was small. The combination of following keywords was used in searching the literature: Schizophrenia AND (BDNF OR C-Reactive Protein) AND (Cogn* OR Neuropsycho*). Reference lists of published reports and review papers were also searched for additional studies. Inclusion criteria for the studies were: (1) correlations between BDNF or CRP and cognition were investigated in an adult (mean age = 18–60) sample of patients with schizophrenia spectrum disorders/first-episode psychosis and (2) reported sufficient data to calculate the effect size and standard error for the strength of relationship between BDNF or CRP and cognition. Exclusion criteria included: (1) sample included high-risk individuals and (2) study shares participants with another study which was already selected.
Cognitive domains included were global cognition, verbal memory, visual memory, working memory, processing speed, verbal fluency, planning/problem solving and speed-based executive functions (EF-Speed). The individual tests summarized under each cognitive domain are provided in online Supplementary eTable 1. The data were coded separately by two individuals. The quality of studies included was assessed using the selection and quality of outcome ascertainment sections (maximum score = 8) of a modified (for cross-sectional studies) version of the Newcastle-Ottawa Scale (Wells et al., Reference Wells, Shea, O'Connell, Peterson, Welch and Tugwell2000).
Statistical analyses
Meta-analyses were performed using the ‘metaphor’ package in R environment. Pearson r correlations were analyzed after Fisher's Z transformation was applied. Effect sizes were weighted using the inverse variance method and a random-effects model. Homogeneity of the distribution of weighted effect sizes was tested with the Q-test, and I 2 (I 2 values <50% indicate low heterogeneity, I 2 >50% indicate moderate heterogeneity and I 2 >75% indicate large heterogeneity). The heterogeneity of random-effects model was also tested with the τ2 test. The possibility of publication bias was assessed by regression analysis of funnel plot of Fisher's Z-transformed correlation coefficient and standard error (Egger's test).
Subgroup analyses were conducted for the phase of the illness (chronic v. first-episode) and state of the illness (stable v. non-stable). Meta-regression analyses were conducted to investigate the effect of demographic (age and ratio of males) and methodological (quality score) on the strength of the association between neurocognition and BDNF or CRP levels.
Results
The selection process is summarized in online Supplementary eFig. 1. Current meta-analysis included 21 studies including 2449 patients with schizophrenia-spectrum disorders (Table 1).
Table 1. Characteristics of the studies included in this meta-analysis

ToL, Tower of London test; WCST, Wisconsin card sorting test; TMT, Trail making test; LNS, Letter number sequencing; BACS, Brief Assessment of cognition in Schizophrenia; RBANS, Repeatable Battery for the Assessment of Neuropsychological Status; Sch, schizophrenia; FEP, first-episode psychosis; FES, first-episode schizophrenia; CRP, C-reactive protein; BDNF, brain-derived neurotrophic factor; CPT, continuous performance test; D-KEFS, Delis–Kaplan Executive Function System; MATRICS, Measurement and Treatment Research to Improve Cognition in Schizophrenia; FE, first episode.
BDNF
The BDNF meta-analysis included 12 studies including 972 (59.9% males) patients with schizophrenia-spectrum disorders (Table 1). Mean age was 37.6 years. Nine of the studies included clinically stable patients. Seven studies included chronic samples and others included first-episode patients (Table 1).
Overall, there was a significant (Table 2) but a very modest relationship between BDNF levels and cognitive functioning in schizophrenia [r = 0.12, confidence interval (CI) 0.04–0.19] (Fig. 1). In meta-analyses of cognitive domains, BDNF levels were significantly associated with verbal memory (r = 0.16, CI 0.09–0.23), working memory (r = 0.14, CI 0.06–0.22), processing speed (r = 0.18, CI 0.10–0.26) and verbal fluency (r = 0.09, CI 0–0.18) performances of schizophrenia patients (Table 2). BDNF levels were not significantly related to EF-speed and planning/problem solving skills in schizophrenia. There was no evidence for heterogeneity of distribution of effect sizes for global cognition (Q = 9.4, p = 0.58, I 2 = 0%) and individual cognitive domains (Q = 3.8–10.2, p = 0.12–0.64, I 2 = 0–46%) except for processing speed (I 2 = 66%). There was no evidence for publication bias for any of the cognitive measures (p = 0.30–0.92).
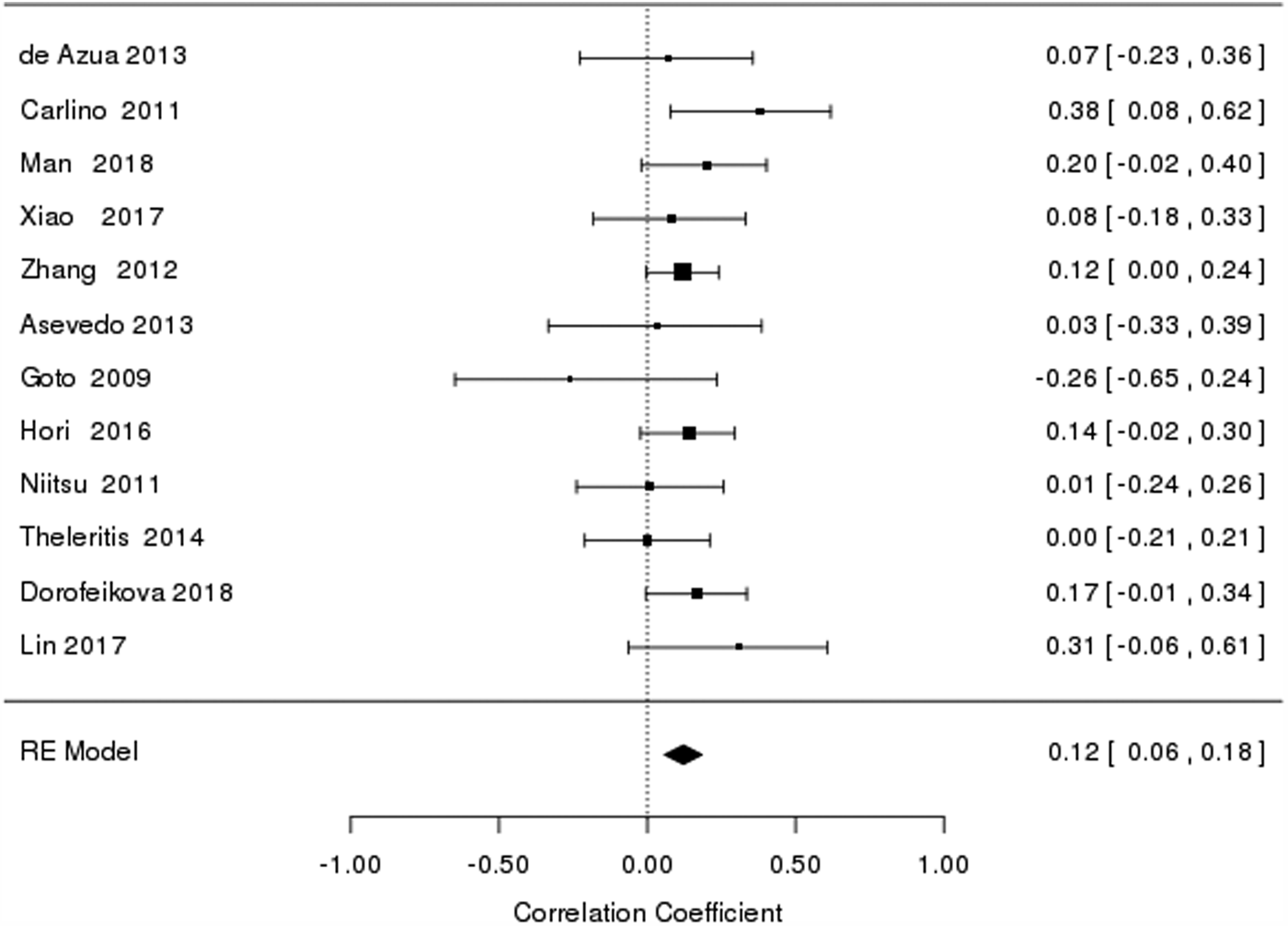
Fig. 1. Forest plot of the meta-analysis of the correlation between BDNF and global cognition.
Table 2. Mean weighted effect sizes for the relationship between BDNF level and severity of cognitive impairment in schizophrenia

r, effect size of correlation; bias, p value of the Egger's test; k, number of studies.
Subgroup analyses suggested that the relationship between BDNF levels and cognition was only significant in chronic samples. Furthermore, there was a statistically significant group-difference between chronic and first-episode patients regarding the level of association between BDNF level and cognitive performance in two domains. These cognitive domains included processing speed (Q bet = 6.6, p = 0.01) and working memory (Q bet = 4.5, p = 0.03). There was no statistically significant group-difference between stable and non-stable patients regarding the level of correlation between BDNF levels and cognitive functioning (Q bet = 0.3, p = 0.62). Meta-regression analyses found no significant effect of age, sex and quality score on the degree of relationship between neurocognition and BDNF levels.
CRP
The CRP meta-analysis included 10 studies including 1602 (65.1% males) patients with schizophrenia-spectrum disorders (Table 1). Mean age was 37.1 years. All of the studies included chronic samples. Seven of the studies included clinically stable patients.
Overall, there was a significant (Table 3) but very modest inverse relationship between elevated CRP levels and cognitive functioning in schizophrenia (r = −0.13, CI −0.08 to −0.18) (Fig. 2). In meta-analyses of cognitive domains, there were significant inverse relationship between elevated CRP levels in nearly all cognitive domains including verbal memory (r = −0.13, CI −0.07 to −0.20), visual memory (r = −0.13, CI −0.04 to −0.22) and working memory (r = −0.13, CI −0.05 to −0.21), processing speed (r = −0.11, CI −0.04 to −0.19), planning/problem solving (r = −0.10, CI 0 to −0.18), EF-speed (r = −0.10, CI −0.03 to −0.18) and attention (r = −0.09, CI 0 to −0.18) (Table 3). CRP levels were not significantly related to verbal fluency in schizophrenia. There was no evidence for heterogeneity of distribution of effect sizes for global cognition (Q = 3.7, p = 0.88, I 2 = 0%) and individual cognitive domains (Q = 0.5–8.8, p = 0.21–0.95, I 2 = 0–37%). There was no evidence for publication bias for any of the cognitive measures (p = 0.15–0.99). Meta-regression analyses found no significant effect of age, sex and quality score on the degree of relationship between neurocognition and CRP levels. There was no statistically significant group-difference between stable and non-stable patients regarding the level of correlation between CRP level and cognitive functioning (Q bet = 1.1, p = 0.28).
Table 3. Mean weighted effect sizes for the relationship between CRP level and cognitive functioning in schizophrenia

r, effect size of correlation; bias, p value of the Egger's test; k, number of studies.

Fig. 2. Forest plot of the meta-analysis of the correlation between CRP and global cognition.
Discussion
The findings of the current meta-analysis suggest that both reduced BDNF levels and elevated CRP levels are significantly related to cognitive impairment in schizophrenia. However, the effect sizes of significant correlations were small.
BDNF is a key regulator of plasticity, synaptogenesis and memory formation, particularly in the medial temporal lobe (Minichiello, Reference Minichiello2009; Leal et al., Reference Leal, Afonso, Salazar and Duarte2015). BDNF might also be important for the integrity of white matter structure (Chiang et al., Reference Chiang, Barysheva, Toga, Medland, Hansell, James, McMahon, de Zubicaray, Martin, Wright and Thompson2011). In this meta-analysis, the strength of the correlation between BDNF was relatively more pronounced for verbal memory, processing speed and working memory. The negative effect of reduced BDNF levels on cortical integrity and efficiency (i.e. medial temporal lobe and other temporal lobe regions) and white matter microstructure (i.e. fronto-temporal dysconnectivity) might play subtle but a significant role in these cognitive deficits in schizophrenia.
However, it is important to note that the nature of the relationship between cognition and BDNF levels in schizophrenia is unknown. The association between reduced BDNF and cognitive impairment in schizophrenia might be mediated with stress, early life trauma, insomnia and loneliness (Aas et al., Reference Aas, Dazzan, Fisher, Morgan, Morgan, Reichenberg, Zanelli, Fearon, Jones, Murray and Pariante2011; Giese et al., Reference Giese, Unternaehrer, Brand, Calabrese, Holsboer-Trachsler and Eckert2013; Meng et al., Reference Meng, Hao, Wei, Sun, Li and Qiu2017) rather than reflecting a direct relationship. Also, the small effect size of the correlations between cognition and BDNF might be interpreted as supporting indirect rather than direct disease related relationship between BDNF and cognition or a direct but marginal relationship between these factors. This might not be surprising as available peripheral biomarkers such as BDNF levels in adults might not be relevant as premorbid neurodevelopmental factors which are the main determinants of cognitive impairment in schizophrenia.
Another important finding in this meta-analysis was the effect of illness stage on the relationship between BDNF and cognition. While the relationship between BDNF level and verbal memory might be apparent in the early stages of the illness, the relationship between BDNF and processing speed/working memory might be evident only in chronic patients. Only cross-sectional studies are included in this meta-analysis as very few studies investigated the relationship between change scores of BDNF and cognition over time (Vinogradov et al., Reference Vinogradov, Fisher, Holland, Shelly, Wolkowitz and Mellon2009). Also, the effect of neurotrophic factors other than BDNF is a neglected area in schizophrenia, other than a very few studies investigating the relationship between glial cell line-derived neurotrophic factor and cognition (Niitsu et al., Reference Niitsu, Shirayama, Matsuzawa, Shimizu, Hashimoto and Iyo2014; Xiao et al., Reference Xiao, Ye, Liu, Tang, Li, Dong, Sha and Zhang2017).
Recently, there has been significant interest in the proposed relationship between inflammation and schizophrenia. Current findings do not support the notion of a major role for inflammation on cognitive impairment in schizophrenia. While the relationship between elevated CRP levels and cognitive impairment was significant for most cognitive domains, the effect sizes of these relationships were quite small. CRP is not the only inflammatory marker associated with schizophrenia (Goldsmith et al., Reference Goldsmith, Rapaport and Miller2016). A meta-analysis of the relationship between cognition in schizophrenia and inflammatory markers such as interleukin (IL)-6, IL-12 and tumor necrosis factor was not possible due to the small number of studies available (Misiak et al., Reference Misiak, Stańczykiewicz, Kotowicz, Rybakowski, Samochowiec and Frydecka2018; Fourier et al., Reference Fourrier, Singhal and Baune2019). However, the findings of the available studies with other inflammatory markers seem to suggest a small-sized relationship between these markers and neurocognition in schizophrenia. However, these findings do not exclude the possibility of an ‘inflammatory subtype' of schizophrenia in which peripheral biomarkers of inflammation might strongly correlate within this subgroup of patients only.
Another consideration is the nature of the relationship between elevated CRP and cognitive impairment in schizophrenia. Elevated CRP levels and other markers of inflammation are not necessarily an illness-related inflammatory process in schizophrenia. Instead, they may reflect co-morbid conditions such as metabolic syndrome, obesity and cardiovascular risk factors (Fond et al., Reference Fond, Lançon, Auquier and Boyer2018) which are more prevalent in schizophrenia compared to healthy adults. Previous studies provided consistent evidence for a significant relationship between metabolic syndrome and cognitive impairment in schizophrenia (Bora et al., Reference Bora, Akdede and Alptekin2017b). Therefore, the small-sized relationship between cognition and CRP levels in schizophrenia might be mediated by other factors. It would be interesting to investigate the specificity of the relationship between cognition and BDNF/CRP to schizophrenia. To date, the outcome of studies investigating peripheral biomarkers and cognitive impairment in bipolar disorder has been less consistent compared to schizophrenia but relatively small number of available studies prevents to conclusively address this issue in bipolar disorder (Bauer et al., Reference Bauer, Pascoe, Wollenhaupt-Aguiar, Kapczinski and Soares2014; Misiak et al., Reference Misiak, Stańczykiewicz, Kotowicz, Rybakowski, Samochowiec and Frydecka2018).
The current report is the first meta-analysis of the relationship between inflammation and neurocognition in schizophrenia. It also aimed to summarize the findings of the studies investigating the relationship between BDNF and cognition in schizophrenia. Current meta-analysis has several limitations. Biomarkers in this meta-analysis were based on the assessment of peripheral blood sample. One may argue that measurement of brain-derived markers in serum or cerebrospinal fluid (i.e. exosomes) might be better candidates of biomarkers in mental disorders (Saeedi et al., Reference Saeedi, Israel, Nagy and Turecki2019). The number of available studies was small for some cognitive domains. Another consideration was the cross-sectional nature of the studies included in this meta-analysis. Also, it was not possible to explore effect of obesity and cardiovascular factors on current findings due to the lack of information in most of the primary studies in this meta-analysis.
In conclusion, reduced BDNF levels and elevated CRP levels are significantly but very modestly related to cognitive impairment in schizophrenia. Inflammation and decreased BDNF levels are not likely to play a major role in cognitive dysfunction in most patients with schizophrenia.
Author ORCIDs
Emre Bora, 0000-0002-1598-6832
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291719001685
Acknowledgements
I would like to thank Berna Yalınçetin for her support in coding the data and literature search.
Conflict of interest
None.







